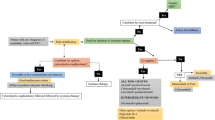
Opinion statement
Treatment of metastatic clear cell renal cancer (mccRCC) has seen substantial progress over the last 20 years, with many regulatory approvals since 2006 culminating in a substantial increase to overall survival (OS). Six therapies are currently available for first-line use, with additional treatments currently being tested in this setting, some of which are expected to be approved soon based on new data from the CABOSUN and CheckMate-214 trials. Based on the available evidence, we strongly believe that vascular endothelial growth factor tyrosine kinase inhibitor (VEGF-TKI) therapy over mechanistic target or rapamycin (mTOR; formerly known as mammalian target of rapamycin) inhibitor therapy is the most effective first-line option regardless of risk category assignment. High-dose interleukin-2 (HDIL-2) therapy remains a reasonable treatment option in patients with Eastern Cooperative Oncology Group (ECOG) performance status 0–1 and have minimal comorbid conditions. In the near future, these agents are likely to be surpassed by cabozantinib and by combination immune checkpoint inhibitor therapy with nivolumab and ipilimumab. Independent review has recently confirmed superiority of first-line cabozantinib over sunitinib in a phase 2 trial of 157 patients with intermediate or poor risk mccRCC (progression-free survival [PFS] 8.6 vs 5.3 months, hazard ratio [HR] 0.48, p = 0.0008). In a separate study of 1096 patients treated with either upfront sunitinib or the combination of nivolumab and ipilimumab, those with intermediate and poor risk had significant improvement in both PFS (11.6 vs 8.4 months, HR 0.82, p = 0.0331) and OS (not reached vs 26 months, p < 0.0001). Responses were greater in patients with positive programmed death receptor ligand-1 (PD-L1) tumor staining, and pending regulatory approval may become standard of care in untreated patients with intermediate to poor risk disease with positive PD-L1 status. This likely represents the beginning of additional novel immunotherapy combinations for the first-line treatment of mccRCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal cell carcinoma is the seventh and the ninth most common in men and women, respectively, with 65,000 new cases and 13,000 deaths in the USA in 2013 [1]. Over 25% of patients with RCC have metastatic disease (mRCC) at presentation, while another 20–40% with localized disease will eventually develop mRCC [2, 3]. Until 2006, the only available treatments were cytokine immunotherapies: interleukin-2 (IL-2) and interferon-α (IFN-α). Overall response rates (ORR) with these two agents were meager at 15–20%, though 7–9% of patients obtained a complete remission (CR) [4, 5]. Fortunately, most patients today experience significantly better clinical outcomes as a result of treatment advances. The WHO pathologic classification defines multiple subtypes of renal cell carcinoma, but this review will only discuss the first-line treatment options for clear cell renal carcinoma. The pathology, prognosis, and treatment of non-clear cell cancer vary from clear cell and has been reviewed previously [6,7,8,9].
Improved understanding of the pathogenic mechanisms of renal cell carcinoma, including the role of the tumor suppressor gene VHL and hypoxia-inducible factor (HIF), has improved the median overall survival (OS). VHL mutations are frequently seen in mccRCC, with a reported incidence of over 50% [10]. In the absence of hypoxia, VHL normally degrades transcription factor HIF which prevents gene expression of the growth factors it encodes including VEGF. In the majority of patients with mccRCC, somatic mutations of VHL prevent ubiquitination of HIF which allows nuclear trafficking and subsequent intracellular HIF accumulation. This leads to growth factor production allowing angiogenesis and glycolysis which promotes tumorigenesis [11]. Other key discoveries of tumor biology include understanding of the PI3K/AKT/mTOR pathway, an important pathogenic driver in mccRCC with an estimated incidence of 28% in the TCGA study [10]. Through production of HIF, mTOR increases expression of proangiogenic growth factors including VEGF [12]. The first VEGF-TKIs approved for treatment of mRCC, sorafenib, and sunitinib were in 2005 and 2006, respectively [13, 14]. FDA approvals of two inhibitors of mTOR (temsirolimus, everolimus), a monoclonal antibody (mAb) against VEGF (bevacizumab), and multiple other VEGF-TKIs (pazopanib, axitinib) have subsequently been approved [15,16,17,18,19,20,21]. Median OS for mRCC has significantly increased during this timeframe [22].
