Opinion statement
Hepatocellular carcinoma is the fourth leading cause of cancer-related deaths worldwide and its associated mortality rate is expected to rise within the next decade. The incidence rate of hepatocellular carcinoma varies significantly across countries and the latter can be attributed to the differences in risk factors that are prevalent across different countries. Some of the risk factors associated with hepatocellular carcinoma include hepatitis B and C infections, non-alcoholic fatty liver disease, and alcoholic liver disease. Regardless of the underlying aetiology, the end result is liver fibrosis and cirrhosis that ultimately progress into carcinoma. The treatment and management of hepatocellular carcinoma is complicated by treatment resistance and high tumor recurrence rates. Early stages of hepatocellular carcinoma are treated with liver resection and other forms of surgical therapy. Advanced stages of hepatocellular carcinoma can be treated with chemotherapy, immunotherapy, and the use of oncolytic viruses and these treatment options can be combined with nanotechnology to improve efficacy and reduce side effects. Moreover, chemotherapy and immunotherapy can be combined to further improve treatment efficacy and overcome resistance. Despite the treatment options available, the high mortality rates provide evidence that current treatment options for advanced-stage hepatocellular carcinoma are not achieving the desired therapeutic goals. Various clinical trials are ongoing to improve treatment efficacy, reduce recurrence rates, and ultimately prolong survival. This narrative review aims to provide an update on our current knowledge and future direction of research on hepatocellular carcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past decade, the incidence and prevalence of liver cancer have been rising. Several studies estimate that by the year 2025, the global incidence will be over one million individuals per year. Thus, liver cancer exerts a significant and challenging health burden worldwide. Specifically, hepatocellular carcinoma (HCC) makes up 90% of primary liver cancer diagnoses and is the fourth most common cause of cancer-related deaths worldwide [1].
A multitude of factors are responsible for the increased prevalence of liver cancer. These factors include, among others, lifestyle factors such as the increased consumption of alcohol which may result in alcoholic liver disease, which may then progress to HCC. Additionally, the increasing rates of obesity and consumption of fat-rich diets have contributed to the rising prevalence of non-alcoholic fatty liver disease (NAFLD), one of the main predisposing conditions to HCC [2].
Initially, patients with HCC may not show any signs or symptoms associated with cancer development. Subsequently, patients present with symptoms related to chronic liver disease and typically have a history of significant exposure to one or more risk factors of HCC. The clinical presentation may include upper abdominal pain and distention due to hepatomegaly, weight loss, fever, poor appetite, early satiety, and diarrhea. Patients may also present with coagulation abnormalities manifesting as hematemesis and signs of acute liver decompensation such as jaundice, hepatic encephalopathy, and ascites should raise suspicion of HCC [3].
In rare cases, patients may present with signs and symptoms attributed to paraneoplastic syndromes. These include hypoglycemia, hypercalcemia, polycythemia, the development of feminization in men, and neurological manifestations such as myasthenia gravis and chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). Unlike most cancers, HCC patients develop hypercholesterolemia rather than hypocholesterolemia, along with weight loss, fatigue, and cachexia. Unfortunately, the development of paraneoplastic syndromes indicates cancer metastasis and has a poor prognosis [4].
In patients that have been diagnosed early, treatment options are highly efficacious and are sometimes even curable. Yet many patients suffer from tumor recurrence. Treatment options include surgical resection, ablation therapy, transarterial chemoembolization (TACE), and liver transplantation. However, most cases of HCC present in advanced stages due to the asymptomatic nature of the disease. Surgical options for these patients are not feasible and therefore, systemic therapy is applied [5].
For many years, chemotherapy has been the standard systemic treatment for advanced HCC; however, these agents were highly unsuccessful, and alternative options were limited. Nonetheless, in recent years progress has been made in developing efficacious chemotherapy agents. Moreover, significant progress has been made in establishing alternative treatments to chemotherapy. These include immunotherapy and nanotechnology which constitute two of the most crucial advancements in the management of HCC [6].
In addition to the lack of efficacious treatment options, medical teams face the problem of treatment resistance. Most cases of advanced HCC that are treated with monotherapy develop resistance. As a result, current research is focused on developing combined treatment regimens in an effort to combat resistance. The results of combination retreatments are promising; yet, further research is still needed [7].
