Opinion statement
Cardiac masses and tumors are a heterogenous group of disorders and include primary tumors (both benign and malignant), metastatic disease, and numerous masquerades such as thrombus. Clinical presentation ranges from incidental discovery on imaging tests ordered for other reasons to life-threatening presentations such as cardiac tamponade, arrhythmia, obstruction, and systemic embolization. Of the available imaging modalities, cardiac MRI is generally the most useful for assessment and helps to delineate the relevant anatomy. Due to the technical difficulties and risk of biopsy of cardiac masses, a presumptive diagnosis is typically made using imaging techniques with surgery serving both a diagnostic and curative role. Because these conditions can vary widely in their management, we recommend early involvement of a multidisciplinary group which should include a cardiologist, cardiac surgeon, and oncologist.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
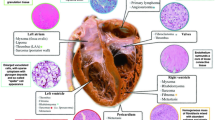
Cardiac masses are uncommonly encountered clinical problems with significant heterogeneity in pathology and clinical presentation. Diagnosis and management can be challenging due to the difficulty in obtaining a biopsy and the reliance on imaging characteristics to make a presumptive diagnosis. Cardiac masses can be classified as benign primary cardiac tumors, primary malignant cardiac tumors, metastases or local invasion from another malignancy, and tumor masquerades such as thrombus or vegetation (Table 1). In this article, we will review the clinical presentation, differential diagnosis, diagnostic modalities, and management and treatment of cardiac tumors.
Epidemiology
Metastatic cancer to the heart is significantly more common than primary cardiac tumors [1]. The most common cancers to metastasize to the heart are lung (37%), breast (7%), and esophageal cancers (6%) and hematologic malignancies such as lymphoma (20%) [2]. Primary cardiac tumors are rare with an incidence in the range of 1.38 to 30 per 100,000 people per year [3]. Of primary cardiac tumors, 80% are benign and 20% are malignant [4]. The three most common primary tumors in patients greater than 16 years of age are myxomas (50%), lipomatous tumors (21%), and papillary fibroelastomas (16%) and account for ~ 85% of benign cardiac tumors in this age group. The four most common tumors in children younger than 16 years of age are rhabdomyomas (55%), teratomas (16%), fibromas (10%), and myxomas (10%) and account for ~ 90% of benign cardiac tumors in this age group.
Clinical presentation
The clinical presentation of cardiac tumors is myriad, ranging from asymptomatic detection on imaging tests ordered for other reasons to shock or aborted sudden cardiac death. When patients have symptoms, they can be categorized as constitutional (fever and weight loss), distal embolization, or direct effects from the tumor. Distal embolization of tumor or thrombus that has aggregated on tumor is common in masses involving the left atrium or left ventricle. Sites of distal embolization determine the clinical presentation which can include stroke, mesenteric ischemia, splenic or renal infarction, or acute limb ischemia. Masses involving the right atrium and right ventricle can result in pulmonary embolism or more rarely systemic embolism in the setting of right-to-left cardiac shunting.
Direct tumor effects of cardiac masses can be further stratified as obstruction, cardiac tamponade, and arrhythmia. Obstructive symptoms such as congestive heart failure or syncope may occur with significant impendence to flow of any cardiac chamber or valve. In the event of a mass in the right atrium or right ventricle, this results in right-sided heart failure symptoms including peripheral edema, ascites, or superior vena cava syndrome. In large left atrial or left ventricular masses, shortness of breath due to pulmonary edema is common. Cardiac tamponade is the most frequent life-threatening presentation of cardiac tumors and occurs due to fluid filling the pericardial space and restricting cardiac filling. This is frequently seen in metastatic disease to the heart or pericardium but is especially common with direct extension of breast or lung cancer. Cardiac tumors can cause arrhythmia due to disruption of normal myocardium. Any arrhythmia can occur, including atrial fibrillation, ventricular tachycardia, and ventricular fibrillation.
The identification of an intracardiac mass should first be placed in the context of the patient’s clinical history. Important factors to consider include age with fibroma or rhabdomyoma more likely in a pediatric patient. Prior cardiac history should be considered, with atrial fibrillation placing patients at risk of atrial thrombus and left ventricular systolic function being associated with left ventricular thrombus [5, 6]. When approaching a valvular mass, risk factors for infective endocarditis such as prosthetic valves or immunodeficiency and thrombotic risk factors such as the antiphospholipid antibody syndrome [7] should be considered. Systemic symptoms like fevers and weight loss may occur with nearly any cardiac mass including myxoma, lymphoma, distant malignancy, and chronic infective endocarditis.
