Abstract
The concentration of coarse and fine indoor particulate matter (PM) was measured along with that of metals (Fe, Cu, Zn, Mn, Pb, Cd, Co, Ni, and Cr) collected from rural and urban residential environments of Pune City with the purpose of examining their free radical generation and its related toxicity. The average mass concentration of indoor PM10 and PM2.5 was ∼3.3 and ∼4.5 times higher than the threshold limits of the Indian National Ambient Air Quality Standards (NAAQS) at urban and rural sites, respectively. The concentration of metals was found to be higher in the fine fraction of PM collected from the rural site. Metal analysis revealed the dominance of Fe concentrations in both PM10 and PM2.5 followed by Zn, Ni, and Cu. Electron paramagnetic resonance (EPR) was used to evaluate the presence of free radicals in samples collected from both the sites. EPR spectra for these particles were attributed to both Fe3+ (g = 1.99∼2.0) and free radicals (g = 2.0023 and g = 2.003) in PM samples. Dithiothreitol (DTT) assay was also performed which supports the presence of higher redox activity in the fine fraction of PM at the rural site. Significant DNA damage was observed by both fractions of PM when tested in the controlled experiment, with averages of 43 and 30.9 % for PM10 and 55.3 and 40.5 % for PM2.5 at the rural and urban sites, respectively. This study articulates a significant amount of evidence to understand the importance of size and composition (especially metals) of indoor PM in the toxicological mechanism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Air pollution is a major environmental issue worldwide and recognized as a leading contributor to the global burden of diseases. There are emerging evidences from numerous scientific investigations that bridge the relation between air pollution and human health (Griffiths 2011; Nawrot et al. 2009). One of the most hazardous air pollutants is inhalable particulate matter (PM) which consists of solid and liquid particles dispersed into air, causing millions of deaths per year. There is a plethora of literature on outdoor PM and its consequences on health risk, while research on indoor PM and related health issues is very minimal and has largely been overlooked.
In recent times, the indoor air problem has become a pivotal concern due to the recognition that people spend a majority of their time indoors. Plenty of potential sources of indoor PM can exceed health thresholds and pose increased life risks (Jonathan et al. 2008). The concentration of indoor PM is an assimilation of various factors viz. sources of indoor particles, movement of pollutants from outdoors to indoors, and removal processes (Nagar et al. 2014; Kwangsam et al. 2004). Indoor PM creates a sense of discomfort and hazards which in turn results into various cardiovascular and respiratory lung diseases and ultimately increases the morbidity and mortality (Goyal and Kumar 2013). It is well documented that toxicological characteristics of indoor PM and the redox activity of these particles have been linked to their chemical composition especially transition metals and organic fractions (Deshmukh et al. 2013; Verma et al. 2009). The redox active transition metals have been identified as a priority concern due to their higher bioavailability and greater toxic potential as they influence both direct and indirect (through inflammation) capacities of particles to generate free radicals which are highly reactive (Wiseman 2015). These free radicals arise from both endogenous (metabolic processes, inflammation) and exogenous (environmental factors) sources (Yang et al. 2015) primarily through three processes: (a) metal-induced free radical production via Fenton reaction, (b) inflammatory responses, and (c) metabolism of xenobiotics by redox cycling processes (Araujo 2011; Sing et al. 2007). Free radicals associated with soluble transition metals are followed by the generation of oxidative stress in airway epithelial cells (Valavandis et al. 2008). This oxidative stress generated by the formation of free radicals by various constituents of PM has been hypothesized as the primary pathway of PM-related health impacts (Yang et al. 2015). Among the various free radicals, the hydroxyl radical is of prime concern (Donaldson et al. 1998) as it is most potent for oxidative DNA damage (Nawrot et al. 2009).
Pune is one of the fastest developing metropolitan cities of the country. Air quality in Pune is deteriorating at a fast pace in terms of PM and becoming a subject of public health concern. Data collected from Central Pollution Control Board (CPCB) India revealed relatively higher levels of PM in Pune, exceeding the prescribed limit of Indian National Ambient Air Quality Standards (NAAQS) (40 μg m−3 for PM2.5 and 60 μg m−3 for PM10) (CPCB 2010). Most of the studies exhibited a higher concentration of PM in the atmosphere of Pune (Sonaje et al. 2013; Satsangi et al. 2014), but few of those have been employed on indoor PM. Alongside, no such research has been found based on the toxicity assessment of indoor air quality in Pune. In order to fill this research gap, the present study is mainly focused on size, chemical composition, presence of free radicals, and toxicity assessment of indoor PM collected from rural and urban sites of Pune City.