The common delays in diagnosis and tumor resistance to treatments have established HCC as a complex and challenging condition that exhibits a severe and substantial burden on the healthcare system and the general population. This review will discuss the recent developments in diagnosis, current treatment options, and ongoing research in the development of innovative treatments for managing HCC.
Methods
Information for this narrative review was obtained from conducting a search on PubMed and ScienceDirect. Keywords used included “Hepatocellular carcinoma,” “HCC Treatment,” “Primary Liver Cancer,” and “Treatment Challenges.” The timeline used during research was from 2013 until 2023. All papers included are in the English language only. Thirty eight journal articles were included in the current review. In addition, information was retrieved from the official website of the European Society of Medical Oncology (ESMO), the official website of the World Health Organization (WHO), and Clinical trials.gov.
Epidemiology
The World Health Organization estimates that more than one million people will die from primary liver cancer in the year 2030 [8]. Primary liver cancer is the sixth most common cancer worldwide with an incidence rate of 9.3 cases per 100,000 person-years (841,000 cases) and is the fourth leading cause of cancer mortality with a reported rate of 8.5 deaths per 100,000 person-years (782,000 deaths) in 2018. HCC makes up 75% of all cases of liver cancer [9].
The incidence of liver cancer is increasing worldwide and the incidence rate of HCC varies significantly across countries. This variation is attributed to each country’s unique demographic characteristics including age, sex, race/ethnicity, and the variety of risk factors associated with HCC. In the USA, the median age at which HCC is diagnosed is between 60 and 64 years in men and 65 and 69 years in women. In contrast, the median age at which HCC is diagnosed in African nations is around 46 years. In most countries worldwide, the incidence rate of HCC is two- to four-fold higher in men than in women. The greatest difference in the incidence rate of HCC between men and women is seen in Europe where men have four-fold higher incidence rates than women. However, in countries such as Uganda, Costa Rica, Ecuador, and Colombia, the differences in incidence rates of HCC among men and women are much smaller [10•]. Despite improved treatment, primary liver cancer still has a relatively poor outcome, with a net 5-year survival rate of 19% and an average of 19 years of life lost per death in the USA [9].
Diagnosis
Imaging modalities such as ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI) play a vital role in the diagnosis of HCC. US is the primary modality used in screening for HCC. It has a specificity of 80–100% and a sensitivity of 51–87%, and with its relative safety and cheap operating costs, it serves as the perfect imaging technique for screening programs [11]. Moreover, the appearance of HCC in US is described as a hypoechoic nodule with hyperenhancement in the arterial phase and washout in the late phase. If such a lesion is found, the patient is sent for a CT or MRI scan, considered the first-line diagnostic imaging modality for HCC. No HCC diagnosis can be made without a CT or MRI scan. Hyperenhancement in the hepatic arterial phase and washout appearance in the portal venous phase are the characteristic features of HCC on CT/MRI scans. If these features are visualized, a diagnosis of HCC can be made without the requirement of a liver biopsy [11]. According to ESMO guidelines, HCC does not require a biopsy for diagnosis unless it develops in a non-cirrhotic liver, as in HBV-associated HCC. In addition, a liver biopsy might be helpful in cases where imaging modalities are inconclusive [12].
Currently, no single staging system for HCC has been established for worldwide use. Many of the existing staging systems are based on their local population, making it difficult for them to be applied internationally. One of the most commonly used staging systems in the USA is the TNM staging system which considers the number of tumors and the extent of tumor vascular invasion as the most important prognostic factors. This system is useful in patients that are candidates for liver transplantation or liver resection. On the other hand, in Japan, the much older Okuda system is most commonly used which considers tumor size as well as the severity of ascites, serum bilirubin level, and albumin level as the main prognostic factors [13]. Moreover, in Europe, the Barcelona Staging classification (BCLC) is the most widely used, and it has been the basis of the current ESMO diagnosis guidelines [14].
Pathophysiology
HCC develops through a stepwise process that progresses as follows: chronic hepatitis, liver fibrosis, liver cirrhosis, and, finally, HCC. Each risk factor associated with HCC results in chronic hepatitis through a different pathophysiological mechanism; however, once chronic liver inflammation develops, the progression to HCC is the same regardless of the initial causative factor [15]. Moreover, genetic mutations and epigenetic modifications with consequent consequences in signaling pathways all contribute to the development of HCC [16].