Treatment
Treatment is highly dependent on the tumor type, diagnostic certainty, presence or absence of metastatic spread, clinical presentation, and comorbidities. For instance, cardiac tamponade with cytopathologic confirmation of adenocarcinoma in the setting of known metastatic breast cancer represents a markedly different clinical scenario than a young person who is presenting with an incidentally discovered right atrial mass. As a result of these complexities, decisions on treatment are best made by an interdisciplinary team including a cardiologist, cardiac surgeon, and oncologist. Important considerations when deciding to send the patient for surgery include the need for a tissue diagnosis, presence of obstructive symptoms and need for relief of obstruction, and expected response to chemotherapy and/or radiotherapy. Given the difficulty of biopsy in cardiac tumors, it is our experience that a presumptive diagnosis must be made based on radiologic appearance with surgery undertaken to provide both definitive diagnosis and therapy. In patients with cardiac tamponade, the initial procedure of choice is typically a pericardiocentesis. In the event of recurrent effusion, a more durable solution such as a surgical pericardial window is needed.
Diagnosis
Imaging
Intracardiac masses are diagnosed and characterized using multimodality non-invasive imaging techniques (Table 2). The goal of cardiac imaging is to provide information about mass size, anatomic location and extent, functional implications in relation to flow obstruction, valvular involvement, regional contractility, presence or absence of pericardial metastasis, and effusion (with assessment for evidence of tamponade), and to identify tissue characteristics implicative of particular diagnoses. With these goals in mind, echocardiography, cardiac MRI, and/or computed tomography serve complimentary roles.
Echocardiography
Transthoracic echocardiography is generally the initial diagnostic test utilized for the evaluation of a suspected cardiac mass. Major advantages include wide availability and expertise, high spatial and temporal resolution to identify small, mobile masses (e.g., valvular vegetations or fibroelastomas), and assessment of intracardiac flow via Doppler echocardiography. Major limitations include inability to determine the full extent and origin of a mass due to restricted imaging planes, the potential for poor acoustic windows, and little capacity for tissue characterization. Contrast echocardiography can improve sensitivity for the identification of intraventricular masses, particularly when acoustic windows are poor. It also offers some ability to distinguish tumor from thrombus, as highly vascularized tumors will enhance when contrast is administered [8].
Transesophageal echocardiography is ideally suited for close evaluation of left-sided structures and is particularly useful in the evaluation of masses within the left atrium or involving the mitral valve. It can also be utilized intraoperatively to evaluate the success of surgical debulking in real time, or assess for new valvular dysfunction after adherent masses are resected.
Computed tomography
Cardiac computed tomography (CT) provides a cross-sectional imaging with high spatial resolution for assessing the heart and surrounding structures (lungs, mediastinum, upper abdomen). This makes it a preferred modality for evaluating patients suspected to have a cardiac metastasis with extracardiac primary malignancy. CT is ideal for tracking locally invasive pulmonary, breast, gastrointestinal, or hematogenous malignancies, or following transvenous renal cell or hepatocellular carcinomas to their origins. ECG gating can be performed to reduce motion artifact of the beating heart and produce cine images that provide insight into function. Coronary CT angiography can be performed concurrently to assess coronary and bypass graft anatomy and screen for coronary artery disease for surgical planning. Major limitations include exposure to ionizing radiation (a particular consideration in pediatric patients) and nephrotoxicity with iodinated contrast. In addition, image quality using ECG-gated techniques may be compromised in the context of irregular rhythms or tachycardia.
Tissue characterization can be performed based on differential tissue attenuation, with fat, fluid, and calcific tumor components demonstrating distinctive radiodensities. Contrast enhancement is generally seen in malignant tumors and in highly vascularized benign tumors such as myxoma and angiomas [9].
Cardiac magnetic resonance
Cardiac magnetic resonance utilizes non-ionizing magnetic field and radiofrequency sequences to create detailed multiplanar still or cine images with spatial resolution that nears that of cardiac CT but with higher soft tissue and temporal resolution [9]. CMR can be used to identify anatomic location and extent (full-chest MR angiography or “black-blood” spin echo), assess mass mobility and perform functional assessment (cine-CMR), and highlight differential tissue properties (using gadolinium perfusion images, delayed gadolinium enhancement, and T1- or T2-weighted imaging). It is therefore the most comprehensive test for identifying and diagnosing cardiac masses [10•] and should be strongly considered when expertise is available. Major limitations include contraindication with glomerular filtration rate < 30 ml/min due to risk of nephrogenic systemic sclerosis, relative contraindication with implantable electronic devices—although new evidence supports safety of MRI in this context under established protocols [11], prolonged supine positioning with repeated breath-holds, and patient claustrophobia.