Materials and methods
Site description
Pune (18º 32′ N, 73º 51′ E), the cultural capital of Maharashtra, is situated on the western mountain ranges of Deccan Plateau. Pune has tropical wet and dry climate with average temperatures ranging between 20 to 41 °C. Due to ample job opportunities in various sectors, the population level of the city is rising day by day. Pune has a mixture of various industries such as agricultural products, steel, glasswork, automobiles, electronic items, and many others. Growing industrializations along with urbanization have resulted in high loads of atmospheric pollution in Pune City.
Sampling and analysis
Sample collection
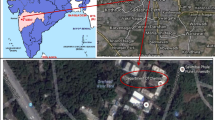
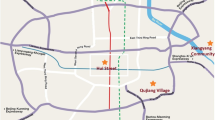
To study the physical, chemical, and toxicological characteristics of indoor PM, sampling of both PM10 and PM2.5 was carried out at two sites of Pune, urban and rural (Fig. 1). Table 1 depicts the detailed sampling profile of the indoor environments of both urban and rural sites. Urban houses are built with bricks and cement, with good flooring and painting, and rural houses are made up of mud, stones, and cow dung. The rural site is located in a region with relatively low traffic density and lots of greenery, whereas urban houses are situated in an area with high vehicular activities approximately about 2 km away from the sampling site. These urban houses use the conventional method for cooking (LPG), whereas at most of the rural houses, cooking is done mostly with traditional fuel such as wood, coal, oil fuels, and others. A low-volume air sampler (MiniVol TAS, Airmetrics, USA, flow rate 5 L min−1) was used for the collection of both PM10 and PM2.5 on pre-weighed quartz and PTFE filters (Whatman, 47 mm diameter), respectively, on a 24-h basis. Sampling was carried out during the period of December 2012–November 2013 from both urban and rural sites of Pune, covering the span of all three seasons, i.e., winter (December–February), summer (March–May), and monsoon (June–August). After collection, the particle-loaded filters were kept in filter cassettes, sealed in plastic bags, and refrigerated at about 4 °C until analysis.
Gravimetric analysis
Before and after sampling, the filters were conditioned for 24 h for accurate measurement of PM. The filters were then weighed by using an analytical five-digit balance until three reproducible values were obtained. To reduce the possibility of contamination, the PM-loaded filters were handled with Teflon tape-coated tweezers.
Inductively coupled plasma-atomic emission spectroscopy analysis
In this study, extraction of half of the filter samples was done by using Milli-Q water at room temperature to determine water-soluble metals. Filters were ultrasonicated for 1 h with 25 mL of Milli-Q water (pH ∼5.8) followed by centrifugation for analyzing the heavy metals (Pb, Zn, Ni, Fe Cr, Mn, Cu, Cd, Co) by inductively coupled plasma-atomic emission spectroscopy (ICP-AES) (ARCOS, Spectro, Germany). The detailed method of the instrument is provided elsewhere (Satsangi et al. 2014).
Quality control
To check the consistency in the flow rate of the sampler, daily flow rate calculations (gas meter reading/timer reading) were done. No significant differences have been found in the flow rate. Cleaning of the sampler was done regularly so that no traces of dust can be counted in the mass concentration of PM samples. In addition to this, background contamination of the heavy metals was also measured in filter blanks. These values were found to be below method detection limits (Satsangi et al. 2014).
Electron paramagnetic resonance study
PM-loaded filters were cut into small strips, rolled, and inserted into a quartz tube, and the electron paramagnetic resonance (EPR) spectra were recorded. The EPR spectra of all the samples were measured by means of a Varian E-109 spectrometer with the following parameters: modulation frequency 100 kHz, X-band microwave frequency 9.1 GHz, modulation amplitude 2 G, time constant 1 s, receiver gain 4000, scan time 4 min, and scan range 400 G. All EPR analyses were done at a low temperature (77 K). The radical concentration was calculated by double integration of the first derivative of the EPR spectra. The g values and radical concentration (spins/g) were determined by comparison with standard 2, 2- diphenyl-1-picrylhydrazyl (DPPH).
Dithiothreitol assay
Dithiothreitol (DTT) assay was used in this study to measure the oxidative potential of indoor PM. This assay is based on the measurement of DTT loss into its disulfide form by the redox active constitutes of PM. DTT was incubated with phosphate-buffered saline (PBS)-extracted PM samples for 30 min at 37 °C followed by 20 min vigorous shaking. Trichloroacetic acid (0.50 mL) was added to stop the reaction, and the remaining DTT was allowed to react with 10 mM 5, 5′-dithiobis-(2-nitro benzoic acid) (DTNB) (made in 0.10 M phosphate buffer; pH 7.4) to form 2-nitro-5-thiobenzoate (TNB) which was detected by a UV-visible spectrophotometer (Shimadzu UV-2501PC) at 412 nm (Charrier and Anastasio 2012).