Viral hepatitis associated with HCV or HBV infection is one of the major causes of HCC in the developing world. Both HCV and HBV cause chronic hepatitis that may progress into HCC, yet via different mechanisms. HCV infects hepatocytes resulting in the activation of hepatic stellate cells that result in fibrosis. The inflammatory response against HCV involves the production of platelet-derived growth factors (PDGF) that are potent stimulators of hepatic stellate cells, further promoting fibrosis. Moreover, the over-expression of HCV proteins has been suggested to promote proliferation, transformation, and tumor formation by directly affecting oncogenic pathways [17].
Unlike HCV, HBV can integrate its genome with the host’s DNA, which explains why some cases of HBV-induced hepatitis progress to HCC without fibrosis or cirrhosis. HBV uses reverse transcription for its replication. Integration of viral DNA occurs early in infection; this also explains why HBV-induced HCC can sometimes have early onset [18]. Integration of the viral genome leads to chromosomal instability, activation of oncogenes, inhibition of tumor suppressor genes, and expression of viral proteins. All these factors drive the pathogenesis of HCC. In addition, HBV infection is accompanied by significant inflammation and fibrosis that contribute to HCC development [19].
As previously mentioned, alcohol is one of the significant risk factors for the development of HCC. Prolonged and excessive ingestion of alcohol leads to the development of alcoholic liver disease (ALD). In excessive alcohol consumption, the metabolites of alcohol metabolism, acetaldehyde and acetate, accumulate in the liver and exert a hepatotoxic effect. Moreover, NADH levels will be significantly reduced, which impairs the body’s protection against oxidative stress. Furthermore, in chronic alcohol abuse, the cytochrome P450 enzymatic system of the liver is upregulated and participates in alcohol metabolism; this results in the excessive generation of reactive oxygen species that contribute to liver damage [20]. On the other hand, liver disease may occur without alcohol-induced damage; such is the case in NAFLD. Patients who are obese, have type 2 diabetes mellitus, or have other metabolic derangements are at risk of developing NAFLD. The development of NAFLD occurs following fat deposition in the liver and cellular and metabolic derangement of hepatocytes that eventually results in hepatitis. Despite the different pathophysiology, both ALD and NAFLD eventually lead to hepatitis, which ultimately can develop into HCC [21].
As it has already been noted, chronic inflammation and fibrosis are two critical steps in the development of HCC. In chronic inflammation, progressive and chronic liver damage induces hepatocyte regeneration and proliferation. As time passes, the more hepatocytes regenerate, the more unstable the genome becomes, and the greater the probability that they gain a cancer-promoting genetic mutation. Furthermore, inflammatory cells, as well as myofibroblasts and endothelial cells of the chronically inflamed liver, secrete cytokines, chemokines, growth factors, and angiogenetic factors that aid in the formation of a tumor-promoting environment in the liver. In addition, these factors activate anti-apoptotic pathways that promote hepatocyte survival [22]. Liver fibrosis involves the uncontrolled deposition of ECM into the liver parenchyma. This excess deposition of ECM disrupts liver architecture resulting in an altered and abnormal liver function. However, multiple studies have revealed that ECM deposition does more than just disrupt liver histology. Deposited ECM promotes tumor development by altering and modulating signaling pathways between components of liver parenchyma, such as epithelial cells, endothelial cells, stromal cells, and inflammatory cells. For example, ECM components can bind to specific growth factors or form complexes with ligands that can bind to them and enhance their activity, thus promoting tumor formation [23].
Management
The treatment of HCC is challenging due to two main reasons. Firstly, HCC has a high recurrence rate despite improved treatment methods. Secondly, many cases of HCC quickly develop resistance to treatment; thus, combined therapy is often required, increasing the treatment burden on the patient. Despite these challenges, extensive research is underway to improve current treatment modalities and introduce new treatment options to overcome these obstacles [24].
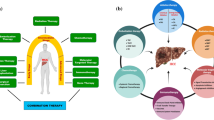
Many guidelines have been established to aid the medical team in determining the most suitable treatment option for the patient. These guidelines consider liver staging, state of liver function, and overall clinical presentation of the patient. Such guidelines include those published by the American Association for the Study of Liver Disease (AASLD) and the European Society for Medical Oncology (ESMO). Figure 1 provides a summary of the main types of treatment implemented in the management of HCC and Table 1 provides an overview of ongoing clinical trials for the management of HCC.