MR angiography and black-blood spin echo sequences provide cross-sectional imaging to evaluate the heart and surrounding structures, notably with lower spatial resolution than CT. Cine sequences provide functional imaging with no limitation to imaging planes. Regarding tissue characterization, first-pass gadolinium perfusion sequences are useful to distinguish highly vascularized tumors from less vascularized tumors and from avascular thrombus [12]. Similarly, delayed enhancement can be used to distinguish tumors that show homogenous uptake of gadolinium (e.g., fibroma) from those with heterogenous uptake (e.g., metastases or angiosarcomas) [13], from avascular thrombi that do not uptake contrast. Such findings, together with mass size and infiltration, can be used to distinguish tumors that are likely malignant from those that are benign [12]. T1-weighted sequences reliably identify fatty tumors such as lipoma or lipomatous hypertrophy [14]. T2-weighted sequences identify masses with high water content, such as myxomas or pericardial cysts.
Positron emission tomography
Positron emission tomography (PET) using 18F-fluoro-D-glucose can be used in combination with CT (or CMR) to assess cardiac masses, with the extent of FDG uptake by tumors differentiating benign and malignant tumors [15]. While dedicated PET scans are rarely performed clinically for evaluating known cardiac masses, cardiac metastases may be identified in staging or screening scans performed to evaluate the primary malignancy. Test characteristics are limited by low sensitivity compared with MRI [16] and potential for false positives such as benign lipomatous hypertrophy being highly metabolically active [17]. A promising application for PET-CMR is in surveilling for intracardiac tumor recurrence, where presence of late gadolinium enhancement by MRI alone may not discriminate between disease recurrence and residual fibrosis [18].
Tissue diagnosis
Pericardial fluid analysis
Pericardial metastasis is common among cancer patients with a prevalence of 10–20% at autopsy and can occur via local invasion, lymphatic spread, or secondary involvement of the pericardium from myocardial metastases [19]. A pericardial effusion—characteristically hemorrhagic—often manifests. Rarely, a malignant pericardial effusion may occur without a concurrent primary lesion (e.g., lymphoma, metachronous metastatic melanoma). In such cases, pericardial fluid analysis to make a tissue diagnosis may be helpful. Pericardiocentesis or pericardial window may be indicated in the case of pericardial effusion with cardiac tamponade, in which case the procedure may be diagnostic and therapeutic.
Tissue biopsy
Preoperative direct tissue biopsy may be achievable via endomyocardial biopsy in the case of right-sided intracardiac masses to guide treatment in those patients for whom surgical resection is not possible and a histologic diagnosis is required to inform non-operative treatment options. More commonly, tissue diagnosis is achieved intraoperatively or following surgical resection of the intracardiac mass.
Tumor masquerades
Thrombus
Thrombus is the most commonly identified intracardiac mass and can be found within the atrial or ventricular cavities. A thrombus may be freely mobile such as with deep venous thrombosis “clot-in-transit,” attached to the endocardial wall on a pedicle, or fully adherent to the wall and sessile.
Atrial thrombi are most commonly located within the left atrial appendage and occur with higher frequency in the setting of atrial enlargement, atrial fibrillation, and mitral valve disease due to stagnation of flow. Left ventricular thrombi are most commonly seen at the ventricular apex in association with systolic dysfunction, particularly in the setting of left ventricular aneurysm. Thrombi in general are also more common among those with hypercoagulable states such as malignancy and the antiphospholipid antibody syndrome or with endomyocardial fibrosis in the hypereosinophilic syndrome. They are also commonly observed in patients with intracardiac prosthetic material such as prosthetic valves or indwelling catheters. Many of these conditions coexist with active malignancy.
Clinical implications
While very large atrial or ventricular thrombi may result in obstructive or restrictive physiology, the most frequent and concerning clinical consequence of thrombus is embolism. Left-sided thrombi with systemic embolization may most dramatically involve the cerebral or coronary circulation resulting in an acute stroke or myocardial infarction, but may also result in renal, splenic, mesenteric, or femoropopliteal infarcts. Right-sided thrombi generally embolize to the pulmonary circuit resulting in pulmonary embolism, though paradoxical systemic embolization may occur in the setting of atrial- or ventricular-level shunts, such as a patent foramen ovale.
