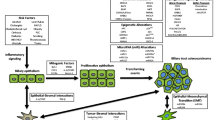
Opinion statement
Biliary tract cancers are relatively uncommon, have an aggressive disease course and a dismal clinical outcome. Until recently, there have been very few clinical advances in the management of these patients and gemcitabine-based chemotherapy has been the only widely accepted systemic therapy. The advent of next generation sequencing technologies can potentially change the treatment paradigm of this disease. Targeted therapy directed against actionable mutations and identification of molecular subsets with distinct prognostic significance is now feasible in clinical practice. Mutation profiling has highlighted the genomic differences between the intra, extrahepatic cholangiocarcinoma, and gallbladder cancer. The mutational spectrum of intrahepatic cholangiocarcinoma differs according to geographic location and ethnicity. There is a higher incidence of chromatin modulating gene mutations in Western patients as compared with Asian patients with liver fluke-associated cholangiocarcinoma. KRAS and p53 mutations are associated with an aggressive disease prognosis while FGFR mutations may signify a relatively indolent disease course of intrahepatic cholangiocarcinoma. FGFR and IDH mutations have promising agents in clinical trials at this time. An estimated 15 % of gallbladder cancers have Her2/neu amplification and can be targeted by trastuzumab. On the other hand, an estimated 10–15 % of cholangiocarcinomas have DNA repair mutations and may be candidates for immune therapies with checkpoint inhibitors. The promise of targeted therapies for biliary tract cancers can be fulfilled with well-designed, prospective, and multi-center clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A genomic era for cancer therapy is rapidly emerging, largely incentivized by next-generation sequencing (NGS) technologies that detect actionable mutations with high sensitivity, deep coverage, and at a fraction of the cost required for traditional sequencing methods just two decades ago. Targeted sequencing panels that investigate high frequency, actionable mutations in cancers are now available at the major cancer centers in the USA. This technology has greatly impacted the management of hematologic malignancies for the past decade and more recently has revolutionized therapy for some solid tumors such as non-small cell lung cancer and melanoma [1]. Biliary tract cancers (BTC), in particular cholangiocarcinoma have a relatively large number of actionable mutations as compared with other gastrointestinal cancers. Recent mutational profiling studies in BTC have indicated that genomic profiling is of profound value for prognostic stratification and for targeted therapy options [2, 3]. In this review, we will discuss mutational spectrum of BTC, clinical correlative findings, and preliminary results from trials of targeted agents.
Epidemiology of biliary tract cancers
As per the American Cancer Society, 39,230 cases of primary liver cancer [which includes intrahepatic cholangiocarcinoma (IHCCA) and hepatocellular cancer] and 11,420 cases of gallbladder cancer (GBC) and extrahepatic cholangiocarcinoma (EHCAA) were diagnosed in the year 2015 [4]. The clinical and molecular epidemiology of these three types of BTC varies markedly.
Higher incident rates of cholangiocarcinoma (CCA) occur in Southeast Asia as compared with the Western population (>80/100,000 vs. 0.3/100,000 in Northeast Thailand and Canada, respectively) [5]. This geographic difference can at least partly be attributed to etiological factors. In western countries, primary sclerosing cholangitis (PSC) is a well-recognized risk factor that is associated with CCA. In Southeast Asia, CCA is more commonly associated with parasitic infection by the liver flukes, Opisthorchis viverrini and Clonorchis sinensis, hepatitis B infection and hepatolithiasis [6, 7]. GBC on the other hand occurs more commonly in South Asia (Northern India and Pakistan), Latin America (including Chile that has the highest incidence rates in the world) and in the Native American, Alaskan Indian, and the Hispanic population in the USA [8, 9]. Risk factors associated with GBC include cholelithiasis, obesity, and diabetes [9].
In most of the western world, GBC is decreasing in incidence in the Caucasian population largely due to increased usage of laparoscopic cholecystectomy [10]. Within the USA, however there is a slight increase in GBC incidence in the younger population [8]. This increase has been attributed to migratory trends. However, IHCCA was previously reported to be increasing in incidence. [11–13]. This increased incidence is being disputed as it is at least partly attributable to a change in the International Classification of Disease classification system wherein cancers of unknown primary in the liver are now being re-classified as IHCCA. Furthermore, Klatskin tumors were previously incorrectly characterized as IHCCA and correction of this anomaly suggests that the incidence of IHCCA is relatively stable from 2000 to 2007 while EHCCA has increased over the same period [14].
Not only are there major epidemiological differences between the three types of BTC, but there are also significant molecular differences as illustrated below.