Surgical strategies
Surgical treatments are usually designated for patients in the early stages of liver cancer, and these treatments tend to be curative. Hepatic resection is the recommended treatment option for patients with good liver function, small masses, and no vascular invasion. Furthermore, liver transplantation is the ideal curative treatment in many early cases of liver cancer, as it removes the tumor and prevents any future cirrhosis. However, due to the few donors, liver transplantation is not always feasible, and other treatment options have to be considered [25]. Patients who present with intermediate-stage liver cancer may be candidates for transarterial chemoembolization (TACE). This method involves inserting a chemotherapeutic agent such as cisplatin mixed with iodized oil into the tumor, followed by embolization of tumor arteries using an embolizing agent. The combined effect of the chemotherapeutic agent and ischemia leads to significant damage to tumor tissue [26].
Chemotherapy
HCC is considered a drug-resistant tumor, and most chemotherapeutic agents have shown to be largely ineffective in its treatment. This remained the case until 2007 when the FDA approved sorafenib as a chemotherapeutic agent for HCC. Since then, sorafenib has been recommended as the first-line treatment for advanced stages of HCC. The initial clinical trial involving sorafenib showed an increase of 3 to 5 months in survival time compared to a placebo group. This outcome is far below the established therapeutic goal; however, it paved the way for future developments [27].
Sorafenib is a multi-kinase inhibitor that exerts a powerful anti-tumor and anti-angiogenic effect by blocking cell signaling pathways such as Raf, MEK, and ERK that promote cellular proliferation. In addition, it blocks the vascular endothelial growth factor receptor (VEGFR) and the platelet-derived growth factor receptor (PDGFR), inhibiting angiogenesis, which disrupts tumor cell growth. Despite its wide use, sorafenib has various adverse effects, such as skin rash, diarrhea, increased blood pressure, and redness of soles or palms [28••]. HCC cells that do not use the pathways mentioned above for their proliferation develop resistance to sorafenib, and it has been shown that HCC patients that are on long-term administration of sorafenib develop resistance as well [24].
Several drugs are currently in clinical trials in an attempt to be used as second-line or sequential therapy in patients that are being treated with first-line agents such as sorafenib and develop resistance. These drugs would be given in an attempt to overcome drug resistance that usually develops during the treatment course. One such drug is regorafenib, a multi-kinase inhibitor. The RESORCE trial (NCT01774344) is a randomized, double-blind, placebo-controlled, and phase III clinical trial that was completed in 2019. The study showed regorafenib as an effective second-line therapy in patients that were on sorafenib treatment. Regorafenib provided a significant survival benefit to patients on sorafenib treatment compared to placebo [29•].
Several other chemotherapeutic drugs have been developed recently and are undergoing extensive clinical trials. One drug that has shown potential is lenvatinib. This drug exhibits anti-tumor activity in multiple ways. Firstly, lenvatinib inhibits the vascular endothelial growth factor (VEGF)/vascular endothelial growth factor receptor (VEGFR) pathway, which is responsible for tumor angiogenesis, a process that is essential for tumor growth and metastasis. This is achieved by the binding of lenvatinib on the VEGFR found on tumor cells, thereby preventing the binding of VEGF to its receptor. Secondly, the fibroblast growth factor (FGF)/fibroblast growth factor receptor (FGFR) pathway is abnormally activated in HCC. Tumor cells secrete a large amount of FGF and express an aberrantly high number of FGFR on their outer membranes. The binding of FGF to FGFR activates multiple different signaling pathways, such as RAS/MAPK and PI3K/AKT pathways that play a role in tumor growth and progression. Lenvatinib binds to and blocks FGFR, inhibiting this aberrant pathway. Finally, the RET signaling pathway is responsible for abnormal cell proliferation seen in HCC. This pathway involves the autophosphorylation of tyrosine kinase residues after ligand binding. Lenvatinib blocks the autophosphorylation of the various tyrosine kinase receptors found on tumor cells thereby directly inhibiting cell proliferation [30••].
The REFLECT clinical trial (NCT01761266) compared the clinical outcomes of lenvatinib with those of sorafenib and revealed that lenvatinib had a slightly higher overall survival than sorafenib. Specifically, lenvatinib had an overall survival of 13.6 months, while sorafenib had an overall survival of 12.2 months. In addition, lenvatinib had a significantly higher progression-free survival than sorafenib, with 7.4 months and 3.7 months, respectively. The results of the REFLECT clinical trial are considered the first breakthrough in the chemotherapy treatment of liver cancer since 2007. Lenvatinib is the first drug to show a beneficial effect on overall survival compared to sorafenib. Previously, many drugs, such as sunitinib and linifanib, had been compared to sorafenib; however, they all failed to have any comparable beneficial effect. Based on the results of the REFLECT trial, in 2018, the FDA approved the use of lenvatinib as a first-line treatment for advanced HCC. Additionally, the EU, China, and Japan have also approved the use lenvatinib [30••].