Anticoagulation with low-molecular weight heparin is preferred over vitamin K agonists to treat thrombus in active malignancy [20]. Recent data suggests that direct-acting oral anticoagulants can safely be used among cancer patients with venous thromboembolism [21], but guidelines do not yet support this practice (updated American Society of Hematology guidelines for management of venous thromboembolism in cancer expected in 2019). Treatment should be continued until follow-up imaging demonstrates thrombus resolution, or longer. Surgical thrombectomy may be indicated in the case of hemodynamic compromise or recurrent embolization despite anticoagulation. Notably, thrombus identified prior to clinical embolization is a treatable condition, and prior studies have observed similar mortality rates to patients matched on disease etiology and extent when identified incidentally [13].
Diagnosis
While CT or echocardiography with contrast can be useful for differentiating between tumor and thrombus, CMR is the gold standard for this indication [22,23,24]. On contrast-enhanced CMR, cardiac thrombus appears dark due to a lack of gadolinium uptake whereas the surrounding blood pool and myocardium appear bright from gadolinium enhancement. Notably, tumor and thrombus are not mutually exclusive and often coexist, as tumor acts as a nidus upon which thrombus may form.
Vegetation
Infective endocarditis most commonly appears on echocardiography, CMR, or CT as left-sided, mobile, and irregular masses which are attached to the atrial aspect of the tricuspid or mitral valves or the ventricular size of the pulmonic or aortic valves. While transesophageal echocardiography demonstrates the highest diagnostic accuracy for left-sided valvular endocarditis, CT and CMR provide more accurate anatomic information regarding perivalvular extent of abscess. Right-sided vegetations are less common but are often observed in patients with indwelling venous catheters or those who inject intravenous drugs.
Infectious vegetations may rarely be adherent to the atrial or ventricular wall, as seen in aspergillomas that may occur in immunosuppressed patients [25]. Non-infective valvular vegetations may also present in the oncologic patient in the form of marantic endocarditis (non-bacterial thrombotic endocarditis) which is treated with systemic anticoagulation [26].
Pericardial cysts and diverticula
Pericardial cysts are benign congenital lesions that are generally observed in the pericardiophrenic spaces and are non-contiguous with the pericardial space. In contrast, pericardial diverticula are contiguous with the pericardial space and therefore may change in size based on respiration or body positioning [27]. Pericardial cysts are typically benign findings of little clinical significance and do not require further work-up or management. However, in rare circumstances, pericardial cysts can lead to cardiac tamponade or impingement on cardiac structures causing right heart or left heart failure in which case surgery can be required. On echocardiography, cardiac CT, or cardiac MRI, both cysts and diverticula are visualized as simple thin-walled structures without septation and without contrast enhancement. Tomographic imaging is helpful to determine the anatomic location and define the full extent of the lesion. On cardiac CT, they are non-enhancing and have attenuation similar to water [28], and on cardiac MRI, they are homogenous and hyperintense on T2-weighted imaging [29].
Normal structures
Pseudomasses with confusing echocardiographic appearances are a common source of referral for tomographic imaging and rarely have clinical consequences. Examples include prominent Eustachian valve or Chiari network within the right atrium, prominent coumadin ridge within the left atrium, prominent pericardiac thymus tissue, or hiatal hernia.
Lipomatous hypertrophy
Lipomatous hypertrophy of the inter-atrial septum is a relatively common neoplastic process, occurring in about 2% of patients, and involves growth of adipocytes interspersed with hypertrophied myocytes within the interatrial septum (larger than 2 cm by definition). Lipomatous hypertrophy typically spares the fossa ovalis, a defining feature that gives the lesion its classic hourglass shape. Because of its prevalence and distinct structural features, lipomatous hypertrophy is a diagnosis that can typically be made by echocardiography, CT, or CMR without the need for tissue biopsy. However, in rare cases, the degree of lipomatous hypertrophy can be profound. In these circumstances, the use of CMR to confirm the fatty content using T1-weighted imaging can be helpful, and serial imaging can be utilized to ensure stability of the lesion [30].
Benign tumors
Myxoma
Myxomas are the most common primary cardiac tumor in adults accounting for 50% of all cases [31, 32]. They typically occur in the middle age, with 90% of patients diagnosed between the ages of 30 and 60 [33]. They affect women more commonly than men [34]. Myxomas are rare in children, accounting for only 10% of benign cardiac tumors in this age group [35].