Many novel agents have recently been approved in the second-line setting and are currently under investigation. These include cabozantinib (targeting VEGFR, c-MET, AXL, c-KIT, and RET) and lenvatinib (targeting VEGFR, PDGFR, FGFR, c-KIT, and RET). Additionally, immunotherapy has had a resurgence with the approval of nivolumab, an immune checkpoint inhibitor directed against programmed death ligand-1 (PD-1). Cabozantinib, lenvatinib, and nivolumab were approved in 2015 in the second-line setting for treatment of mRCC [23]. Recently published data may also lead to regulatory approvals for cabozantinib and combination nivolumab with ipilimumab for the treatment-naïve patient [24••, 25••].
In this review, we discuss the background, clinical trials, and appropriate use of approved first-line agents including HDIL-2, VEGF-TKIs, and mTOR inhibitors. Finally, we review the potential for future first-line therapies and the role of genetic biomarkers in personalizing first-line treatment selection.
Current first-line therapy
High-dose interleukin-2 (HDIL-2)
IL-2 is a cytokine produced by T cells which induces both cytotoxic and helper T cells which can restore immunocompetence by generating lymphokine-activated killer cells targeted against tumor cells [26]. Initial human studies of 255 patients with mRCC treated with HDIL-2 reported ORR of 14% with 5% obtaining a CR [27]. Today, the treatment-related mortality with HDIL-2 has decreased as a consequence of improved supportive care and better patient selection. In the PROCLAIM registry of 352 patients treated with HDIL-2 between 2011 and 2014, the treatment-related mortality was just 1.4% (5/352) with an ORR of 17% and stable disease (SD) in 39% (129/352) [28]. In connection with the University of Michigan, we recently reported our institutional experience with no treatment-related mortalities in 362 patients [29]. Importantly, we also found no difference in PFS between those who obtained partial response (PR) or SD (HR 0.74, 95% CI 0.48–1.10). Similar results were seen with OS (HR 0.66, 95% CI 0.39–1.09) [30]. However, those obtaining SD compared to progressive disease had significantly improved PFS (HR 0.13, 95% CI 0.09–0.22) and OS (HR 0.33, 95% CI 0.23–0.48) [30]. Therefore, approximately half of appropriately selected HDIL-2-treated patients will achieve some clinical benefit.
In the present era, many have questioned how HDIL-2 interacts with targeted therapy. Two recent studies have shown that response to HDIL-2 after targeted therapy and response to targeted therapy after HDIL-2 are independent, and patients still derive benefit from either treatment [28, 31]. HDIL-2 still has a role in contemporary treatment of mRCC and can be offered to select patients with excellent performance status (ECOG of 0–1) and appropriate organ function receiving care at treatment centers experienced with HDIL-2 administration.
VEGF-TKIs
Up to 80% of sporadic clear cell mRCC have inactivating mutations in VHL. Inactivated VHL leads to elevated levels of HIF and its downstream products, including VEGF and PDGF [11]. The first VEGF-TKI to obtain FDA approval was sorafenib in 2005. Sorafenib is a multi-kinase inhibitor of VEGFR, PDGFRβ, FLT-3, c-Kit, and RET [13]. The phase 3 TARGET trial compared sorafenib to placebo in the first-line setting for mRCC. Median PFS was improved in the sorafenib arm compared to placebo (5.5 vs 2.8 m, HR 0.44, 95% CI 0.35–0.55, p < 0.01), although improvement in OS was only significant when post-cross-over placebo data was censored (17.8 vs 14.3 m, HR 0.78, p = 0.03) [13, 32]. In 2006, sunitinib obtained approval after a phase 3 trial of 750 patients randomized to either sunitinib or IFN-α demonstrated improved median PFS (11.0 vs 5.0 months, HR 0.42, p < 0.001) and ORR (31 vs 6%, p < 0.01) in the sunitinib arm [14]. Follow-up data also reported superior OS in the sunitinib arm (26.4 vs 21.8 m, HR 0.82, p = 0.51) [33]. Pazopanib, a VEGF-TKI targeting VEGFR, PDGFR, FGFR, c-Kit, and RET, was compared to placebo for untreated mccRCC and those previously treated with cytokines [18]. In treatment naïve mccRCC, pazopanib exhibited superior median PFS compared to placebo (11.1 vs 2.8 m, HR 0.40, p < 0.0001). It subsequently obtained FDA approval in 2009. Axitinib, targeting VEGFR, PDGFR, and c-Kit, was approved for second-line mRCC treatment but is not approved for upfront use [19].