Plasmid DNA assay
Particles were separated from the filter substrate by immersing one fourth of the filter in 1 mL PBS (1×). The samples were first ultrasonicated for 15 min followed by vortexing. Agarose gel (0.8 %) and ethidium bromide (0.25 %) were put into the electrophoresis unit filled with Tris borate EDTA (TBE) buffer. PBS-extracted samples (10 μL) were incubated with 0.8 μL of Phi X174 supercoiled phage DNA (Bangalore GeNei) at 37 °C for 20 min. The mixture was carefully loaded into the wells of gel along with 2 μL of tracking dye (bromophenol blue). The same treatment was done with the OH radical scavenger (mannitol 4 mM). The gel was run at 80 V for 8 h. The gel image was visualized through Imagining System (EC3 Chemi HR 410), and the percent DNA damage induced by indoor particles was calculated with respect to control.
Results and discussion
Indoor air quality depends on numerous factors such as ventilation, pollutant sources, and infiltration of ambient pollution which makes indoor air pollution among the top category of environmental risks (Gibson et al. 2013; DeBrouwere et al. 2007). This study is based on the assessment of indoor air quality of residential areas: rural and urban sites of Pune.
Mass concentration of PM10 and PM2.5
Table 2 depicts the average mass concentration of PM10 and PM2.5 at both urban and rural sites of Pune City. The mass concentration of PM10 varied from 45.8 to 250 μg m−3 for the urban site with a mean value of 176 ± 57 μg m−3 and 83.3 to 630 μg m−3 for the rural site with a mean value of 283 ± 185 μg m−3 while the PM2.5 concentration varied from 27.8 to 263 μg m−3 for the urban site with a mean value of 105 ± 61 μg m−3 and 27.8 to 397 μg m−3 for the rural site with a mean value of 176 ± 126 μg m−3. The average mass concentration of PM10 and PM2.5 was significantly higher at the rural site in comparison to the urban site during the study period. On comparing the average mass concentration of PM10 and PM2.5 with Indian NAAQS (40 μg m−3 for PM2.5 and 60 μg m−3 for PM10), our results exceeded 4.5 times for the rural site and 3.3 times for the urban site which indicates poor air quality of Pune in terms of PM. PM10 and PM2.5 concentrations were 3.5 and 7 times higher for the urban site and 5.6 and 3.5 times higher for the rural site, respectively, than the prescribed standard values of the US Environmental Protection Agency (USEPA) (50 μg m−3 for PM10 and 15 μg m−3 for PM2.5). The average PM2.5 to PM10 ratio at the urban and rural sites was found to be 0.60 and 0.62, respectively. It suggests that PM10 is composed of 60 % PM2.5 at the urban area and 62 % PM2.5 at the rural site, indicating fine particles comprised a large fraction in PM10 at both sites. As regards health impact, slight domination of fine particles over coarse ones can be a threat as fine particles penetrate deep into the alveoli of human lungs through inhalation (Habil et al. 2013; Pipal et al. 2011). Table 3 represents the comparative study of mass concentration of indoor PM collected from different geographical locations. The mass concentration of PM10 and PM2.5 at both urban and rural sites of Delhi City (Kulshreshtha and Khare 2011) is much higher than the mass concentration of the present study. The possible reasons for high concentration of PM found by them were cooking and improper ventilation for the rural site and infiltration of outdoor air at the urban site. The mass concentration of both fractions of PM at the rural site in the present study was higher than corresponding values at the rural site of Agra City (Kulshrestha et al. 2014). In addition to this, the overall mass concentration of PM is higher in the present study than the concentration reported at the United Arab Emirates (Funk et al. 2014), Malaysia (Nazariah et al. 2013), and China (Jiang and Bell 2008) and lower than the concentration reported in Pakistan (Nasir et al. 2013).
Seasonal analysis in mass concentration of PM10 and PM2.5 showed significant variation at both sampling sites. Mass concentration of both sized PM was higher during winter (157.4 μg m−3 for PM10 and 135.8 μg m−3 for PM2.5) followed by monsoon (124 μg m−3 for PM10 and 64.7 μg m−3 for PM2.5) and summer (82.45 μg m−3 for PM10 and 62.91 μg m−3 for PM2.5) at the urban environment. This could be due to the lack of ventilation as windows are kept closed during the winter which results in the saturation of PM inside the houses, whereas for the rural site, mass concentration was maximum in monsoon season (753.4 μg m−3 for PM10 and 284.7 μg m−3 for PM2.5) followed by winter (250.94 μg m−3 for PM10 and 209.88 μg m−3 for PM2.5) and exhibited the lowest during summer (97.70 μg m−3 for PM10 and 92.59 μg m−3 for PM2.5). During monsoon at the rural site, cooking activities are performed inside which may result in a higher concentration of PM indoors (Satsangi et al. 2014).