Myxomas usually arise in the region of the atrial fossa ovalis, with 75% arising in the left atrium and 10–15% arising in the right atrium. They rarely occur in the ventricles (3–4% in each ventricle) or on the heart valves [36]. More than 90% of myxomas are sporadic and solitary [35]. Less than 10% occur in familial clusters as part of the Carney complex. The Carney complex is an autosomal dominant condition characterized by a combination of cardiac and cutaneous myxomas, endocrine hyperfunction, and distinctive pigmented lesions of the skin and mucosal surfaces (specifically lentiginosis and blue nevi) [37]. The myxomas of Carney complex are typically multicentric, atypically located, occur at younger ages (peak incidence in the third decade), and tend to recur after resection.
Myxomas are neoplasms of multipotent mesenchymal cells in the subendocardial tissue. Myxomas can be round, oval, or polypoid. They are often gelatinous with a smooth or gently lobulated surface. They can be pedunculated or sessile. Myxomas range from sub-cm to 15 cm in diameter [36].
Clinical implications
As with other cardiac masses, the presentation of myxoma is determined by location, size, and mobility. Most patients present either incidentally when the tumor is picked up on imaging performed for another indication or as the result of embolism, intracardiac obstruction, or constitutional symptoms.
Diagnosis
Myxomas are most commonly diagnosed by their typical appearance on echocardiography (Fig. 1). CT and MRI have a role in atypical cases where the diagnosis is unclear.
Treatment
Once a myxoma is diagnosed, the treatment of choice is surgical excision. The overall risk of recurrence for sporadic myxoma is less than 3% whereas the risk is significantly higher at about 12 and 22% for familial and complex myxomas, respectively [38, 39]. Interval echocardiographic follow-up examinations are indicated in all patients who have had prior myxoma resection.
Fibroelastoma
Fibroelastoma (papillary fibroelastoma) is a benign tumor of the endocardium (most commonly the valvular surface). It accounts for three fourths of all tumors of the cardiac valves [40]. It can occur at any age but the mean age for detection is 60 years old. On histopathology, the tumor is gelatinous, having a characteristic frond-like appearance, with multiple long and narrow papillary branches. Fibroelastomas may be asymptomatic or can result in systemic or peripheral embolization, cardiac obstruction, or interference with valve function.
Treatment
The decision to proceed with surgical resection of a fibroelastoma is based upon the size, location, mobility, and association of the tumor with symptoms [41]. Surgical excision is favored for symptomatic fibroelastomas, or if they are large (> 1 cm), left-sided, and/or mobile in appearance. Fibroelastomas that do not meet the criteria for surgery should be followed longitudinally on imaging studies, and patients should be considered for antiplatelet/anticoagulation therapy to lower thrombosis and embolic risk.
Lipoma
Lipomas differ from lipomatous hypertrophy of the interatrial septum in that they are typically encapsulated [42]. Lipomas are usually asymptomatic and thus most commonly discovered incidentally. Symptoms depend upon the site of origin. Lipomas are most commonly found in the right atrium or left ventricle. They can originate from the subendocardium (50%), subepicardium (25%), and myocardium (25%). Lipomas of the subendocardium typically cause obstructive symptoms while those originating in the myocardium can be arrhythmogenic. Embolization is atypical because the tumors are encapsulated.
Rhabdomyoma
Rhabdomyoma is the most common primary cardiac tumor in children accounting for 50–60% of cases. Rhabdomyoma is a tumor of cardiac myocytes and is considered by some to be a hamartoma occurring exclusively in the heart. Rhabdomyomas can be sporadic or associated with tuberous sclerosis (30–50% of cases) or in association with congenital heart malformations [43]. Rhabdomyomas can be solitary but more commonly multiple and are typically located in the interventricular septum or the ventricular free wall. Symptoms are commonly related to obstruction or cardiac arrhythmias [44]. Because up to 50% of cases of rhabdomyomas regress spontaneously, surgery is reserved for patients with significant symptoms [45].
Malignant tumors
Sarcoma
Sarcomas represent the majority of primary malignant cardiac tumors and are pathologically categorized as undifferentiated high-grade pleomorphic sarcoma, angiosarcoma, rhabdomyosarcoma, leiomyosarcoma, and fibrosarcoma. As a group, sarcomas may originate in either the left or right heart chambers and the pulmonary arteries. Typically, sarcomas are diagnosed in young adults (mean age of 46 years) and near equally divided between genders [46]. The overall prognosis of cardiac sarcoma is poor with a median overall survival of 6 to 12 months and metastatic rate at the time of diagnosis from 26 to 48.2% [47, 48]. Known risk factors include a history of chest irradiation. CMR provides the best characterization of cardiac sarcomas, and in some circumstances, some imaging findings can be supportive of specific diagnoses. For example, a sunray pattern can be seen in angiosarcoma with gadolinium-enhanced CMR where the mass will be mostly non-enhancing but with enhancing lines traveling from epicardium to pericardium.