Immunotherapy
In cases of liver cancer where surgical resection or liver transplantation is not feasible, attention is turned toward other treatment options, such as immunotherapy. This treatment modulates the immune system and enhances its ability to detect and kill cancer cells. One method of achieving this involves inhibiting immune checkpoints. For example, the PDL-1 pathway inhibits T cell activation, and this pathway is highly active in tumor tissue. The anti-PDL-1 antibody pembrolizumab, inhibits this pathway, resulting in increased immune system activity against tumor cells [31]. The KEYNOTE 224 clinical trial (NCT02702414) demonstrated that pembrolizumab had a promising response and reasonable adverse effects. According to the trial’s results, the median overall survival in patients that took pembrolizumab was 12.9 months, and 54% of patients were alive after 12 months. However, the trial lacked a randomized control group, one of its main limitations. These promising results led to larger studies being conducted, i.e., the KEYNOTE-240 (NCT02702401) and the KEYNOTE-394 (NCT03062358) clinical trials which showed promising results regarding the efficacy of pembrolizumab in the treatment of HCC [32••].
The CTLA-4 pathway inhibits T cell responses and has also been shown to be excessively activated in tumor cells. Drugs such as ipilimumab inhibit this pathway by blocking the activity of CTLA-4. These drugs have been FDA approved in the treatment of other cancers, such as melanoma and Hodgkin lymphoma, and clinical trials are underway to determine their efficacy in the treatment of liver cancer [33]. In addition to targeting immune checkpoints, tumor cells’ surface markers such as CD133, CD13, and CD44 are being used to target and destroy cancer stem cells via monoclonal antibodies. Since HCC originates from these stem cells, destroying them would significantly reduce the risk of recurrence, one of the main challenges in liver cancer treatment [34].
Combination of chemotherapy and immunotherapy
Combinations of chemotherapy and immunotherapy are in development as a means to combat resistance and improve efficacy. Recently, a clinical trial (An Open-Label Phase 1b Trial of Lenvatinib Plus Pembrolizumab in Subjects With Hepatocellular Carcinoma—NCT03006926) assessed the efficacy of combining lenvatinib with pembrolizumab. The clinical trial was an interventional study that assessed a single group of patients that suffered from HCC but lacked a control group. This study provided evidence that the overall response rate (ORR) was 46% in 100 patients that were evaluated and reported that the tumor completely disappeared in 11% of the patients [30••]. When comparing these results with results from other clinical trials where lenvatinib and pembrolizumab were used as monotherapy, it is evident that combination treatment has much higher efficacy than monotherapy. To illustrate this, lenvatinib in the REFLECT trial (NCT01761266) showed an ORR of 24.1%, and pembrolizumab in the KEYNOTE-224 trial (NCT02702414) showed an ORR of 17%. These results demonstrate the superiority of a combined regimen compared to monotherapy [30••].
The recently published results of the IMbrave150 clinical trial (NCT03434379) revealed that the combination treatment of bevacizumab, an anti-VGEF antibody, and atezolizumab, an anti-PD-L1 antibody, had a higher overall survival when compared with sorafenib monotherapy. Specifically, the combination therapy involving bevacizumab and atezolizumab increased overall survival by 12.6% at 12 months and prolonged the progression-free survival interval by 2.5 months compared to the use of sorafenib. Specifically, overall mean survival with atezolizumab plus bevacizumab was 19.2 months on average, while overall mean survival with sorafenib was 13.4 months. Additionally, progression-free survival was 6.9 months with atezolizumab plus bevacizumab and 4.3 months with sorafenib. Based on these results, in 2020, the FDA approved the use of bevacizumab plus atezolizumab in patients with unresectable HCC [32••].
The evolving role of nanotechnology
In order to overcome drug resistance, it is important to enhance drug delivery to cancer cells. Nanoparticles that can help achieve a highly accurate and precise drug delivery are currently under development. Nanoparticles are highly stable and have a variable structural design, low immunogenicity, and specific tissue/cell targeting abilities, making them a highly attractive modality for medical application and research [35].