The COMPARZ trial was the first prospective head-to-head comparison of pazopanib and sunitinib and reported non-inferior of pazopanib to sunitinib in efficacy, but had a lower incidence of reported adverse events (AEs) and better quality of life [34]. However, some have critiqued the study results since more patients discontinued pazopanib for toxicity and a relatively high cutoff for non-inferiority at 25%, suggesting tolerance of a 25% reduction in clinical outcomes as the definition for equivalency between the two therapies [35]. In summary, however, this study does strongly argue for clinical equivalence between pazopanib and sunitinib. The PISCES trial was an innovative clinical trial where patients were randomized to pazopanib for 10 weeks followed by a 2-week washout period then sunitinib for 10 weeks or the reverse [36]. The primary outcome was patient preference assessed by a questionnaire. Seventy percent of patients preferred pazopanib, whereas only 8% preferred sunitinib. However, some have criticized the trial for administering questionnaires prior to the sunitinib washout period and for using a dosing schedule which is less common in clinical practice. Currently, both sunitinib and pazopanib are approved first-line VEGF-TKI options and appear to have equal efficacy. With the available data, we agree with the established clinical guidelines which state that sunitinib and pazopanib are reasonable first-line options and are preferred over sorafenib given the superior tolerability of pazopanib or sunitinib.
In an attempt to increase tolerability of VEGF-TKI therapy, a phase 2 trial of intermittent therapy was conducted recently [37]. All patients received up to 4 cycles of sunitinib at standard dosing (50 mg 4 weeks on, 2 weeks off). Patients were eligible for intermittent dosing if overall tumor burden decreased by 10% or more at the end of 4 cycles, and treatment was restarted if tumor burden increased by at least 10%. Of 20 eligible patients, median PFS was 22.4 months. This potentially attractive strategy may improve the quality of life for patients with mRCC; however, it is a small study of selected patients with relatively indolent disease and should be validated prior to changing current practice.
Retrospective studies have also evaluated dosing sunitinib at 50 mg daily for 2 weeks followed by a 1-week break in order to improve patient tolerability and satisfaction [38]. Compared to the standard dosing (4 weeks on, 2 weeks off), these results demonstrate a lower incidence of diarrhea and other side effects with similar clinical outcomes. Though randomized data is lacking, the results are compelling and in our opinion represents a reasonable standard of care.
Bevacizumab and IFN-α
Bevacizumab is a mAb targeting VEGF. Results from the AVOREN trial led to FDA approval of bevacizumab in combination with IFN-α for previously untreated patients. PFS was improved compared to IFN-α monotherapy (10.2 vs 5.4 months, HR 0.63, 95% CI 0.52–0.75, p = 0.0001) [17]. There was also a trend towards improvement in OS with combination bevacizumab and IFN-α (median 23.3 vs 21.3, HR 0.86, 95% CI 0.72–1.04) at the expense of greater toxicity with combination therapy [39]. Though IFN-α is infrequently used compared with other first-line options, bevacizumab with IFN-α continues to be recognized by NCCN guidelines as an option for first-line treatment of mRCC.
ECOG led a clinical trial evaluating doublet combinations of bevacizumab with VEGF-TKI or mTOR therapies [40]. Four arms were compared: bevacizumab monotherapy (reference arm), bevacizumab plus temsirolimus, bevacizumab plus sorafenib, and sorafenib plus temsirolimus. The median PFS was statistically similar between all treatment arms at 7.5, 7.6, 9.2, and 7.4 months, respectively. Each combination arm did have statistically significant more AEs than single agent bevacizumab. Currently, no combinations are approved for first-line therapy except for bevacizumab with IFN-α.
mTOR inhibitors
Temsirolimus forms a complex with FKBP-12 which then inhibits mTOR signaling. Disruption of mTOR inhibits cell cycle progression and angiogenesis [41, 42]. A 2007 phase 3 trial of 626 patients with previously untreated, poor-risk mRCC was randomized to receive temsirolimus, IFN-α, or both [15]. Temsirolimus achieved longer median OS (10.9 vs 7.3 m, HR 0.73, 95% CI 0.58–0.92, p = 0.008) and PFS (p < 0.001) than IFN-α and was associated with fewer serious AEs than IFN-α (p = 0.02). Per current NCCN guidelines, it is reasonable to use temsirolimus for first-line treatment of patients with poor-risk mRCC; however, a population-based study using the International mRCC Database Consortium (IMDC) dataset found that temsirolimus is only used in 3% of patient in the first-line setting (33/1014) [43, 44]. Everolimus, a different mTOR inhibitor, is approved for previously treated mRCC, and previously was a standard second-line therapy, but it is now generally reserved for salvage therapy as monotherapy or in combination with other novel agents, such as lenvatinib [16, 43]. Though no phase 3 trial has directly compared the two, some evidence suggests greater clinical efficacy of VEGF-TKIs over mTOR inhibitors. This includes data from a sequencing study in which patients who received a VEGF-TKI prior to an mTOR inhibitor had superior outcomes compared to those treated with the reverse [45].