Clinical presentation of cardiac sarcoma may vary pending the anatomical location. Left heart sarcoma is more common in the left atrium as compared with the left ventricle (92% versus 8%, respectively) and can present with heart failure due to obstruction [47]. Right heart sarcomas tend to have more tumor burden, though less intracardiac obstruction; therefore, clinical symptoms are more non-specific and unfortunately are more likely metastatic at the time of diagnosis. Sarcomas that originate in the pulmonary arteries are the rarest group and commonly present with shortness of breath. Typically, advanced cardiac imaging techniques are required to differentiate them from pulmonary embolism.
Treatment
A multidisciplinary team approach is needed to effectively diagnose, prognosticate, and treat primary cardiac sarcomas. Improved outcomes have consistently been seen following complete surgical resection with tumor-free margins [49, 50•]. This requires both surgical experience and adequate tumor exposure in situ. Cardiac auto-transplantation with ex vivo tumor resection and reconstruction has also been done for complete tumor resection [51]. In addition, the use of multimodal treatment (neoadjuvant chemotherapy and surgical resection) has been shown to improve survival as compared with either surgery or chemotherapy alone, 36.5 months compared with 14.1 months [50•, 52••].
Conclusions
Cardiac tumors are a heterogenous group of disorders and include primary benign and malignant tumors, metastatic disease, and numerous masquerades such as thrombus or infective endocarditis. Echocardiography is typically the first imaging test performed, but cardiac MRI provides a superior tissue characterization and is helpful in making a presumptive diagnosis. Because treatment varies widely depending on the clinical presentation and pathology, a multidisciplinary approach is essential to manage these patients.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Al-Mamgani A, Baartman L, Baaijens M, et al. Cardiac metastases. Int J Clin Oncol. 2008;13:369–72.
Klatt EC, Heitz DR. Cardiac metastases. Cancer. 1990;65:1456–9.
Primary tumors of the heart. In Bonow RO, Mann DL, Zipes DP, Libby P (eds): Braunwald’s heart disease. 9th ed. Philadelphia, Elsevier Saunders, 2011, pp 1638–1650.
Cardiac tumors. In Crawford MH, DiMarco JP and Paulus WJ (eds): Cardiology. 3rd ed. Philadelphia, Elsevier Saunders, 2010, pp 1743–1751.
Stollberger C, Chnupa P, Kronik G, Brainin M, Finsterer J, Schneider B, et al. Transesophageal echocardiography to assess embolic risk in patients with atrial fibrillation. ELAT Study Group. Embolism in left atrial thrombi. Ann Intern Med. 1998;128:630–8.
Sharma ND, McCullough PA, Philbin EF, Weaver WD. Left ventricular thrombus and subsequent thromboembolism in patients with severe systolic dysfunction. Chest. 2000;117:314–20.
Gertner E, Leatherman JW. Intracardiac mural thrombus mimicking atrial myxoma in the antiphospholipid syndrome. J Rheumatol. 1992;19:1293–8.
Kirkpatrick JN, Wong T, Bednarz JE, Spencer KT, Sugeng L, Ward RP, et al. Differential diagnosis of cardiac masses using contrast echocardiographic perfusion imaging. J Am Coll Cardiol. 2004;43:1412–9.
Kassop D, Donovan MS, Cheezum MK, Nguyen BT, Gambill NB, Blankstein R, et al. Cardiac masses on cardiac CT: a review. Curr Cardiovasc Imaging Rep. 2014;7:9281.
• Giusca S, Mereles D, Ochs A, Buss S, Andre F, Seitz S, et al. Incremental value of cardiac magnetic resonance for the evaluation of cardiac tumors in adults: experience of a high volume tertiary cardiology centre. Int J Card Imaging. 2017;33:879–88 This is a retrospective analysis of a large number of cardiac masses evaluated by cardiac MRI and shows superior mass identification as compared with echocardiography.
Nazarian S, Hansford R, Rahsepar AA, Weltin V, McVeigh D, Gucuk Ipek E, et al. Safety of magnetic resonance imaging in patients with cardiac devices. N Engl J Med. 2017;377:2555–64.
Pazos-Lopez P, Pozo E, Siqueira ME, Garcia-Lunar I, Cham M, Jacobi A, et al. Value of CMR for the differential diagnosis of cardiac masses. JACC Cardiovasc Imaging. 2014;7:896–905.