Table 4 presents a comparative study of seasonal variation in mass concentration of PM obtained in the present study with worldwide data. The average mass concentration (present study) exhibited higher values during winter for both PM10 and PM2.5 in comparison with Pittsburg (USA) (Tunno et al. 2015), Athens (Greece) (Diapouli et al. 2011), Tallinn (Estonia) (Orru et al. 2014), and Agra (India) (Massey et al. 2012), whereas these values are lower in Pakistan (Ali et al. 2015) and Delhi (Kulshrestha and Khare 2011). In summer, the measured average mass concentration for both fractions of PM was higher than those of the USA, Greece, Estonia, and Pakistan but lower than those of Agra and Delhi (PM10). The mass concentration of PM10 and PM2.5 throughout monsoon was only measured in Agra. Therefore, the comparison of both sized PM in the present study has been done only with respect to Agra City and was found to be higher.
Heavy metal concentration
Residential particulate matter was found strongly contaminated with Fe, Zn, Ni, and Cu followed by Mn, Co, Cd, Pb, and Cr. Table 2 shows average concentrations of metals in PM10 and PM2.5 at both urban and rural sites during the entire study period. At both sites, Fe was the highest contributor for both PM10 and PM2.5 while Cd and Mn provided the least contribution in both size fractions of PM. On average, the decreasing metal concentration trend in PM10 was Fe > Zn > Cu > Ni > Pb > Cr > Co > Mn > Cd for the rural site and Fe > Zn > Ni > Mn > Co > Cr > Cu > Pb > Cd for the urban site, while in PM2.5, it was Fe > Zn > Ni > Pb > Cr > Cu > Co > Cd > Mn for the rural site and Fe > Cu > Ni > Zn > Co > Cd > Cr > Pb > Mn for the urban site. In both PM10 and PM2.5, Fe exhibited the highest concentration among all the analyzed elements at both the sampling sites. It could be a result of excessive usage of utensils and may also originate from re-suspension of dust from indoor human activities (Kwangsam et al. 2004).
Factor analysis (using SPSS 16) was applied to identify the principal sources which influence the concentration of metals at both the sampling sites. The urban site of Pune consisted of well-planned and modern types of houses with proper ventilation, many commercial activities going on in the surrounding areas, and high population density. Table 5 represents two rotated factors explaining more than 90 and 88 % of the total variance for PM10 and PM2.5 at the urban site, respectively. Factor 1 with variance of 43.3 and 25.3 % in PM10 and PM2.5 shows high loading of Co, Cr, Cu, Mn, Ni, and Pb, suggesting their emission from common sources like excessive usage of electric appliances and metallurgical products used in indoor environments (Nazir et al. 2011). Sources of Mn, Pb, Ni, and Cr are expected originate from household dust emitted by walls, cleaning, and other human activities. Possible sources of Cu are primarily metallic cooking utensils because of the combination of corrosion, wear, and abrasion or emitted from the food during cooking (Balasubramanian et al. 2012). Additionally, PM2.5 was also loaded with Zn and Fe in factor 1. Factor 2, with variance of 47.4 and 17.4 %, was dominated by Cr, Zn, and Mn in PM10 and Zn in PM2.5. Zn is mostly released from cooking fumes and various other indoor building materials like paint and plastics (Taner et al. 2013) whereas Cr is derived from smoking of cigarettes, cosmetics, and paint chips (Madany et al. 1994). Mn is used in unleaded gasoline as antiknocking agent and can be also used as an additive in petroleum products (Kulshrestha et al. 2014). This metal may be profoundly influenced by ambient air that infiltrates into houses as this site is located near the roadside.
Rural sites are characterized by low traffic density and agricultural activities in close vicinity with improper ventilation. At rural site, the factor analysis suggested contribution from two factors for PM10 with a total variance of 85.9 %. Candles and fuel lamps are used as alternative methods of lightening as this site usually faces power outage and three factors for PM2.5 with the total variance of 89.2 % (Table 3). Elements like Cr, Cu, Co, Pb, Zn, and Mn represent factor 1 with 65.7 % variance in PM10 whereas Cu, Cr, and Mn represent 43.2 % variance in PM2.5. Pb, Mn, and Zn may be attributed to vehicular activities that are generally used for agricultural purposes at these sites (Park and Dam 2010). Stainless steel used indoors primarily consists of Cu and Cr. Therefore, the probable common sources of these two metals may be associated with cooking utensils. Cd and Fe represent factor 2 with 20.2 % variance in PM10. Both of these species predominantly originate from fossil fuel combustion and biomass burning which are frequently carried out at this site (Watson et al. 2001), whereas in PM2.5, factor 2 was enriched with Cd, Ni, and Pb with a variance of 34.1 %. The candles with lead wicks have the potential to generate indoor airborne Pb particles. Combustion of coal and other fuels used for cooking and lighting purposes can be the possible common origin of Ni and Cd at this site. Factor 3 (PM2.5) represents 11.9 % of total variance for the loading of Zn and Fe attributed to the infiltration of dust.