Future first-line therapy
Clinical trials are ongoing investigating anti-angiogenic agents and immunotherapies both in combination and as monotherapy. As the treatment paradigm for mRCC continues to evolve, trial design will become increasingly important. While currently approved first-line agents showed superior outcomes to placebo or IFN-α, most trials now utilize sunitinib as the control arm. In the second-line setting, lenvatinib, cabozantinib, and nivolumab all showed superior survival outcomes to everolimus in the second-line setting, suggesting that these therapies might be effective as first-line therapies as well.
Anti-angiogenic agents
Cabozantinib may be the next drug approved in the first-line setting for mccRCC based on promising results from Alliance A031203 CABOSUN trial. In a randomized, phase 2 open-label trial of 157 good performance status patients with IMDC intermediate or poor risk disease, cabozantinib 60 mg once daily was compared to sunitinib 50 mg once daily for 4 weeks followed by 2 weeks off therapy. Grade 3/4 toxicities were similar between the two arms (cabozantinib, 67% vs sunitinib 68%). Notably, grade 3/4 hypertension was more frequent in the cabozantinib arm (28.2 vs 22.2%) while rates of anemia, neutropenia, leukopenia, and thrombocytopenia were all more frequent in the sunitinib arm. In total, grade 3/4 hematologic toxicities occurred in 22% of patients in the sunitinib arm vs 3% treated with cabozantinib [46]. Recently presented data from an independent review confirms that median PFS, the primary endpoint, was significantly increased in the cabozantinib arm (8.6 vs 5.3 months, HR 0.48, 95% CI 0.31–0.74, two-sided p value 0.0008). Median OS, a secondary endpoint, was increased in the cabozantinib arm but did not reach statistical significance (26.6 vs 21.2 months; HR 0.80, 95% CI 0.53 to 1.21) [24••]. Cabozantinib is currently being evaluated by the FDA for approval in the first-line setting.
Combination anti-angiogenic agents + immunotherapy
IMmotion150 is a phase 2 study (NCT02420821) comparing atezolizumab with or without bevacizumab to sunitinib in previously untreated patients with mRCC. Results, announced February 2017, reported that the 164 of 305 patients with at least 1% PD-L1 expression on tumor-infiltrating cells (IC) trended towards improved median PFS with combination atezolizumab and bevacizumab compared to sunitinib (14.7 vs 7.8 months, HR 0.64, 95% CI 0.38–1.08, p = 0.095). PD-L1 positive patients had similar median PFS with atezolizumab monotherapy compared to sunitinib (median PFS 5.5 vs 7.8 months, HR 1.03, 95% CI 0.63–1.67, p = 0.917). Notably, in the 12 patients with at least 10% PD-L1 IC expression, the HR versus sunitinib improved to 0.23 with atezolizumab and bevacizumab combination therapy compared to HR 0.48 with atezolizumab monotherapy. Toxicity was similar between sunitinib and combination therapy [47]. KEYNOTE-426 is comparing sunitinib monotherapy to combination pembrolizumab and axitinib (NCT02853331), and another trial is investigating avelumab and axitinib (NCT02684006).
In an attempt to avoid the toxicity associated with other immunotherapy agents, AGS-003 is an autologous dendritic cell therapy which utilizes leukapheresis to co-electroporate dendritic cells with amplified RNA from the patient’s own tumor cells and CD40L in order to stimulate a CD8+ T cell response [48]. A phase 2 study of 22 patients reported promising results with 43% obtaining PR and 24% obtained 5-year OS with AGS-003 in combination with sunitinib [48]. Subsequently, the phase 3 ADAPT trial (NCT01582672) investigated sunitinib with AGS-003 versus sunitinib alone in 462 patients with mRCC. Unfortunately, an independent data monitoring committee has recommended stopping the trial based on futility and low likelihood of improving OS.