Molecular heterogeneity of BTC
Significant differences exist in mutational spectrum of GBC vs. CCA. For instance, Isocitrate Dehydrogenase 1 or 2 (IDH1/2), BAP1 mutations and FGFR fusions are more likely to occur in IHCCA while KRAS, p53, and SMAD4 mutations are more common in EHCCA. GBC on the other hand has the high frequency of ERBB2 mutations [2, 15]. Furthermore, mutational aberrations vary with etiology of CCA. In patients with non-liver fluke-related CCAs, BAP1 and IDH1, IDH2 are more commonly seen whereas in liver fluke--related CCA, p53 and SMAD4 mutations are observed [16]. IDH1/2 mutation frequency varies depending upon ethnicity—lower in Asians (7.5 %) than in Caucasians (25 %) [17]. A difference in the mutational landscape is also observed when IHCCA is associated with chronic liver disease as compared with IHCCA that occurs in normal liver. In chronic liver disease associated IHCCA, higher frequency of EGFR and lower KRAS, MLH1, and GNAS mutations are observed as compared with normal liver associated IHCCA [18].
Genomic profiling in clinical practice
Since 2012, a rapidly increasing pace of genetic sequencing studies have begun to illuminate the landscape of deregulated genes and pathways in cholangiocarcinoma. As a result of technical advances in molecular pathology, high-throughput sequencing, also referred to as next- generation sequencing (NGS) has now become feasible in standard clinical practice. Standard formalin-fixed paraffin-embedded specimens can now be used for NGS. Several academic centers and commercial vendors have developed analytic protocols for the interpretation of NGS data. Simultaneous developments of novel therapeutics that target the somatic mutations (drivers) have together made significant advances towards precision oncology. A significant caveat in this field is the need for core needle biopsies for high-throughput NGS studies. Biopsies in BTC are often limited to fine needle aspirates and cytology and therefore NGS is feasible in less than 50 % of the new BTC cases in our clinical experience.
Before the advent of NGS, tumor genotyping was performed for few select oncogenes (hotspots). The present assay options include cancer ‘gene panels’, whole exome genome, or transcriptome sequencing. There are several potential uses for NGS besides targeted therapeutics: these include diagnostics, identification of resistance mechanisms, and prognostic stratification. The most recurrent and functionally validated mutations discovered in IHCCA occur in five broad categories are as follows: (1) fusions affecting the receptor tyrosine kinase FGFR2; (2) MAPK pathway mutations, particularly KRAS, NRAS, BRAF, and ARAF; (3) IDH1 and IDH2 mutations; (4) chromatin-modifying genes, particularly ARID1A, ARID1B, PBRM1, BAP1, and MLL3; and (5) cell cycle-related genes including TP53, mutation or deletion of CDKN2A, and amplification of CCND1. Tables 1 and 2 illustrate the most common mutations found in BTCs.
Mutational spectrum and prognosis
Correlation between mutational burden and prognosis has the potential of guiding clinical interventions. Our group recently profiled 321 cases with BTC and correlated the mutational spectrum with clinical outcome [19]. In this study, 224 were IHCCA, 42 were EHCCA, and 55 had GBCA. Treatments administered included surgery (n = 130), radiation (n = 83), while the rest received systemic therapy. In IHCCA, p53 and KRAS mutations were associated with poor overall survival (OS), whereas FGFR mutations were associated with a significantly improved OS. On the other hand, IDH1 or IDH2 mutations were not prognostic in this study confirming a similar prior report from Goyal et al. [20]. CDKN2A mutations were significantly associated with poor progression-free survival (PFS) in both IHCCA and EHCCA. On multivariate analysis, only p53 and FGFR mutations were significantly associated with OS, with a HR of 1.64 (p = 0.015) and 0.478 (p = 0.03), respectively. The FGFR mutant subgroup in particular appears to be a unique and relatively indolent subtype of CCA, occurring more frequently in women, some <40 years of age at diagnosis. BAP1 mutant CCA appears to be an aggressive subtype, with a predilection for bony metastases although the number of cases were limited in our series [2].
Actionable mutations and targeted therapy
BTC may have more actionable mutations than any other gastrointestinal malignancy. In IHCCA in particular, over 30 % of cases have FGFR, IDH or BRAF mutations, all of which have small molecule inhibitors in clinical trials [2, 21, 22]. In GBC, 15 % of cases have HER2/Neu amplification that can be targeted by trastuzumab and newer inhibitors of this pathway [23]. These mutations and early clinical trial results are discussed below.
FGFR signaling pathway
The FGFR pathway is a complex signaling pathway consisting of four transmembrane tyrosine kinase receptors FGFR (1 to 4) along with 18 FGF ligands. These ligands are polypeptide growth factors that bind to receptors expressed on the surface of target cells. Upon binding, the receptor-ligand combination is responsible for the regulation of several key cell processes including cell proliferation, survival, migration, and angiogenesis [24, 25]. For instance, FGF mediated activation of MAPK signaling pathway is responsible for cellular proliferation and activation of the PI3K/AKT pathway which prevents apoptosis [26]. Aberrant FGFR pathway activity caused by genetic alterations such as activating mutations, amplifications, or chromosomal translocation can initiate malignant transformation.