Chan AT, Plodkowski AJ, Pun SC, Lakhman Y, Halpenny DF, Kim J, et al. Prognostic utility of differential tissue characterization of cardiac neoplasm and thrombus via late gadolinium enhancement cardiovascular magnetic resonance among patients with advanced systemic cancer. J Cardiovasc Magn Reson. 2017;19:76.
Ganame J, Wright J, Bogaert J. Cardiac lipoma diagnosed by cardiac magnetic resonance imaging. Eur Heart J. 2008;29:697.
Rahbar K, Seifarth H, Schafers M, Stegger L, Hoffmeier A, Spieker T, et al. Differentiation of malignant and benign cardiac tumors using 18F-FDG PET/CT. J Nucl Med. 2012;53:856–63.
Chan ATF, J. Perez Johnston, R. Plodkowski, A. Pollie, M. Moskowitz, C. Steingart, R. Weinsaft, J.W. Biologic and prognostic validation of delayed enhancement (DE-) CMR for cancer-associated cardiac masses - multimodality comparison to positron emission tomography (PET) [ABSTRACT]. Society for Cardiovascular Magnetic Resonance Scientific Sessions. 2018:22.
Klein MA, Scalcione LR, Youn T, Shah RA, Katz DS, Sung WW, et al. Intensely hypermetabolic lipomatous hypertrophy of the interatrial septum on 18-FDG PET with MRI and CT correlation. Clin Nucl Med. 2010;35:972–3.
Nensa F, Tezgah E, Poeppel TD, Jensen CJ, Schelhorn J, Kohler J, et al. Integrated 18F-FDG PET/MR imaging in the assessment of cardiac masses: a pilot study. J Nucl Med. 2015;56:255–60.
Theologides A. Neoplastic cardiac tamponade. Semin Oncol. 1978;5:181–92.
Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, et al. Randomized comparison of low-molecular-weight heparin versus oral anticoagulant therapy for the prevention of recurrent venous thromboembolism in patients with cancer I. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–53.
Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378:615–24.
Srichai MB, Junor C, Rodriguez LL, Stillman AE, Grimm RA, Lieber ML, Weaver JA, Smedira NG and White RD. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J. 2006;152:75-84.
Hong YJ, Hur J, Kim YJ, Lee HJ, Nam JE, Kim HY, Choe KO and Choi BW. The usefulness of delayed contrast-enhanced cardiovascular magnetic resonance imaging in differentiating cardiac tumors from thrombi in stroke patients. Int J Cardiovasc Imaging. 2011;27 Suppl 1:89-95.
Weinsaft JW, Kim HW, Crowley AL, Klem I, Shenoy C, Van Assche L, Brosnan R, Shah DJ, Velazquez EJ, Parker M, Judd RM and Kim RJ. LV thrombus detection by routine echocardiography: insights into performance characteristics using delayed enhancement CMR. JACC Cardiovasc Imaging. 2011;4:702-12.
Chen-Milhone CS, Chakravarthy Potu K, Mungee S. Cardiac aspergilloma: a rare case of a cardiac mass involving the native tricuspid valve, right atrium, and right ventricle in an immunocompromised patient. Case Rep Cardiol. 2018;2018:6927436.
Biller J, Challa VR, Toole JF, Howard VJ. Nonbacterial thrombotic endocarditis. A neurologic perspective of clinicopathologic correlations of 99 patients. Arch Neurol. 1982;39:95–8.
Tower-Rader A, Kwon D. Pericardial masses, cysts and diverticula: a comprehensive review using multimodality imaging. Prog Cardiovasc Dis. 2017;59:389–97.
Yared K, Baggish AL, Picard MH, Hoffmann U, Hung J. Multimodality imaging of pericardial diseases. JACC Cardiovasc Imaging. 2010;3:650–60.
Frank H, Globits S. Magnetic resonance imaging evaluation of myocardial and pericardial disease. J Magn Reson Imaging. 1999;10:617–26.
Heyer CM, Kagel T, Lemburg SP, Bauer TT, Nicolas V. Lipomatous hypertrophy of the interatrial septum: a prospective study of incidence, imaging findings, and clinical symptoms. Chest. 2003;124:2068–73.
Reynen K. Frequency of primary tumors of the heart. Am J Cardiol. 1996;77:107.
Centofanti P, Di Rosa E, Deorsola L, et al. Primary cardiac neoplasms: early and late results of surgical treatment in 91 patients. Ann Thorac Surg. 1999;68:1236–41.
Sarjeant JM, Butany J, Cusimano RJ. Cancer of the heart: epidemiology and management of primary neoplasms and metastases. Am J Cardiovasc Drugs. 2003;3:407–21.
Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma: a series of 112 consecutive cases. Medicine. 2001;80:159–72.