Two randomized phase 3 trials are currently investigating the combination of an immune checkpoint inhibitor with axitinib against sunitinib. NCT02684006 is combining axitinib with avelumab, a PD-L1 mAb, while NCT02853331 is combining axitinib with pembrolizumab, a PD-1 mAb. NCT02811861, another randomized phase 3 trial, is currently investigating the combination of either lenvatinib and everolimus or lenvatinib and pembrolizumab versus sunitinib in previously untreated mRCC. All three of these trials have accrued at least 500 patients and are anticipating completion by the end of 2020.
Immunotherapy
RCC is an immunogenic tumor, as evidenced from response to immunotherapy-based therapies [49, 50]. It is expected that immune therapy with checkpoint inhibitors will also be effective in the first-line setting. While nivolumab has improved outcomes in the second-line setting, until recently, the only data supporting the use of immune checkpoint inhibitors in the first-line setting for mRCC is from the aforementioned IMmotion150 trial [47]. However, results from the CheckMate-214 trial which investigated the role of combination checkpoint inhibitor therapy in the first-line setting were presented at the European Society of Medical Oncology (ESMO) meeting in September 2017. In this phase, 3b/4 trial of 1096 patients randomized to either sunitinib or the combination of nivolumab and ipilimumab (Table 1). Though ORR and PFS were improved with sunitinib in patients with IMDC good risk disease, in those with intermediate and good risk disease, nivolumab and ipilimumab demonstrated superior ORR (42 vs 27%, 95% CI 37–47 vs 22–31%, p < 0.0001), PFS (11.6 vs 8.4 months, HR 0.82, p = 0.0331), and OS (not reached vs 26 months, p < 0.0001). Patients with ≥ 1% PD-L1 staining with intermediate or poor risk by IMDC criteria showed 16% complete response rate with an ORR 58% (95% CI 48–68%) compared to 1% complete response and ORR 22% (95% CI 15–31%) when treated with sunitinib, p < 0.0001 [25••]. Notably, health-related quality of life was also better in the immunotherapy arm.
The ECHO-202/KEYNOTE-037 trial is combining epacadostat, an immune checkpoint inhibitor directed against indoleamine 2,3 dioxygenase (IDO) to reverse immune suppression and tolerance, with pembrolizumab compared against standard of care in a phase 3 trial of 630 patients [53]. Recently presented interim results of 33 patients reported in those who have received less than two previous therapies, the ORR was 47%. There was no response in the cohort who had received at least two previous therapies [52].
Two phase 2 trials seek to better understand the role of sequencing immunotherapy and anti-angiogenic agents in treatment-naïve patients. NCT02959554 (NIVOSWITCH) is a phase 2 study of 244 patients treated with VEGF-TKI for 3 months and then randomized to nivolumab versus their previous VEGF-TKI with a primary endpoint of OS. NCT03035630 is randomizing patients to treatment with sunitinib and then avelumab on progression or the reverse sequence, with a primary endpoint of PFS. These novel designs will hopefully aid the clinician as the number of therapeutic options continue to grow.
Future directions
While we await data from ongoing trials, and as more therapies become available in the first-line setting, it will become increasingly important to identify additional predictive biomarkers and increase our understanding of the tumor microenvironment to further improve care for patients with this disease. With many different potential first-line options likely available for patients in the future, optimal treatment selection of efficacious therapy will be a critical need for patients with mccRCC.
Biomarkers
Currently, the only validated predictive genetic biomarker available for first-line therapy is FLT1 C/C, which is a SNP predictive of inferior PFS and ORR to sunitinib [54, 55]. While PD-L1 expression may be associated with a poor prognosis in some settings, the presence of PD-L1 staining appears to predict response to immune checkpoint inhibitors to some extent [25••, 56•, 57]. Other retrospective data suggests that mTOR mutations may predict response to therapy [58]. Fortunately, recent technologic advances have allowed much broader access to SNP, whole genome and epigenetic sequencing for researchers. Termed a “liquid biopsy,” researchers also now have the ability to analyze genomic and mitochondrial circulating cell-free DNA of a patient’s tumor with a blood draw rather than a biopsy [59]. These techniques are early in their clinical development, with additional validation and understanding needed to fully determine the value of this technology regarding the prognostic and predictive potential in guiding treatment selection for broad clinical use.
References and Recommended Reading
Paper of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Cho E, Adami HO, Lindblad P. Epidemiology of renal cell cancer. Hematol Oncol Clin North Am. 2011;25(4):651–65. https://doi.org/10.1016/j.hoc.2011.04.002.
Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335(12):865–75. https://doi.org/10.1056/NEJM199609193351207.
Janzen NK, Kim HL, Figlin RA, Belldegrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin N Am. 2003;30(4):843–52. https://doi.org/10.1016/S0094-0143(03)00056-9.
Fisher RI, Rosenberg SA, Fyfe G. Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. Cancer J Sci Am. 2000;6(Suppl 1):S55–7.
Fossa SD. Interferon in metastatic renal cell carcinoma. Semin Oncol. 2000;27(2):187–93.
Albiges L, Escudier B. Current and future strategies in nonclear-cell metastatic renal cell carcinoma. Curr Opin Urol. 2015;25(5):367–73. https://doi.org/10.1097/MOU.0000000000000197.
Giles RH, Choueiri TK, Heng DY, et al. Recommendations for the management of rare kidney cancers. Eur Urol. 2017.
Valenca LB, Hirsch MS, Choueiri TK, Harshman LC. Non-clear cell renal cell carcinoma, part 1: histology. Clin Adv Hematol Oncol. 2015;13(5):308–13.
Valenca LB, Hirsch MS, Choueiri TK, Harshman LC. Non-clear cell renal cell carcinoma, part 2: therapy. Clin Adv Hematol Oncol. 2015;13(6):383–91.
Network CGAR. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499:43.
Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373(9669):1119–32. https://doi.org/10.1016/S0140-6736(09)60229-4.
Gnarra JR, Zhou S, Merrill MJ, Wagner JR, Krumm A, Papavassiliou E, et al. Post-transcriptional regulation of vascular endothelial growth factor mRNA by the product of the VHL tumor suppressor gene. Proc Natl Acad Sci U S A. 1996;93(20):10589–94. https://doi.org/10.1073/pnas.93.20.10589.
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–34. https://doi.org/10.1056/NEJMoa060655.
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–24. https://doi.org/10.1056/NEJMoa065044.
Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–81. https://doi.org/10.1056/NEJMoa066838.
Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–56. https://doi.org/10.1016/S0140-6736(08)61039-9.
Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370(9605):2103–11. https://doi.org/10.1016/S0140-6736(07)61904-7.
Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28(6):1061–8. https://doi.org/10.1200/JCO.2009.23.9764.
Rini B, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line therapy for metastatic renal cell carcinoma (mRCC): results of phase III AXIS trial. J Clin Oncol. 2011;29(15_suppl):4503. https://doi.org/10.1200/jco.2011.29.15_suppl.4503.
Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1814–23. https://doi.org/10.1056/NEJMoa1510016.
Motzer RJ, Hutson TE, Glen H, Michaelson MD, Molina A, Eisen T, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16(15):1473–82. https://doi.org/10.1016/S1470-2045(15)00290-9.
Pal SK, Nelson RA, Vogelzang N. Disease-specific survival in de novo metastatic renal cell carcinoma in the cytokine and targeted therapy era. PLoS One. 2013;8(5):e63341. https://doi.org/10.1371/journal.pone.0063341.
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus Everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–13. https://doi.org/10.1056/NEJMoa1510665.
Choueiri T, Hessel C, Halabi S, et al. LBA38Progression-free survival (PFS) by independent review and updated overall survival (OS) results from Alliance A031203 trial (CABOSUN): Cabozantinib versus sunitinib as initial targeted therapy for patients (pts) with metastatic renal cell carcinoma (mRCC). Ann Oncol 28, 2017. Phase 2 trial reporting superioer PFS for cabozantinib over sunitinib in the first-line setting for patients with IMDC intermediate or poor risk mRCC.
Escudier B, Tannir N, McDermott D, et al: LBA5CheckMate 214: Efficacy and safety of nivolumab+ ipilimumab (N+ I) v sunitinib (S) for treatment-naïve advanced or metastatic renal cell carcinoma (mRCC), including IMDC risk and PD-L1 expression subgroups. Ann Oncol 28, 2017. Phase 3 trial reporting superior PFS, ORR, and OS for patients treated with nivolumab and ipilimumab compared to sunitinib in the first-line setting for patients with IMDC intermediate or poor risk mRCC.
Rosenberg SA, Mule JJ, Spiess PJ, et al. Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin 2. J Exp Med. 1985;161(5):1169–88. https://doi.org/10.1084/jem.161.5.1169.
Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13(3):688–96. https://doi.org/10.1200/JCO.1995.13.3.688.