FGFR1amplifications have been implicated in several human solid tumors like squamous and adenocarcinoma non-small cell lung cancer (NSCLC), small cell carcinoma lung, squamous carcinoma head and neck, breast, ovarian, and esophageal cancer. Additionally, hepatocellular carcinoma is associated with FGFR4 overexpression [25]. In IHCCA, FGFR fusions have been observed more commonly [21]. About 13–14 % of IHCCAs have FGFR2 fusions/ translocations and these may be mutually exclusive with KRAS mutations [2, 3, 21, 27]. FGFR2 fusions have been observed more commonly in women, with an improved disease-free and overall survival and relatively indolent disease course [2, 27]. Common FGFR2 fusions observed are FGFR2- AHCYL1, FGFR2-BICC1, FGFR2-PARK2, FGFR2-MGEA5, FGFR2-TACC3, and FGFR2-KIAA1598 [2, 21, 28–30] Cancers with FGFR2 gene fusions have demonstrated an enhanced susceptibility to FGFR inhibitors as compared to cancers having FGFR point mutations [21, 30]. On the other hand, FGFR4 overexpression is associated with proliferation, invasion and epithelial-mesenchymal transition leading to a more aggressive disease type with poorer survival in IHCCA [24]. Anti-FGFR agents include small molecule tyrosine kinase inhibitors (TKIs) that act at the receptor level inhibiting downstream signaling and the FGFR antibody drug conjugate LY3076226. Selective FGFR-targeted TKIs include BGJ398 and JNJ42756493. BGJ398 is a selective pan-FGFR inhibitor with potent activity against FGFR1-3 and has been the furthest along in clinical development in CCA. A recent phase II study of BGJ398 in CCA was reported at the Gastrointestinal Cancers Symposium. This study showed that BGJ398 was well tolerated other than for hyperphosphatemia and resulted in a response rate of 14 % with the disease control rate of 82 % [31]. Non-selective FGFR inhibitors include brivanib, nintedanib, pazopanib, regorafenib, dovitinib, and ponatinib. Phase II trial of ponatinib for advanced IHCAA is ongoing [24].
IDH1/2 mutations in CCA
IDH1 and IDH2 are the cytosolic and mitochondrial variants of metabolic enzymes, respectively and both have very similar functions. These enzymes normally lead to the conversion of a-ketoglutarate (aKG) into isocitrate but the mutant enzymes convert aKG to D-2-hydroxyglutarate (2-HG), which is oncogenic [22]. Wang et al., established that IDH1 mutations in cholangiocarcinomas impair the activity of TET family of DNA dioxygenases which are aKG-dependent dioxygenases, resulting in a decrease of cytosine hydroxymethylation with a concurrent increase of DNA methylation [17]. The epigenetic alterations caused by IDH1/2 mutations lead to a blockade of cellular differentiation, causing an increase in the progenitor cells, which eventually results in tumorigenesis, most commonly in gliomas and acute myeloid leukemia. A similar mechanism may be responsible in IHCCA [17]. IDH mutations occur primarily in IHCCA (ranging from 10 to 43 % cases) and rarely in EHCCA and GBC [32]. Several studies have demonstrated the presence of IDH1/2 mutations exclusively in IHCCA. A meta-analysis demonstrated that IDH1/2 mutations are mutually exclusive with KRAS/NRAS mutations in CCA (p = 0.055) but may co-exist frequently with BAP1 mutations (p = 0.002) [33]. IDH and FGFR mutations also almost never co-exist and may be mutually exclusive. The most common IDH mutations in IHCCA are IDH1-R132C and IDH1-R132G (44 and 14 %, respectively) [33]. Circulating 2-HG level has been reported as a surrogate biomarker for IDH-mutant CCA. IDH inhibitors AG-120, AG-881, and IDH-305 are in clinical trials at the current time for the treatment of IDH-mutant CCA, acute myeloid leukemia, and glioma [34]. Burris et al., recently presented the clinical efficacy data of the IDH1 inhibitor AG-120, in patients with IDH1 mutant cancers, including IHCCA [35]. In their study, one out of 20 patients with IDH1-mutant IHCCA had a partial response while 11 patients had sustained stable disease. While the preliminary signal from IDH1-directed therapies is encouraging, further studies are required to clarify their role in the management of BTC.