The materials that constitute the nanoparticles affect their ability to deliver the drug. The most widely used material is polylactic acid (PLA) because of its ability to actively target tumor tissue as well as its biodegradability allowing it to be administered orally. Another factor contributing to the efficacy of drug delivery via nanoparticles is the method by which these drugs are delivered. One method is targeted delivery, which is split into passive and active targeted delivery systems. Passive targeting involves the leakage of the drug continuously from the nanoparticles into the tumor. On the other hand, active targeting involves an interaction between a specific receptor on the tumor cells and the nanoparticle, which results in drug release into tumor cells. Furthermore, researchers took advantage of the physiological differences in pH between tumor tissue and normal tissue, and designed nanoparticles that release their content in the setting of pathological pH, sparing healthy tissue. Overall, nanoparticles can help resolve the resistance issue by improving drug specificity and targeting. Additionally, nanoparticles limit the amount of healthy tissue exposed to the drug, which helps minimize side effects [28••].
Oncolytic viruses
With the advancements in technology and genetic engineering, a new therapeutic approach to liver cancer has started to emerge, which involves the use of oncolytic viruses which are genetically modified viruses that aim to infect and destroy cancer cells. These viruses exhibit their antitumor effects through two mechanisms. Firstly, they selectively replicate in cancer cells resulting in subsequent cell lysis. Secondly, they induce a systemic immune response that exhibits antitumor effects [36]. For example, ONYX-015 is a genetically engineered adenovirus in which the E1B gene has been deleted. This deletion prevents the virus from replicating in healthy tissue and allows it to replicate only in p53-deficient cells. Another example is the vaccinia virus which is derived from a poxvirus strain similar to smallpox. Initial research suggests that this oncolytic virus might be part of the treatment of liver cancer in the future; however, it is currently in clinical trials involving animal models and would require further extensive research before determining its efficacy [37].
Herpes simplex viruses (HSVs) are another group of viruses being investigated for their efficacy in the treatment of HCC. Given their strong replication ability and their large genome size that can accommodate multiple exogenous genes, HSVs have a promising potential as a treatment agent. Currently, a phase 1 clinical trial (Study of RP2 Monotherapy and RP2 in Combination With Nivolumab in Patients With Solid Tumours (NCT04336241)) is ongoing to assess the efficacy of genetically modified HSV1 in the treatment of HCC. As of now, no results have been published yet [38].
Conclusions
HCC is a complex and challenging cancer with a bleak survival outcome. The 2007 breakthrough in chemotherapy treatment with sorafenib provided hope and paved the way for the development of new chemotherapy agents in the past few years. Moreover, newly developed immunotherapy agents offer physicians the ability to take advantage of the powerful immune system and modulate it to fight off cancer cells. Combined therapy involving chemotherapy and immunotherapy has shown extremely promising results in recent clinical trials and may become the first-line management for treating HCC. Additionally, with the rise of nanotechnology drug delivery systems, a future with reduced cancer resistance and drug side effects becomes even closer. Future studies will provide evidence on whether these advancements will be fruitful. The rate at which treatments are being developed is remarkable; however, it is not enough to meet current needs and challenges such as chemoresistance. Therefore, more clinical trials and studies are required to expand on our current knowledge and provide more treatment options that are both efficacious and safe.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Philips CA, Rajesh S, Nair DC, Ahamed R, Abduljaleel JK, Augustine P. Hepatocellular carcinoma in 2021: an exhaustive update, Cureus. 2021. https://doi.org/10.7759/cureus.19274
Maurice J, Manousou P. Non-alcoholic fatty liver disease. Clin Med. 2018;18(3):245–50. https://doi.org/10.7861/clinmedicine.18-3-245.
Lotfollahzadeh S, Recio-Boiles A, Babiker H. Liver cancer, StatPearls Publishing, 2022. Available at: https://www.ncbi.nlm.nih.gov/books/NBK4. Accessed 20 Dec 2022
Nancy F. Primary liver cancer: surveillance, diagnosis, and treatment. 1st ed. Connecticut: Humana Press; 2012.
Kim E, Viatour P. Hepatocellular carcinoma: old friends and new tricks. Exp Mol Med. 2020;52(12):1898–907. https://doi.org/10.1038/s12276-020-00527-1.