Clark JI, Wong MK, Kaufman HL, et al. Impact of sequencing targeted therapies with high-dose Interleukin-2 immunotherapy: an analysis of outcome and survival of patients with metastatic renal cell carcinoma from an on-going observational IL-2 clinical trial: PROCLAIMSM. Clin Genitourin Cancer. 2017;15(1):31–41.e4. https://doi.org/10.1016/j.clgc.2016.10.008.
Alva A, Daniels GA, Wong MK, et al. Contemporary experience with high-dose interleukin-2 therapy and impact on survival in patients with metastatic melanoma and metastatic renal cell carcinoma. Cancer immunology. Immunotherapy. 2016;65(12):1533–44. https://doi.org/10.1007/s00262-016-1910-x.
Stenehjem DD, Toole M, Merriman J, et al: Extension of overall survival beyond objective responses in patients with metastatic renal cell carcinoma treated with high-dose interleukin-2. Cancer Immunol, Immunotherapy:1–9, 2016.
Gills J, Parker WP, Pate S, et al: The role of high dose interleukin-2 in the Era of Targeted Therapy. J Urol. 2017.
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Staehler M, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27(20):3312–8. https://doi.org/10.1200/JCO.2008.19.5511.
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27(22):3584–90. https://doi.org/10.1200/JCO.2008.20.1293.
Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369(8):722–31. https://doi.org/10.1056/NEJMoa1303989.
Ouzaid I, Rini B. Words of wisdom: re: Pazopanib versus sunitinib in metastatic renal-cell carcinoma. Eur Urol. 2014;65(3):667–8. https://doi.org/10.1016/j.eururo.2013.11.023.
Escudier B, Porta C, Bono P, Powles T, Eisen T, Sternberg CN, et al. Randomized, controlled, double-blind, cross-over trial assessing treatment preference for pazopanib versus sunitinib in patients with metastatic renal cell carcinoma: PISCES study. J Clin Oncol. 2014;32(14):1412–8. https://doi.org/10.1200/JCO.2013.50.8267.
Ornstein MC, Wood LS, Elson P, et al. A phase II study of intermittent sunitinib in previously untreated patients with metastatic renal cell carcinoma. J Clin Oncol:Jco2016711184, 2017.
Guida FM, Santoni M, Conti A, Burattini L, Savini A, Zeppola T, et al. Alternative dosing schedules for sunitinib as a treatment of patients with metastatic renal cell carcinoma. Crit Rev Oncol Hematol. 2014;92(3):208–17. https://doi.org/10.1016/j.critrevonc.2014.07.006.
Escudier B, Bellmunt J, Négrier S, Bajetta E, Melichar B, Bracarda S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol. 2010;28(13):2144–50. https://doi.org/10.1200/JCO.2009.26.7849.
Flaherty KT, Manola JB, Pins M, et al. BEST: a randomized phase II study of vascular endothelial growth factor, raf kinase, and mammalian target of rapamycin combination targeted therapy with bevacizumab, sorafenib, and temsirolimus in advanced renal cell carcinoma—A trial of the ECOG–ACRIN Cancer Research Group (E2804). J Clin Oncol:JCO. 2015.60. 9727, 2015.
Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18(16):1926–45. https://doi.org/10.1101/gad.1212704.
Del Bufalo D, Ciuffreda L, Trisciuoglio D, Desideri M, Cognetti F, Zupi G, et al. Antiangiogenic potential of the mammalian target of rapamycin inhibitor temsirolimus. Cancer Res. 2006;66(11):5549–54. https://doi.org/10.1158/0008-5472.CAN-05-2825.
Motzer RJ, Jonasch E, Agarwal N, Beard C, Bhayani S, Bolger GB, et al. Kidney cancer, version 3.2015. J Natl Compr Cancer Netw. 2015;13(2):151–9. https://doi.org/10.6004/jnccn.2015.0022.
Ko JJ, Xie W, Kroeger N, Lee J, Rini BI, Knox JJ, et al. The international metastatic renal cell carcinoma database consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study. Lancet Oncol. 2015;16(3):293–300. https://doi.org/10.1016/S1470-2045(14)71222-7.
Motzer RJ, Barrios CH, Kim TM, et al. Phase II randomized trial comparing sequential first-line everolimus and second-line sunitinib versus first-line sunitinib and second-line everolimus in patients with metastatic renal cell carcinoma. J Clin Oncol:JCO. 2013.54. 6911, 2014.
Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the alliance A031203 CABOSUN trial. J Clin Oncol:JCO. 2016.70. 7398, 2016.