DNA repair mutations in CCA
DNA repair mechanisms play a critical role in the maintenance of genomic stability. Germline mutations are the cause of cancer, for instance in hereditary breast and ovarian cancer, Fanconi anemia, and xeroderma pigmentosum syndromes. Somatic mutations in DNA repair genes are present quite commonly in tumors and may represent targets for therapeutic intervention with specific inhibitors or DNA damaging chemotherapeutic agents. The presence of incorrect DNA repair in tumor cells predisposes them to accumulate even more genetic alterations. For example, colorectal and endometrial cancers with defective DNA mismatch repair (MMR) due to mutations in the MLH1 and MSH2 genes have very high single nucleotide changes and small insertions/deletions. This high mutational burden makes Microsatellite Instability-high (MSI-H) tumors susceptible to immune blockade using checkpoint inhibitors [36]. In our series of 321 BTC with mutational profiling discussed earlier, DNA repair mutations (in MSH6, BRCA1, BRCA2, ATM, MLH1, or MSH2 genes) occurred in 12 % IHCCA, 26 % in EHCCA, and 5 % of GBC cases [19]. Data in regards to MMR in BTC is very limited. Goyal et al., reported a 9 % rate of MMR protein loss in CCA patients on immunohistochemistry [37]. The incidence is even lower in Asia, particularly in liver fluke-associated IHCCA in Thailand [38]. These results suggest that DNA repair mutations may be an important area of investigation for targeted therapeutics with specific DNA repair inhibitors or immunotherapy in a subset of IHCCA patients.
HER2/Neu in BTC
HER2/neu gene is a key driver of oncogenesis and its overexpression as a result of gene amplification is a critical target for therapy in breast cancer and gastric cancer. HER 2/neu overexpression has also been reported in BTC. Our group recently studied HER2/neu expression in 187 cases of gallbladder cancer; this is the largest reported series to date using the commonly accepted American Society of Clinical Oncology criteria [15]. We noted that 13 % of patients have HER2/neu overexpression (3+ by immunohistochemistry) and radiological partial responses were noted with HER2/neu directed therapies [23]. HER2/neu amplification is uncommon in CCA, although mutations in the kinase domain (V777 L) or in the extracellular domain (S310F) were noted in EHCCA [39]. HER2/neu amplifications can be successfully targeted by trastuzumab reversible small molecule tyrosine kinase inhibitors (TKIs), including afatinib, neratinib, and dacomatinib. Fig. 1 illustrates the benefits of HER 2/neu targeted therapy, noted in a case of GBC that was invading the liver arising in a 61-year-old female. After 3 months of treatment with trastuzumab and FOLFOX, follow-up scans demonstrated resolution of the gallbladder mass and decrease in the size of the liver mass. Other EGFR pathway-directed agents include erlotinib, cetuximab, and panitumumab. EGFR overexpression is common in BTC and these agents have been investigated in prospective clinical trials. Philip et al., demonstrated the benefits of erlotinib as a single agent in 42 patients with advanced BTC. A 10 % response rate was noted [40]. Lee at al, studied the effect of gemcitabine, oxaliplatin + erlotinib in a phase III randomized trial conducted in 268 South Korean patients with BTC. They noted a significantly higher response rate with the addition of erlotinib. However, this did not translate to a survival improvement [41]. In the gemcitabine, oxaliplatin, with or without cetuximab, in advanced biliary-tract cancer (BINGO) phase II randomized trial, the effect cetuximab addition to standard first-line chemotherapy was explored. Unfortunately, Malka et al. noted that cetuximab did not improve the patient outcome as measured by 4-month progression survival [42]. Other EGFR-directed therapies are discussed in detail by Merla et al. [34].
Her2/neu directed therapy for advanced gallbladder carcinoma. Axial contrast-enhanced CT images demonstrate a. a mass in the gallbladder neck causing gallbladder obstruction. There is contiguous extension from the mass to the liver b after 3 months of Her2/neu directed therapy with trastuzumab, the polypoid gallbladder mass is no longer visualized on the scans, and the liver mass is decreased. The patient was then treated with en bloc cholecystectomy and extended right hepatectomy followed by adjuvant chemotherapy
Mutations in chromatin remodeling genes
Chromatin remodeling allows the genomic DNA to access regulatory transcriptional proteins and thereby controlling gene expression. Genes involved in chromatin remodeling including ARID, BAP1, PBRM, and MLL are mutated in several cancer types and the resultant alterations induce carcinogenesis. BAP1 encodes for a nuclear deubiquitinase involved in chromatin remodeling and mutations in this gene are associated with uveal melanoma, mesothelioma, and renal cancers, while PBRM1 and ARID1A both encode a subunit of the ATP dependent SWI/SNF chromatin remodeling complexes [43, 44]. Jiao et al., performed exome sequencing in 32 IHCCA cases and reported frequent mutations in BAP1, ARID1A, and PBRM1 [3]. In fact, mutation in at least one of these genes occurred in almost half of the carcinomas sequenced. Interestingly, exome sequencing studies by Ong et al., in liver fluke-associated CCA did not report a high incidence of these mutations, thereby further highlighting the differences between these two types of CCA (liver fluke-associated CCA in Asia and sporadic CCA in western patients) [45••]. Furthermore, a Chinese study of IHCCA detected very low BAP1 and PBRM1 mutations (1 % each) compared to other studies, suggesting possible geographic/demographic influences as seen in Table 1. In our series, we found no correlation between mutations in chromatin remodeling genes and prognosis. However, BAP1 mutation was associated with an aggressive outcome in some cases of IHCCA and a predilection for bone metastases [2]. Germline BAP1 mutations confer increased susceptibility for the above cancers as well as epithelioid atypical Spitz tumors and cutaneous melanomas [46]. At the current time, there are no proven therapies for cancers having these mutations. However, histone deacetylase (HDAC) inhibitors may offer therapeutic value and need further investigation.
