Opinion statement
At present, sipuleucel-T represents the only approved immunotherapy for prostate cancer. Sipuleucel-T is an autologous cellular therapy, which primes autologous antigen-presenting cells against the prostatic acid phosphatase (PAP) antigen. For patients with metastatic castrate-resistant prostate cancer (CRPC) who are asymptomatic or minimally symptomatic, sipuleucel-T monotherapy is one of the standard of care treatment options pre- or postdocetaxel. With the approval of new treatments, including abiraterone and enzatutamide, sequencing and combination of these treatments with sipuleucel-T represent unanswered questions facing the field. Whereas steroids that are coadministered with abiraterone and chemotherapy have long been thought to be immunosuppressive, early results show that concurrent abiraterone and prednisone does not significantly impact the ability to develop immune responses to this treatment. Additional clinical data are needed to elucidate optimal sequencing of therapeutic agents in CRPC. Several novel immunotherapies are currently in development, and enrollment in clinical trials should be considered. These include PROSTVAC-VF, a viral vaccine that encodes PSA and T-cell costimulatory molecules, which is currently undergoing phase III clinical trials. DNA plasmid-based vaccines targeting different antigens, including PAP, also are under investigation. Immune checkpoint blockade with ipilimumab, a monoclonal antibody against CTLA-4, which is approved for metastatic melanoma, also is being evaluated. Whereas this treatment failed to show significant improvement in overall survival in CRPC patients treated with docetaxel, results from a phase III trial in the predocetaxel setting are pending. Conventional therapies for prostate cancer, such as radiation and hormonal therapy, may have immunomodulatory effects. Future areas for research include the sequencing and combination of immunotherapies as well as other conventional therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is estimated to have an incidence of 238,590 new cases and projected to be responsible for 29,720 deaths in the United States during 2013. The disease is the most common noncutaneous cancer and the second leading cause of cancer death for American men [1]. The management of localized disease is stratified by prognostic group, which is defined by PSA level, Gleason score, and primary tumor stage. Modalities include active surveillance, radical prostatectomy, lymph node dissection, radiotherapy, brachytherapy, and neoadjuvant or adjuvant androgen deprivation therapy. The disease is primarily driven by signaling through the androgen receptor, and initial therapy for metastatic disease is primary androgen deprivation [2•]. The disease becomes lethal when progression occurs in the low testosterone environment, which is then referred to as castration-resistant prostate cancer (CRPC). Median survival for patients with CRPC is around 2 years [3]. The treatment of CRPC has significantly changed during the past few years. The clinical state of CRPC patients has been previously classified by pre- and postdocetaxel treatment. With the approval of novel agents, CRPC now can be clinically classified differently based on symptoms as well as before and after these newer agents (Table 1). Sipuleucel-T was approved for patients pre- and postdocetaxel so long as they are asymptomatic or minimally symptomatic. Agents that target secondary hormonal manipulation have been approved since, including abiraterone, an androgen biosynthesis inhibitor, and enzalutamide, an androgen receptor inhibitor. Abiraterone is approved for use in the pre- and postdocetaxel settings [4]. Enzalutamide is currently approved in the postdocetaxel setting and is currently under evaluation in the predocetaxel setting [5]. Cabazitaxel is a second-generation taxane approved for use postdocetaxel [6]. Radium-223 now also is approved for CRPC patients with symptomatic bone metastasis pre- and postdocetaxel [7]. Optimal sequencing of these new therapies is not known at present. Nevertheless, sipuleucel-T is currently being evaluated not just relative to these approved treatments for CRPC but also is being evaluated in earlier clinic settings, including in the neoadjuvant and biochemical relapse settings.
Multiple immune approaches beyond sipuleucel-T are under development and include antigen-directed therapies as well as monoclonal antibodies against immune checkpoints. Moreover, combinations of these immunotherapies with other immunotherapies and conventional therapies are under investigation. Finally, studying immunotherapies in earlier settings of disease, where the immune systems of patients may be more intact, also are being studied.
Treatment
-
Multiple immune therapies are under development for the treatment of CRPC; however, the only commercially available agent is currently sipuleucel-T. Enrollment in clinical trials should be considered for eligible patients as an alternative to sipuleucel-T treatment.
Pharmacologic treatment
Sipuleucel-T
-
Sipuleucel-T is an autologous cellular immunotherapy. Peripheral blood mononuclear cells are obtained from the patient via leukapheresis and then cultured ex vivo with a fusion protein containing prostatic acid phosphatase (PAP) and granulocyte monocyte colony stimulating factor (GM-CSF). The product, containing activated antigen-presenting cells (APCs), are subsequently reinfused in three doses at approximately 2-week intervals, resulting in activation and proliferation of T cells with antigen-specific reactivity against PAP [8].