Atkins MB, McDermott DF, Powles T, et al. IMmotion150: a phase II trial in untreated metastatic renal cell carcinoma (mRCC) patients (pts) of atezolizumab (atezo) and bevacizumab (bev) vs and following atezo or sunitinib (sun), Am Soc Clin Oncol, 2017.
Amin A, Dudek AZ, Logan TF, Lance RS, Holzbeierlein JM, Knox JJ, et al. Survival with AGS-003, an autologous dendritic cell–based immunotherapy, in combination with sunitinib in unfavorable risk patients with advanced renal cell carcinoma (RCC): phase 2 study results. J Immunotherapy Cancer. 2015;3(1):14. https://doi.org/10.1186/s40425-015-0055-3.
De Meerleer G, Khoo V, Escudier B, Joniau S, Bossi A, Ost P, et al. Radiotherapy for renal-cell carcinoma. Lancet Oncol. 2014;15(4):e170–7. https://doi.org/10.1016/S1470-2045(13)70569-2.
Gill D, Hahn AW, Sonpavde G, Agarwal N. Immunotherapy of advanced renal cell carcinoma: current and future therapies. Hum Vaccin Immunother. 2016;12(12):2997–3004. https://doi.org/10.1080/21645515.2016.1212794.
Atkins M, Plimack E, Puzanov I, et al. Axitinib in combination with pembrolizumab in patients (pts) with advanced renal cell carcinoma (aRCC): Preliminary safety and efficacy results. Eur Soc Med Oncol. 2016.
Lara P, Bauer TM, Hamid O, et al. Epacadostat plus pembrolizumab in patients with advanced RCC: preliminary phase I/II results from ECHO-202/KEYNOTE-037. Am Soc Clin Oncol. 2017.
Moon YW, Hajjar J, Hwu P, Naing A. Targeting the indoleamine 2, 3-dioxygenase pathway in cancer. Journal for immunotherapy of cancer. 2015;3(1):51. https://doi.org/10.1186/s40425-015-0094-9.
Beuselinck B, Karadimou A, Lambrechts D, Claes B, Wolter P, Couchy G, et al. VEGFR1 single nucleotide polymorphisms associated with outcome in patients with metastatic renal cell carcinoma treated with sunitinib - a multicentric retrospective analysis. Acta Oncol. 2014;53(1):103–12. https://doi.org/10.3109/0284186X.2013.770600.
Beuselinck B, Jean-Baptiste J, Schoffski P, et al. Validation of VEGFR1 rs9582036 as predictive biomarker in metastatic clear-cell renal cell carcinoma patients treated with sunitinib. BJU Int. 2016;118(6):890–901. https://doi.org/10.1111/bju.13585.
McDermott DF, Atkins MB, Motzer RJ, et al. A phase II study of atezolizumab (atezo) with or without bevacizumab (bev) versus sunitinib (sun) in untreated metastatic renal cell carcinoma (mRCC) patients (pts). Am Soc Clin Oncol. 2017. Phase 2 trial reporting a trend toward improved PFS with combination atezolizumab and bevacizumab over sunitinib in the first-line setting for mRCC. Outcomes were improved in patients with positive PD-L1 staining
Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66(7):3381–5. https://doi.org/10.1158/0008-5472.CAN-05-4303.
Kwiatkowski DJ, Choueiri TK, Fay AP, Rini BI, Thorner AR, de Velasco G, et al. Mutations in TSC1, TSC2, and MTOR are associated with response to rapalogs in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2016;22(10):2445–52. https://doi.org/10.1158/1078-0432.CCR-15-2631.
Lu H, Busch J, Jung M, Rabenhorst S, Ralla B, Kilic E, et al. Diagnostic and prognostic potential of circulating cell-free genomic and mitochondrial DNA fragments in clear cell renal cell carcinoma patients. Clin Chim Acta. 2016;452:109–19. https://doi.org/10.1016/j.cca.2015.11.009.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
David M. Gill, Andrew W. Hahn, Peter Hale, and Benjamin L. Maughan declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Genitourinary Cancers
Rights and permissions
About this article
Cite this article
Gill, D.M., Hahn, A.W., Hale, P. et al. Overview of Current and Future First-Line Systemic Therapy for Metastatic Clear Cell Renal Cell Carcinoma. Curr. Treat. Options in Oncol. 19, 6 (2018). https://doi.org/10.1007/s11864-018-0517-1
Published:
DOI: https://doi.org/10.1007/s11864-018-0517-1