McAllister H, Fenoglio J. Tumors of the cardiovascular system. In: Hartmannn W, Cowan W, editors. Atlas of tumor pathology. Washington, DC: Armed Forces Institute of Pathology; 1978. p. 1–20.
Reynen K. Cardiac myxomas. N Engl J Med. 1995;333:1610–7.
Carney J, Gordon H, Carpenter P, Shenoy B, Go V. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine. 1985;64(4):270–83.
McCarthy PM, Piehler JM, Schaff HV, Pluth JR, Orszulak TA, Vidaillet HJ Jr, et al. The significance of multiple, recurrent, and “complex” cardiac myxomas. J Thorac Cardiovasc Surg. 1986;91:389–96.
Waller DA, Ettles DF, Saunders NR, Williams G. Recurrent cardiac myxoma: the surgical implications of two distinct groups of patients. Thorac Cardiovasc Surg. 1989;37:226–30.
Mariscalco G, Bruno VD, Borsani P, Dominici C, Sala A. Papillary fibroelastoma: insight to a primary cardiac valve tumor. J Card Surg. 2010;25:198–205.
Yee HC, Nwosu JE, Lii AD, Velasco M, Millman A. Echocardiographic features of papillary fibroelastoma and their consequences and management. Am J Cardiol. 1997;80:811–4.
Jain D, Maleszewski JJ, Halushka MK. Benign cardiac tumors and tumorlike conditions. Ann Diagn Pathol. 2010;14:215–30.
Freedom RM, Lee KJ, MacDonald C, Taylor G. Selected aspects of cardiac tumors in infancy and childhood. Pediatr Cardiol. 2000;21:299–316.
Uzun O, Wilson DG, Vulanic GM, et al. Cardiac tumours in children. Orphanet J Rare Dis. 2007;2:11.
Gunther T, Schreiber C, Noebauer C, Eicken A, Lange R. Treatment strategies for pediatric patients with primary cardiac and pericardial tumors: a 30-year review. Pediatr Cardiol. 2008;29:1071–6.
Chen TW, Loong HH, Srikanthan A, et al. Primary cardiac sarcomas: a multinational retrospective review. Cancer Med 2018; 1–7.
Ramlawi B, Leja MJ, Abu Saleh WK, al Jabbari O, Benjamin R, Ravi V, et al. Surgical treatment of primary cardiac sarcomas: review of a single-institution experience. Ann Thorac Surg. 2016;101:698–702.
Hamidi M, Moody JS, Weigel TL, Kozak KR. Primary cardiac sarcoma. Ann Thorac Surg. 2010;90:176–81.
Isambert N, Ray-Coquard I, Italiano A, Rios M, Kerbrat P, Gauthier M, et al. Primary cardiac sarcomas: a retrospective study of the French Sarcoma Group. Eur J Cancer. 2014;50(1):128–36.
• Randhawa JS, Budd GT, Randhawa M, Ahluwalia M, Jia X, Daw H, et al. Primary cardiac sarcoma: 25-year Cleveland Clinic experience. Am J Clin Oncol. 2016;39(6):593–99. This is a retrospective review of 42 cases of primary cardiac sarcoma and described a benefit in patients who received multimodality therapy.
Reardon MJ, DeFelice CA, Sheinbaum R, Baldwin J. Cardiac autotransplant for surgical treatment of a malignant neoplasm. Ann Thorac Surg. 1999;67:1793–5.
•• Abu Saleh WK, Ramlawi B, Shapira OM, et al. Improved outcomes with the evolution of neoadjuvant chemotherapy approach to right heart sarcoma. Ann Thorac Surg. 2017;104(1):90–6. This is a single-center study of 133 primary cardiac sarcoma cases with a focus on the management of right-sided sarcomas. This study demonstrated a marked survival benefit with complete resection, leading to a recommendation for neoadjuvant therapy to increase the probability of achieving complete resection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Timothy J. Poterucha owns a stock in Abbott Laboratories and AbbVie, Inc.
Jonathan Kochav declares that he has no conflict of interest.
Daniel S. O’Connor declares that he has no conflict of interest.
Gregg F. Rosner declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardio-oncology
Rights and permissions
About this article
Cite this article
Poterucha, T.J., Kochav, J., O’Connor, D.S. et al. Cardiac Tumors: Clinical Presentation, Diagnosis, and Management. Curr. Treat. Options in Oncol. 20, 66 (2019). https://doi.org/10.1007/s11864-019-0662-1
Published:
DOI: https://doi.org/10.1007/s11864-019-0662-1