Linking genomics with immunotherapy in BTC
There is a well-recognized link between tumor genomics and immune response. Hypermutated tumors, such as those with microsatellite instability result in a heavy burden of neoantigens that contribute significantly to the success of clinical immunotherapies in this setting [47]. DNA repair mutations in CCA including those resulting in MSI were discussed earlier. Investigators at National Institute of Health used a unique approach to immunotherapy for CCA. They used a whole-exomic-sequencing-based approach to demonstrate that tumor-infiltrating lymphocytes (TIL) from a patient with metastatic cholangiocarcinoma contained CD4+ T helper 1 (TH1) cells recognizing a mutation in erbb2 interacting protein (ERBB2IP). After adoptive transfer of TIL containing mutation-specific polyfunctional TH1 cells, the patient achieved prolonged partial response. Upon disease progression, the patient was retreated with mutation-reactive TH1 cells and again experienced tumor regression [48]. These results provide evidence that a CD4+ T-cell response against a mutated antigen can lead to tumor regression. Identification of immunogenic epitopes in the mutations seen in CCA will be key to success with checkpoint inhibitors. From an immunological perspective, IDH1 (R132H) represents a potential target for immunotherapy as it is a tumor-specific potential neoantigen expressed in tumor cells. Schumacher et al., demonstrated that IDH1 (R132H) contains an immunogenic epitope suitable for mutation-specific vaccination. Peptides from the mutated region induce CD4+ immune response that can potentially be exploited by mutation-specific anti-IDH1 (R132H) vaccines [49]. Further immunologic profiling of CCA and correlation with mutational spectrum of this cancer is required. Recent results with pembrolizumab in pre-treated BTC having Programmed death-ligand 1 (PD-L1) expression indicated that 17 % of patients have a meaningful response to checkpoint blockade [50]. These results have reinforced the potential role of immune therapy in this disease.
Conclusions
BTC are enriched with actionable mutations and with the advent of modern NGS technologies, precision medicine may soon become a reality and alter the treatment landscape of these cancers. The use of NGS is no longer limited to research settings and this technology can now be incorporated into standard clinical practice for the management of BTC patients. Given the genomic heterogeneity between the different BTC tumors, NGS argues for a need for molecular classification of BTC as against the traditional classification based on the site of the primary. The current American Joint Committee on Cancer (AJCC) classification is based on tumor location and extent and is an effective tool for estimating prognosis and treatment selection. In the case of BTC, we hypothesize that molecular information could add to the clinical utility of the AJCC classification. For instance, KRAS mutations and FGFR fusions may represent polar ends of the clinical prognostic spectrum at a given stage of intrahepatic CCA. An integrated clinical and molecular classification system consisting of AJCC staging in addition to validated molecular biomarkers could alter the treatment paradigm for BTC, and an international collaborative effort is required to advance this field. Our charge now is to make NGS feasible using limited biopsy samples and accessible to a larger population of BTC patients at a lower cost.
References and recommended reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Rovigatti U. Cancer modelling in the NGS era—part I: emerging technology and initial modelling. Crit Rev Oncol Hematol. 2015;96(2):274–307. doi:10.1016/j.critrevonc.2015.05.017.
Churi CR, Shroff R, Wang Y, Rashid A, Kang HC, Weatherly J, et al. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLoS One. 2014;9(12):e115383. doi:10.1371/journal.pone.0115383 .This article correlates clinical outcome with somatic mutations in BTC and is therefore of significance
Jiao Y, Pawlik TM, Anders RA, Selaru FM, Streppel MM, Lucas DJ, et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet. 2013;45(12):1470–3 .First experience with exome sequening in BTC that idnitifies mutations in chromatin modulating genes
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. A cancer journal for clinicians: CA; 2015.