Chidambaranathan-Reghupaty S, Fisher PB, Sarkar D. Hepatocellular carcinoma (HCC): epidemiology, etiology and molecular classification, Adv Cancer Res. 2021;1–61. doi: https://doi.org/10.1016/bs.acr.2020.10.001
Lurje I, Czigany Z, Bednarsch J, Roderburg C, Isfort P, Neumann UP, et al. Treatment strategies for hepatocellular carcinoma – a multidisciplinary approach. Int J Mol Sci. 2019;20(6):1465. https://doi.org/10.3390/ijms20061465.
Samant H, Amiri H, Zibari G. Addressing the worldwide hepatocellular carcinoma: epidemiology, prevention and management. J Gastrointest Oncol. 2021;12(S2):S361–73. https://doi.org/10.21037/jgo.2020.02.08.
Dasgupta P, Henshaw C, Youlden DR, Clark PJ, Aitken JF, and Baade PD. Global trends in incidence rates of primary adult liver cancers: a systematic review and meta-analysis. Front Oncol. 2020;10. https://doi.org/10.3389/fonc.2020.00171
• McGlynn K, Petrick J, El‐Serag H. Epidemiology of hepatocellular carcinoma. Hepatology. 2020;73(S1):4–13. https://doi.org/10.1002/hep.31288. This review demonstrated the impact of population’s demographic characteristics on the risk factors and incidence of HCC. In addition, it provided an overview on the epidemiology of HCC.
Jiang H, Chen J, Xia C, Cao L, Duan T, Song B. Non-invasive imaging of hepatocellular carcinoma: from diagnosis to prognosis. World J Gastroenterol. 2018;24(22):2348–62. https://doi.org/10.3748/wjg.v24.i22.2348.
Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv238–55. https://doi.org/10.1093/annonc/mdy308.
Fenton S, Burns M, Kalyan A. Epidemiology, mutational landscape and staging of hepatocellular carcinoma. Chin Clin Oncol. 2021;10(1):2–2. https://doi.org/10.21037/cco-20-162.
Bednarsch J, Czigany Z, Heise D, Joechle K, Luedde T, Heij L, et al. Prognostic evaluation of HCC patients undergoing surgical resection: an analysis of 8 different staging systems. Langenbeck’s Arch Surg. 2020;406(1):75–86. https://doi.org/10.1007/s00423-020-02052-1.
Pinter M, Trauner M, Peck-Radosavlijec M, Seighart W. Cancer and liver cirrhosis: implications on prognosis and management. ESMO Open. 2016;1(2):e000042. https://doi.org/10.1136/esmoopen-2016-000042.
Khemlina G, Ikeda S, Kurzrock R. The biology of hepatocellular carcinoma: implications for genomic and immune therapies. Mol Cancer. 2017;16(1). https://doi.org/10.1186/s12943-017-0712-x.
Goossens N, Hoshida Y. Hepatitis C virus-induced hepatocellular carcinoma. Clin Mol Hepatol. 2015;21(2):105. https://doi.org/10.3350/cmh.2015.21.2.105.
Nguyen MH, Wong G, Gane E, Kao J, Dushkeiko G. Hepatitis B virus: advances in prevention, diagnosis, and therapy. Clin Microbiol Rev. 2020;33(2). https://doi.org/10.1128/cmr.00046-19
D’souza S, Lau KC, Coffin CS, Patel TR. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J Gastroenterol. 2020;26(38):5759–83. https://doi.org/10.3748/wjg.v26.i38.5759.
Rocco A, Compare D, Angrisani D, SanduzziZamparelli M, Nardone G. Alcoholic disease: liver and beyond. World J Gastroenterol. 2014;20(40):14652. https://doi.org/10.3748/wjg.v20.i40.14652.
Neuschwander-Tetri B. Non-alcoholic fatty liver disease. BMC Med. 2017;15(1). https://doi.org/10.1186/s12916-017-0806-8.
Affo S, Yu L, Schwabe R. The role of cancer-associated fibroblasts and fibrosis in liver cancer. Annu Rev Pathol. 2017;12(1):153–86. https://doi.org/10.1146/annurev-pathol-052016-100322.
Dhar D, Baglieri J, Kisseleva T, Brenner DA. Mechanisms of liver fibrosis and its role in liver cancer. Exp Biol Med. 2020;245(2):96–108. https://doi.org/10.1177/1535370219898141.
Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochimica et Biophysica Acta (BBA) - Rev Cancer. 2020;1873(1),188314. https://doi.org/10.1016/j.bbcan.2019.188314.