Free radical generation and EPR
Generation of free radicals in PM has been suggested as one of the most important mechanisms underlying the toxicity of particulate matter. For assessment of this activity, EPR method was used to characterize PM according to their paramagnetic properties. Figure 2a, b shows the EPR spectra of unexposed filters, where no signals were found. Figure 3a–d shows EPR examination of both PM10 and PM2.5 which display a singly unstructured similar kind of spectra with only differences in peak intensities. The average ∆Hp–p (peak to peak width) was found to be 8–10 Gausses. The broadness of these EPR signals indicates the presence of several paramagnetic radical species and organic-metal interaction (Gehling et al. 2013). g values for all the spectra were calculated. For PM10, the observed g value in all samples was 1.99 (i.e., ∼2.0) which indicates the presence of high-spin ferric ion (Fe3+), whereas in PM2.5, a set of different g values, i.e., 1.99 (i.e., ∼2.0), 2.0023, and 2.003, were observed. EPR data revealed that both particulate sizes contain Fe3+ which is corroborated by the ICP data (Table 2). In presence of Fe, formation of hydroxyl radicals (·OH) via Fenton-type reactions has been suggested as a key feature of the PM toxicity (Schins et al. 2004). In addition to this, PM2.5 contains both purely C-centered radical (g = 2.0023) and C-centered radical with vicinal oxygen atom (g = 2.003). In the present study, radical concentration of the paramagnetic center was also calculated with reference to the stable free radical, i.e., DPPH. In indoor samples, radical concentration was found in the range of 1017∼1018 radicals/g of PM for both PM10 and PM2.5. Therefore, these calculated values indicate that indoor PM of Pune is composed of free radicals in high concentration at both urban and rural sites.
EPR gives direct quantification of radical species in the samples, but the complexity and high price of this instrument makes its use very limited (Hedayat et al. 2015). In addition to this, EPR gives only the qualitative information about the free radical generation but, in order to quantify redox-active moieties in PM responsible for the oxidative stress through ROS generation, DTT assay was done. The DTT assay is considered biologically relevant because DTT loss has been correlated with cellular oxidative stress in vitro. In this study, DTT activity has been measured in terms of DTT loss for both PM10 and PM2.5 at the urban and rural sites of Pune. For both fractions of PM, significant rise in DTT loss (in μM) was observed as compared to the control, suggesting that collected indoor PM samples are highly oxidative in nature. Figure 4 shows DTT loss of both the fraction of PM at two sampling sites. DTT loss exhibited the highest value in PM2.5 mode at both sites. Various studies have reported similar observations regarding the effect of particle size on per mass DTT loss (Ntziachristos et al. 2007). Among these two sites, DTT loss was found to be maximum at the rural site for both sized PM which is directly influenced by the high concentration of metals at this site (Table 2). It is speculated that DTT loss increases with the presence of more redox active species in samples; that is why it is needed to assess the significance of metals towards this loss of indoor particles. Pearson’s coefficients also have been calculated for both PM10 and PM2.5 (Table 6). A significant correlation of water-soluble Cu and Ni concentration with DTT loss was observed with r values of 0.64 and 0.67, respectively. Our results indicate the significant contribution of metals in DTT loss of indoor PM (Kam et al. 2011) as these metals have potential to form ROS such as OH· and HOOH by participating in Fenton reaction (Charrier and Anastasio 2012). In contrast to Cu and Ni, Fe concentration was not correlated with DTT loss, even though Fe is known to form reactive oxygen species (ROS). The possible reason for this may be due to the binding of Fe with organic ligand, altering the redox activity of Fe on formation of Fe-DTT complexes. Similar results were also reported by Lin and Yus (2011), suggesting the loss of DTT due to Cu metal and not by Fe. In the context of this present study, the significant correlation of DTT loss with Cu and Ni concentrations emphasizes the significant contribution of these metals to the generation of free radicals.