Bragazzi MC, Cardinale V, Carpino G, Venere R, Semeraro R, Gentile R, et al. Cholangiocarcinoma: epidemiology and risk factors. Translational Gastrointestinal Cancer. 2011;1(1):21–32.
Rizvi S, Borad MJ, Patel T, Gores GJ, editors. Cholangiocarcinoma: molecular pathways and therapeutic opportunities. Seminars in liver disease; 2014: NIH Public Access.
Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology (Baltimore, Md). 2011;54(1):173–84.
Henley SJ, Weir HK, Jim MA, Watson M, Richardson LC. Gallbladder cancer incidence and mortality, United States 1999–2011. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24(9):1319–26. doi:10.1158/1055-9965.epi-15-0199.
Castro FA, Koshiol J, Hsing AW, Devesa SS. Biliary tract cancer incidence in the United States—demographic and temporal variations by anatomic site. Int J Cancer. 2013;133(7):1664–71 .Description of changes in the incidnce of BTC over time. Cholangiocarcinoma may not be increasing as rapidly as previously described
Witjes CD, van den Akker SA, Visser O, Karim-Kos HE, de Vries E, Ijzermans JN, et al. Gallbladder cancer in the Netherlands: incidence, treatment and survival patterns since 1989. Dig Surg. 2012;29(2):92–8. doi:10.1159/000336217.
Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: liver, biliary tract, and pancreas. Gastroenterology. 2009;136(4):1134–44.
Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology (Baltimore, Md). 2001;33(6):1353–7.
Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40(3):472–7.
Tyson GL, Ilyas JA, Duan Z, Green LK, Younes M, El-Serag HB, et al. Secular trends in the incidence of cholangiocarcinoma in the USA and the impact of misclassification. Dig Dis Sci. 2014;59(12):3103–10 .Description of changes in the incidnce of BTC over time. Cholangiocarcinoma may not be increasing as rapidly as previously described
Roa I, de Toro G, Schalper K, de Aretxabala X, Churi C, Javle M. Overexpression of the HER2/neu gene: a new therapeutic possibility for patients with advanced gallbladder cancer. Gastrointestinal cancer research: GCR. 2014;7(2):42–8 .Largest experience with her2/neu IHC in GB Cancer suggesting a need for targeted therapy in this disease
Chan-On W, Nairismägi M-L, Ong CK, Lim WK, Dima S, Pairojkul C, et al. Exome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancers. Nat Genet. 2013;45(12):1474–8 .Exome sequencing of asian patients with BTC shows differences when compared with western population
Wang P, Dong Q, Zhang C, Kuan PF, Liu Y, Jeck WR, et al. Mutations in isocitrate dehydrogenase 1 and 2 occur frequently in intrahepatic cholangiocarcinomas and share hypermethylation targets with glioblastomas. Oncogene. 2013;32(25):3091–100. doi:10.1038/onc.2012.315 .IDH1 and 2 mutations occur in cholangiocarcinoma and gliomas and may represent targets for therapy in both these diseases
Jang S, Chun S-M, Hong S-M, Sung CO, Park H, Kang HJ, et al. High throughput molecular profiling reveals differential mutation patterns in intrahepatic cholangiocarcinomas arising in chronic advanced liver diseases. Mod Pathol. 2014;27(5).
Ross JS, Wang K, Javle MM, Catenacci DVT, Shroff RT, Ali SM et al. Comprehensive genomic profiling of biliary tract cancers to reveal tumor-specific differences and frequency of clinically relevant genomic alterations. ASCO Meeting Abstracts. 2015; 33(15_suppl):4009.
Goyal L, Govindan A, Sheth RA, Nardi V, Blaszkowsky LS, Faris JE, et al. Prognosis and clinicopathologic features of patients with advanced stage isocitrate dehydrogenase (IDH) mutant and IDH wild-type intrahepatic cholangiocarcinoma. Oncologist. 2015;20(9):1019–27. doi:10.1634/theoncologist.2015-0210 .Description of patients with IDH mutant cholangiocarcinoma in the U.S
Arai Y, Totoki Y, Hosoda F, Shirota T, Hama N, Nakamura H, et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology (Baltimore, Md). 2014;59(4):1427–34. doi:10.1002/hep.26890.
Borger DR, Goyal L, Yau T, Poon RT, Ancukiewicz M, Deshpande V, et al. Circulating oncometabolite 2-hydroxyglutarate is a potential surrogate biomarker in patients with isocitrate dehydrogenase-mutant intrahepatic cholangiocarcinoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2014;20(7):1884–90. doi:10.1158/1078-0432.ccr-13-2649 .Circulating 2HG may be an important PD biomarker for iDH-directed therapies in BTC
Javle M, Churi C, Kang HC, Shroff R, Janku F, Surapaneni R, et al. HER2/neu-directed therapy for biliary tract cancer. J Hematol Oncol. 2015;8:58. doi:10.1186/s13045-015-0155-z.