Chen Z, Xie H, Hu M, Huang T, Hu Y, Sang N, and Zhao Y. Recent progress in treatment of hepatocellular carcinoma. Am J Cancer Res. 2020;10(9):2993. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7539784/ (Accessed: 31 October 2022).
Petruzzi P, Crocetti L, Lencioni R. Chemoembolization of hepatocellular carcinoma. Semin Interv Radiol. 2013;30(01):003–11. https://doi.org/10.1055/s-0033-1333648.
Qiu Z, Li H, Zhang Z, Zhu Z, He S, Wang X, et al. A Pharmacogenomic landscape in human liver cancers. Cancer Cell. 2019;36(2):179-193.e11. https://doi.org/10.1016/j.ccell.2019.07.001.
•• Kong FH, Ye QF, Miao XY, Liu X, Haung SQ, Xiong L, et al. Current status of sorafenib nanoparticle delivery systems in the treatment of hepatocellular carcinoma. Theranostics. 2021;11(11):5464–90. https://doi.org/10.7150/thno.54822. This review illustrates the constituents of nanoparticles and how they influence drug delivery. In addition, this paper provided insight into the different drug delivery systems with regard to sorafenib in the treatment of HCC.
• Huang A, Yang XR, Chung WY, Dennison AR, and Zhou J. Targeted therapy for hepatocellular carcinoma. Sig Transduct Target Ther. 2020;5(1). https://doi.org/10.1038/s41392-020-00264-x. This paper provides an overview on different chemotherapy and immunotherapy agents available for the treatment of HCC and provides examples of chemotherapy agents used as sequential treatment to sorafenib
•• Zhao Y, Zhang YN, Wang KT, and Chen L. Lenvatinib for hepatocellular carcinoma: from preclinical mechanisms to anti-cancer therapy, Biochimica et Biophysica Acta (BBA) Rev Cancer. 2020;1874(1):188391. https://doi.org/10.1016/j.bbcan.2020.188391. This paper provides a summary of the mechanism of action of lenvatinib and describes clinical trials comparing lenvatinib with standard chemotherapy treatment. Additionally, this paper describes the results of clinical trials in which different combination therapy regimens are compared.
Sangro B, Sarobe P, Herves-Stubbs, and Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18(8), 525-543. https://doi.org/10.1038/s41575-021-00438-0.
•• Luo, X., Wu, K. and He, X. Advances in drug development for hepatocellular carcinoma: clinical trials and potential therapeutic targets, J Exp Clin Cancer Res. 2021;40(1). https://doi.org/10.1186/s13046-021-01968-w. This review demonstrates the different therapy options available for HCC in terms of first-line and second-line treatments that are currently in use as well as therapy options that are at clinical trial stage.
Xu F, Jin T, Zhu Y, and Dai C. Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res. 2018;37(1). https://doi.org/10.1186/s13046-018-0777-4.
Sun JH, Luo Q, Liu LL. and Song GB Liver cancer stem cell markers: progression and therapeutic implications. World J Gastroenterol. 2016;22(13):3547. https://doi.org/10.3748/wjg.v22.i13.3547.
Cao L, Zhu YQ, Wu ZX, Wang GX, Cheng HW. Engineering nanotheranostic strategies for liver cancer. World J Gastrointest Oncol. 2021;13(10):1213–28. https://doi.org/10.4251/wjgo.v13.i10.1213.
Kaufman H, Kohlhapp F, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discovery. 2015;4(9):642–62. https://doi.org/10.1038/nrd4663.
Li Y, Shen Y, Zhao R, Samudio I, Jia W, Bai X, et al. Oncolytic virotherapy in hepato-bilio-pancreatic cancer: the key to breaking the log jam? Cancer Med. 2020;9(9):2943–59. https://doi.org/10.1002/cam4.2949.
Li X, Sun X, Wang B, Li Y, Tong J. Oncolytic virus-based hepatocellular carcinoma treatment: current status, intravenous delivery strategies, and emerging combination therapeutic solutions. Asian J Pharm Sci. 2023;18(1):100771. https://doi.org/10.1016/j.ajps.2022.100771.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None of the authors has any potential conflicts of interest to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alawyia, B., Constantinou, C. Hepatocellular Carcinoma: a Narrative Review on Current Knowledge and Future Prospects. Curr. Treat. Options in Oncol. 24, 711–724 (2023). https://doi.org/10.1007/s11864-023-01098-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-023-01098-9