Plasmid DNA assay
Metals are implicated as one of the possible causes of the adverse effects of PM on human health. The presence of a high concentration of metals in PM is one of the main contributors to free radical generation and these radicals cause oxidative stress and ultimately damage the DNA. To evaluate the genotoxic effect of Pune PM, indoor samples have been extracted in PBS and DNA strand breakage was quantified by gel electrophoresis technique. Figure 5 depicts the typical output from DNA plasmid assay where there is a strand breakage of DNA by indoor PM samples and protection effect by mannitol. Among various ROS, the ·OH radical is the most potent to cause DNA damage formed via Fenton reaction in the presence of H2O2 and metals (Halliwell and Gutteridge 1999). In the present study, mannitol has been used as ·OH radical scavenger and tested to determine whether its presence could protect DNA scission and measure the role of ·OH in the degradation of DNA. On treatment of Phi X174 supercoiled phage DNA with PM, samples collected from urban and rural sites resulted in significant strand breakage with an average of 43 and 30.9 % for PM10 and 55.3 and 40.5 % for PM2.5 at the rural and urban sites, respectively (Fig. 6). On the basis of these findings, PM2.5 caused more oxidative DNA damage than PM10. Fine particles are generally considered as more toxic because of the abundant presence of reactive oxygen species (Hung and Santschi 2001). In comparison to the rural PM samples, urban PM samples were found to be less potent in inducing oxidative DNA damage due to the low concentration of metals (total metal concentration was 736–795 ng m−3 in urban and 829–1306 ng m−3 in rural). It can be visualized in Fig. 6 that co-incubation with mannitol caused a significant reduction of DNA strand breakage. The average DNA strand breakage was reduced to 28.5 and 20.9 % in PM10 and 10.5 and 8.3 % in PM2.5 for the rural and urban sites, respectively, suggesting the involvement of ·OH in mediating a proportion of DNA damage via Fenton-type reaction. In the present study, the observed DNA breakage showed significant correlation with the concentration of soluble metals such as Fe (r = 0.68), Cu (r = 0.58), and Ni (r = 0.76), indicating the ability of PM to cause DNA damage. It may be due to the water-soluble metals which promote radical generation via Fenton reaction (Table 6). Fe is a well-known Fenton active metal and considered to exert its effect via formation of ·OH radical (Charrier and Anastasio 2011). The ICP-AES analysis indicated that Fe concentration in PM10 and PM2.5 samples was much higher than that of other metals which suggests that Fe may be responsible for DNA damage through radical generation. However, other metals such as Cu and Ni in PM have potential to perform Fenton chemistry and have been reported to elicit DNA damage (Oikawa et al. 2002). Our data demonstrated the key role for Fenton-driven ·OH in induction of DNA damage. DNA scission was not completely protected by the mannitol, as it is an effective scavenger for OH· and not capable of scavenging other radicals (like peroxide, superoxide) during the reaction..
Conclusions
The quality of indoor air and the resultant risk associated with regular exposure to particle-bound metals are major concerns for building occupants. This paper is aimed at expanding the present knowledge about the physico-chemical and toxicological characteristics of indoor particles in both coarse and fine size ranges at urban and rural sites of Pune City. The average mass concentration of indoor PM10 and PM2.5 was higher than the Indian NAAQS and USEPA standards. The monitoring data revealed that the mass concentration of PM10 and PM2.5 was maximum in winter season at the urban site whereas for the rural site it showed a higher value in monsoon. Among the metals, Fe showed the highest concentration for both particulate sizes at both sampling sites followed by Zn, Ni, and Cu. Heavy metal concentration was observed to be higher in fine fraction of PM at the rural site. Factor analysis identified the possible common sources such as combustion of fossil fuels, coal, re-suspension of household dust, waste dumping, paint, candle burning, and outdoor impact influencing the metal concentration at the sampling site. EPR study divulged the presence of free radicals in PM samples collected from both the sites of Pune. In both PM10 and PM2.5, the g value of 1.9 corresponding to Fe3+ shows that Fe plays a pivotal role for oxidant-generating activity. The oxidative capacity measured by DTT assay exhibited fine PM as highly oxidative in nature as compared to PM10. Toxicity of indoor PM was evaluated by gel electrophoresis, and significant DNA strand breakage was caused by both fractions of particulate matter, slightly higher in the fine fraction, which was inhibited to some extent in the presence of mannitol suggesting the OH· radical was the main cause of this damage. Presented results suggest a mechanism of genotoxicity of indoor PM in Pune is based on OH radical generation through Fenton reaction. Plasmid DNA breakage showed significant correlation with metals such as Fe, Cu, and Ni, indicating that oxidative DNA damage induced by PM10 and PM2.5 was mainly due to the water-soluble metals.