Ang C. Role of the fibroblast growth factor receptor axis in cholangiocarcinoma. J Gastroenterol Hepatol. 2015;30(7):1116–22.
Hierro C, Rodon J, Tabernero J. Fibroblast growth factor (FGF) receptor/FGF inhibitors: novel targets and strategies for optimization of response of solid tumors. Semin Oncol. 2015;42(6):801–19. doi:10.1053/j.seminoncol.2015.09.027.
Wesche J, Haglund K, Haugsten EM. Fibroblast growth factors and their receptors in cancer. The Biochemical journal. 2011;437(2):199–213. doi:10.1042/bj20101603.
Graham RP, Fritcher EGB, Pestova E, Schulz J, Sitailo LA, Vasmatzis G, et al. Fibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinoma. Hum Pathol. 2014;45(8):1630–8.
Borad MJ, Champion MD, Egan JB, Liang WS, Fonseca R, Bryce AH, et al. Integrated genomic characterization reveals novel, therapeutically relevant drug targets in FGFR and EGFR pathways in sporadic intrahepatic cholangiocarcinoma. PLoS Genet. 2014;10(2):e1004135.
Ross JS, Wang K, Gay L, Al-Rohil R, Rand JV, Jones DM, et al. New routes to targeted therapy of intrahepatic cholangiocarcinomas revealed by next-generation sequencing. Oncologist. 2014;19(3):235–42.
Wu YM, Su F, Kalyana-Sundaram S, Khazanov N, Ateeq B, Cao X, et al. Identification of targetable FGFR gene fusions in diverse cancers. Cancer discovery. 2013;3(6):636–47. doi:10.1158/2159-8290.cd-13-0050 .First description of FGFR mutations in cholangiocarcinoma
Javle MM, Shroff RT, Zhu A, Sadeghi S, Choo S, Borad MJ et al. A phase 2 study of BGJ398 in patients (pts) with advanced or metastatic FGFR-altered cholangiocarcinoma (CCA) who failed or are intolerant to platinum-based chemotherapy. ASCO Meeting Abstracts. 2016; 34(4_suppl):335.
Kipp BR, Voss JS, Kerr SE, Barr Fritcher EG, Graham RP, Zhang L, et al. Isocitrate dehydrogenase 1 and 2 mutations in cholangiocarcinoma. Hum Pathol. 2012;43(10):1552–8. doi:10.1016/j.humpath.2011.12.007.
Grassian AR, Pagliarini R, Chiang DY. Mutations of isocitrate dehydrogenase 1 and 2 in intrahepatic cholangiocarcinoma. Curr Opin Gastroenterol. 2014;30(3):295–302.
Merla A, Liu KG, Rajdev L. Targeted therapy in biliary tract cancers. Curr Treat Options in Oncol. 2015;16(10):48. doi:10.1007/s11864-015-0366-0.
Burris H, Mellinghoff I, Maher E, Wen P, Beeram M, Touat M, et al. Abstract PL04-05: the first reported results of AG-120, a first-in-class, potent inhibitor of the IDH1 mutant protein, in a phase I study of patients with advanced IDH1-mutant solid tumors, including gliomas. Mol Cancer Ther. 2015;14(12 Supplement 2):PL04–5. doi:10.1158/1535-7163.targ-15-pl04-05.
Maletzki C, Schmidt F, Dirks WG, Schmitt M, Linnebacher M. Frameshift-derived neoantigens constitute immunotherapeutic targets for patients with microsatellite-instable haematological malignancies: frameshift peptides for treating MSI+ blood cancers. Eur J Cancer. 2013;49(11):2587–95. doi:10.1016/j.ejca.2013.02.035.
Goyal L, Deshpande V, Chung DC, Groeschl RT, Gamblin TC, Zhu AX. Mismatch repair protein loss and microsatellite instability in cholangiocarcinoma. ASCO Meeting Abstracts. 2014; 32(3_suppl):237.
Liengswangwong U, Karalak A, Morishita Y, Noguchi M, Khuhaprema T, Srivatanakul P, et al. Immunohistochemical expression of mismatch repair genes: a screening tool for predicting mutator phenotype in liver fluke infection-associated intrahepatic cholangiocarcinoma. World journal of gastroenterology: WJG. 2006;12(23):3740–5.
Lee H, Wang K, Johnson A, Jones DM, Ali SM, Elvin JA, et al. Comprehensive genomic profiling of extrahepatic cholangiocarcinoma reveals a long tail of therapeutic targets. J Clin Pathol. 2016;69(5):403–8. doi:10.1136/jclinpath-2015-203394.