References
Ali Z, Naz F, Nasir A, Sidra S, Colbech I (2015) Particulate pollution in urban residential built environment during winter and summer seasons in Lahore, Pakistan. J Anim Plant Sci 2:660–665
Araujo JA (2011) Particulate air pollution, systematic oxidative stress, inflammation and atherosclerosis. Air Qual Atmos Health 4:79–93
Balasubramanian J, Shahul HMK, Tamilselvan R, Vijaykumar N (2012) Artificial neural network: a forecast in pharmaceutical science. Nerve 1:7–12
Charrier JG, Anastasio C (2011) Impacts of antioxidants on hydroxyl radical production from individual and mixed transition metals in a surrogate lung fluid. Atmos Environ 45:7555–7562
Charrier JG, Anastasio C (2012) On dithiothreitol (DTT) as a measure of oxidative potential for ambient particles: evidence for the importance of soluble transition metals. Atmos Chem Phys 9321–9333
CPCB (Central Pollution Control Board) (2010) Air quality monitoring and emission source apportionment study for Pune [ARAI/IOCL-AQM/R-12/2009-10]
DeBrouwere KE, Goelen M, Spruyt RT (2007) Ranking indoor air health problems using health impact assessment. Final report. Service Contract for the European Commission. DG. Environment: contract 061651.2007/1MS/R/394
Deshmukh DK, Deb MK, MKoma SL (2013) Size distribution and seasonal variation of size segregated particulate matter in ambient air of Raipur City, India. Air Qual Atmos Health 6:259–276
Diapouli E, Eleftheriadis K, Karanasion AA, Vratolis S, Hermansen O, Colbeck I, Lazaridis M (2011) Indoor and outdoor particle number and mass concentrations in Athens. Aerosol Air Qual Res 11:632–642
Donaldson K, Li XY, MacNee W (1998) Ultrafine (nanometre) particle mediated lung injury. J Aerosol Sci 29:553–560
Funk WE, Pleil JD, Pedit JA, Boundy MG, Yeatis KB, Nash DG, Trent CB, Sadig MEI, Devidson CA, Leith D (2014) Indoor air quality in the United Arab Emirates. J Environ Prot 5:709–722
Gehling WM, Khachatryan L, Dellinger B (2013) Hydroxyl radical generation from environmentally persistent free radicals (EPFRs) in PM2.5. Environ Sci Technol DOI: 10.1021/es401770y
Gibson JM, Bramer A, Davidson C, Folley T, Lauvay F, Thomson J (2013) Environmental burden of diseases. Environ Sci Technol Libr Springer 110. doi:10.1007/978-94-007-5925-1
Goyal R, Kumar P (2013) Indoor-outdoor concentrations of particulate matter in nine microenvironments of a nix-use commercial building in megacity, Delhi. Air Qual Atmos Health 6:747–757
Griffiths SJ (2011) Implications of individual particulate matter component toxicity for population exposure. Air Qual Atmos Health 4:189–197
Habil M, Massey DD, Taneja A (2013) Exposure of children studying in school of India to PM levels and metal contamination: sources and their identification. Air Qual Atmos Health. doi:10.1007/S11869-073-0201-3
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine. Oxford Univ. Press, Oxford, U.K.
Hedayat F, Stevanovic S, Miljevic B, Bottle S, Ristovski ZD (2015) Review- evaluating the molecular assays for measuring the oxidative potential of particulate matter. Chem Ind Chem Eng Q 2(1):201–210
Hung CC, Santschi PH (2001) Spectrophotometric determination of total uronic acids in seawater using cation-exchange separation and pre-concentration by lyophilization. Anal Chim Acta 427:111–117
Jiang R, Bell ML (2008) A comparison of particulate matter from biomass- burning rural and non-biomass-burning urban households in northeastern China. Environ Health Persp 116(7):907–914
Jonathan G, Kulkarni N, Pierse N, Rushton L, Callaghan CO, Rutman A (2008) Black-pigmented material in airway macrophages from healthy children: association with lung function and modeled PM10. Res Rep Health Eff Inst 134
Kam W, Ning Z, Shafer MM, Schauer JJ, Sioutas C (2011) Chemical characterization and redox potential of coarse and fine particulate matter (PM) in underground and ground–level rail systems of the Los Angeles metro. Environ Sci Technol 45:6769–6776
Kulshreshtha P, Khare M (2011) Indoor exploratory analysis of gaseous pollutants and respirable particulate matter at residential homes of Delhi, India. Atmos Pollut Res 2:337–350
Kulshrestha A, Massey DD, Masih J, Taneja A (2014) Source characterization of trace elements in indoor environments at urban, rural and roadside sites in a semi arid region of India. Aerosol Air Qual Res 14:1738–1751
Kwangsam N, Sawant AA, David R (2004) Trace elements in fine particulate matter within a community in western riverside county, CA: focus on residential sites and a local high school. Atmos Environ 38:2867–2287
Lin P, Yu JZ (2011) Generation of reactive oxygen species mediated by humic-like substances in atmospheric aerosols. Environ Sci Technol 45:10362–10368
Madany IM, Akhter S, Jowder OAA (1994) The correlations between heavy metals in residential indoor dust and outdoor street dust in Bahrain. Environ Int 20:483–449
Massey D, Kulshrestha A, Masih J, Taneja A (2012) Seasonal trens of PM10, PM5.0, PM2.5, & PM1.0 in indoor and outdoor environments of residential homes located in north-central India. Build Environ 47(0):22–231
Nagar JK, Akolkar AB, Kumar R (2014) A review on airborne particulate matter and its sources, chemical composition and impact on human respiratory system. Int J Environ Sci 5(2):447–463
Nasir ZA, Colbeck I, Ali Z, Ahmad S (2013) Indoor particulate matter in developing countries: a case study in Pakistan and potential intervention strategies. Environ Res Lett 8:024002
Nawrot TS, Künzli N, Sunyer J, Shi T, Moreno T, Viana M (2009) Oxidative properties of ambient PM2.5 and elemental composition: heterogeneous associations in 19 European cities. Atmos Environ 43(30):4595–4460
Nazariah SSN, Suliana S, Abdah MA (2013) Interleukin-6 via spectrum induction as biomarker of inflammation for indoor particulate matter among primary school children in Klang Valley, Malaysia. Global J Health Service 5(4)
Nazir R, Shaheen N, Shah MH (2011) Indoor/outdoor relationship of trace metals in the atmospheric particulate matter of an industrial area. Atmos Res 101:765–772
Ntziachristos L, Froines JR, Cho AK, Sioutas C (2007) Relationship between redox activity and chemical speciation of size- fractionated particulate matter. Fibre Toxicol 4
Oikawa H, Fujita T, Satomi M, Suzuki T, Kotani Y, Yano Y (2002) Accumulation of paralytic shell wash poisoning toxins in the edible shore crab. Telmessus Acutidens Toxicon 40:1593–1599
Orru H, Mikola A, Upam M, Koiv TA (2014) Variation of indoor/outdoor particulates in Tallinn, Estonia—the role of ventilation, heating systems & lifestyle. J Environ Pollut Hum Health 2(2):52–57
Park K, Dam HD (2010) Characterization of metal aerosols in PM10 from urban, industrial, and Asian dust sources. Environ Monit Assess 160:289–300
Pipal AS, Kulshrestha A, Taneja A (2011) Characterization and morphological analysis of airborne PM2.5 and PM10 in Agra located in North Central India. Atmos Enviorn 45:3621–3630
Satsangi PG, Yadav S, Pipal AS, Kumbhar N (2014) Characteristics of trace metals in fine (PM2.5) and inhalable (PM10) particles and its health assessment along with in-silico approach on indoor environment of India. Atmos Enviorn 92:384–393
Schins RP, Lightbody JH, Borm PJ, et al. (2004) Inflammatory effects of coarse and fine particulate matter in relation to chemical and biological constituents. Toxicol Appl Pharm 195:1–11
Sing R, Sramb RJ, Binkov B, Kalinac I, Popov TA, Georgievad T, Gartee S, Taiolig E, Farmer PB (2007) The relationship between biomarkers of oxidative DNA damage, polycyclic aromatic hydrocarbon DNA adducts, antioxidant status and genetic susceptibility following exposure to environmental air pollution in humans. Mutat Res 620:83–92
Sonaje NP, Mane SJ, Kote AS (2013) Modelling of respirable suspended particulate matter concentration using artificial neural networks in an urban area. Eng Sci Technol 3(4):694
Taner S, Pekey B, Pekey H (2013) Fine particulate matter in the indoor air of barbeque restaurants: elemental compositions, sources and health risks. Sci Total Environ 454-455:79–87
Tunno BL, Shields KN, Cambal L, Tripathy S, Holguin F, Lioy P, Clougherty JE (2015) Indoor air sampling for fine particulate matter and black carbon in industrial communities in Pittsburgh. Sci Total Environ 536:108–115
Valavandis A, Fiotakis K, Vlachogianni T (2008) Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J Environ Sci Health C Environ Carcin Ecotoxicol Rev 26(4):339–362
Verma V, Polidori A, Cassee FR, Schauer JJ, Shafer MM, Sioutas C (2009) Physiochemical and toxicological profiles of particulate matter in Los Angeles during the October 2007 Southern California wildfires. Environ Sci Technol 43:954–960
Watson JG, Chow JC, Chen Li, Wang X, Merrifields TM, Fine PM, Barkers K (2001) Measurement system evaluation for upwind/downwind sampling of fugitive dust emissions. Aerosol Air Qual Res11: 331–350
Wiseman CLS (2015) Analytical methods for assessing metal bioaccessibility in airborne particulate matter: a scoping review. Anal Chim Acta. doi:10.1016/j.aca.2015.01.024
Yang A, Hellack B, Leseman D, Brunekreef B, Kuhlbusch TAJ, Cassee FR, Janssen NAH (2015) Temporal and spatial variation of the metal-related oxidative potential of PM2.5 and its relation to PM2.5 mass and elemental composition. Atmos Environ 102:62–69
Acknowledgments
The authors wish to thank University Grants Commission (UGC-41-324/2012 (SR)), New Delhi, and BCUD, SPPU, Pune, for financial assistance. The authors also express their gratitude to the Head, Department of Chemistry, Savitribai Phule Pune University, for encouragement. IIT, SAIF—Mumbai is also acknowledged for analyzing the samples on ICP-AES and EPR.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roy, R., Jan, R., Yadav, S. et al. Study of metals in radical-mediated toxicity of particulate matter in indoor environments of Pune, India. Air Qual Atmos Health 9, 669–680 (2016). https://doi.org/10.1007/s11869-015-0376-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-015-0376-x