Philip PA, Mahoney MR, Allmer C, Thomas J, Pitot HC, Kim G, et al. Phase II study of erlotinib in patients with advanced biliary cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2006;24(19):3069–74. doi:10.1200/jco.2005.05.3579.
Lee J, Park SH, Chang HM, Kim JS, Choi HJ, Lee MA, et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. The Lancet Oncology. 2012;13(2):181–8. doi:10.1016/s1470-2045(11)70301-1.
Malka D, Cervera P, Foulon S, Trarbach T, de la Fouchardière C, Boucher E, et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. The lancet oncology. 2014;15(8):819–28.
Wu RC, Wang TL, Shih IM. The emerging roles of ARID1A in tumor suppression. Cancer biology & therapy. 2014;15(6):655–64. doi:10.4161/cbt.28411.
Chiang NJ, Shan YS, Hung WC, Chen LT. Epigenetic regulation in the carcinogenesis of cholangiocarcinoma. Int J Biochem Cell Biol. 2015;67:110–4. doi:10.1016/j.biocel.2015.06.012.
Ong CK, Subimerb C, Pairojkul C, Wongkham S, Cutcutache I, Yu W, et al. Exome sequencing of liver fluke-associated cholangiocarcinoma. Nat Genet. 2012;44(6):690–3. doi:10.1038/ng.2273 .Exome sequencing of population of cholangiocarcinoma, particularly in Thailand suggests a different disease biology
Murali R, Wiesner T, Scolyer RA. Tumours associated with BAP1 mutations. Pathology. 2013;45(2):116–26. doi:10.1097/PAT.0b013e32835d0efb.
Dudley JC, Lin MT, Le DT, Eshleman JR. Microsatellite instability as a biomarker for PD-1 blockade. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016;22(4):813–20. doi:10.1158/1078-0432.CCR-15-1678.
Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344(6184):641–5. doi:10.1126/science.1251102.
Schumacher T, Bunse L, Pusch S, Sahm F, Wiestler B, Quandt J, et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 2014;512(7514):324–7. doi:10.1038/nature13387.
Bang YJ, Doi T, Braud FD, Piha-Paul S, Hollebecque A, Razak ARA, et al. 525 safety and efficacy of pembrolizumab (MK-3475) in patients (pts) with advanced biliary tract cancer: interim results of KEYNOTE-028. Eur J Cancer. 51:S112. doi:10.1016/S0959-8049(16)30326-4.
Nakamura H, Arai Y, Totoki Y, Shirota T, Elzawahry A, Kato M et al. Genomic spectra of biliary tract cancer. Nat Genet 2015; 47(9):1003–10. doi: 10.1038/ng.3375 http://www.nature.com/ng/journal/v47/n9/abs/ng.3375.html#supplementary-information.
Zou S, Li J, Zhou H, Frech C, Jiang X, Chu JS, et al. Mutational landscape of intrahepatic cholangiocarcinoma. Nat Commun. 2014;5:5696. doi:10.1038/ncomms6696.
Simbolo M, Fassan M, Ruzzenente A, Mafficini A, Wood LD, Corbo V, et al. Multigene mutational profiling of cholangiocarcinomas identifies actionable molecular subgroups. Oncotarget. 2014;5(9):2839–52. doi:10.18632/oncotarget.1943.
Lee H, Wang K, Johnson A, Jones DM, Ali SM, Elvin JA, et al. Comprehensive genomic profiling of extrahepatic cholangiocarcinoma reveals a long tail of therapeutic targets. J Clin Pathol. 2015. doi:10.1136/jclinpath-2015-203394.
Li M, Zhang Z, Li X, Ye J, Wu X, Tan Z, et al. Whole-exome and targeted gene sequencing of gallbladder carcinoma identifies recurrent mutations in the ErbB pathway. Nat Genet. 2014;46(8):872–6. doi:10.1038/ng.3030.
Stephens P, Wang K, Palma NA, Chmielecki J, Shroff RT, Churl C et al., editors. Comprehensive genomic profiling of gallbladder adenocarcinoma and frequent genomic-derived targets of therapy. JOURNAL OF CLINICAL ONCOLOGY; 2014: AMER SOC CLINICAL ONCOLOGY 2318 MILL ROAD, STE 800, ALEXANDRIA, VA 22314 USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Topical Collection on Upper Gastrointestinal Cancers
Rights and permissions
About this article
Cite this article
Jain, A., Kwong, L.N. & Javle, M. Genomic Profiling of Biliary Tract Cancers and Implications for Clinical Practice. Curr. Treat. Options in Oncol. 17, 58 (2016). https://doi.org/10.1007/s11864-016-0432-2
Published:
DOI: https://doi.org/10.1007/s11864-016-0432-2