-
Sipuleucel-T was evaluated in three Phase III studies. Study D9901, which enrolled 127 patients, demonstrated a significant 4.5-month survival advantage in the sipuleucel-T group compared with control [9]. Study D9902A, which enrolled 98 patients, demonstrated a trend towards overall survival (OS) of 3.3 months but was discontinued early [10]. The pivotal trial leading to the agent’s approval was the IMPACT study, which enrolled 512 subjects in a 2:1 ratio to treatment or control. Trial participants had ECOG performance statuses of zero or one did not have visceral metastases and had not received other treatment within 28 days. The sipuleucel-T group demonstrated a 4.1-month median improvement in overall survival, the primary endpoint. No difference in the secondary endpoint, objective disease progression, was seen. The therapy was well-tolerated. Chills, pyrexia, headache, and myalgias were major adverse events more frequently seen in the treatment group, generally occurring in the postinfusion period [11••]. Analysis of immunological responses in the three phase III trials demonstrated that sipuleucel-T product parameters (APC activation, APC number, and total nucleated cell count) and antigen-specific immune responses were significantly correlated with OS [12]. In an exploratory subgroup analysis of the IMPACT trial, lower baseline PSA values were correlated with increased sipuleucel-T treatment effect, suggesting greatest benefit in less advanced disease states [13].
-
Improvement in overall survival occurs in the absence of changes in progression-free survival. Evaluation of the therapeutic benefit of sipuleucel-T at earlier points in treatment course is challenging, as traditional endpoints, such as RECIST criteria or PSA responses, are not reliable. Numerous bioassays to evaluate antigen-specific responses are under development, and immune-related response criteria (irRC) are increasingly employed during radiographic monitoring [14].
-
Sipuleucel-T is recommended (category 1) as first-line treatment for asymptomatic or minimally symptomatic CRPC in the most recent NCCN guidelines [2]. The therapy is approved in both the pre- and postdocetaxel settings, and efficacy was similar in each during the IMPACT trial. However, sipuleucel-T should be employed as an earlier treatment modality. Also, following the approval of abiraterone and enzalutmide, postdocetaxel patients are more likely to have aggressive, symptomatic disease. Additionally, sipuleucel-T should be considered for use before abiraterone, because immune effects of corticosteroids coadministered with abiraterone are still being evaluated and furthermore to avoid loss of treatment eligibility secondary to symptom progression.
-
Given the duration required to develop an immune response and the potential for durable benefit, sipuleucel-T has been evaluated in earlier stages of disease. The PROTECT trial evaluated androgen-dependent prostate cancer patients with detectable PSA following prostatectomy. There was no change in the primary endpoint, time to biochemical failure (PSA ≥ 3.0 ng/ml). However a 48 % increase in PSA doubling time after testosterone recovery was seen in the sipuleucel-T group, although this endpoint has not been definitively linked to clinical outcomes [15]. A phase II neoadjuvant study, NeoACT, is ongoing. There is evidence of increased T-cell infiltration in prostate tissue at the interface of malignant and benign glands in this setting [16•].
-
Combination strategies with abiraterone represent an important area of investigation. The therapies, both approved in the predocetaxel setting, have unique mechanisms of action and dual therapy may be synergistic. However, potential immunosuppressive effects of prednisone, which must be coadministered with abiraterone, are of concern. A phase II trial (NCT01487863) evaluated sipuleucel-T in combination with concurrent or sequential abiraterone and prednisone. Preliminary findings suggest that sipuleucel-T product parameters, APC activation, cytokine profiles, and safety are not significantly altered with concurrent administration of abiraterone and prednisone [17•].
Emerging therapies (vaccines)
PROSTVAC-VF
-
Other tumor vaccines have been investigated in prostate cancer. PROSTVAC-VF employs a recombinant poxvirus-based vector encoded with PSA and TRICOM, a triad of costimulatory molecules consisting of B7-1, ICAM-1, and LFA-3. The therapy has been studied in two phase II trials.
-
One phase II trial enrolled 32 patients and evaluated PSA-specific T-cell responses with the ELISPOT assay as the primary endpoint. A trend towards increased OS was seen in patients with a greater than sixfold increase in T-cell response. Additionally, a decrease in regulatory T-cell (Treg) suppression was seen in patients surviving longer than expected using the Halabi prognostic model. No difference was seen with GM-CSF coadministration [18].
-
The largest phase II trial to date randomized 125 patients in a 2:1 ratio to treatment or control vectors. PROSTVAC-VF was associated with an 8.5-month survival advantage, and there was a 43 % reduction in death at 3-year follow-up. T-cell immune responses were not evaluated in this study. The therapy was well-tolerated. Major adverse effects more frequently seen in the treatment group included injection site reactions, nausea, and fatigue. Two patients withdrew for possible treatment-related adverse effects [19•].
-
A phase III trial is ongoing, with and without GM-CSF, in asymptomatic or minimally symptomatic men with metastatic CRPC (NCT01322490).
Personalized peptide vaccines
-
Cancer immunity is likely heterogeneous and dependent on diverse host- and tumor-factors, and vaccination with single antigens may not elicit responses in all patients. Personalized peptide vaccination (PPV), in which antigen targets are selected based on preexisting host reactivity, also have been studied. One phase II study immunized 20 docetaxel-resistant and 22 docetaxel-naive CRPC patients with 2–4 peptide targets selected from 31 available antigens based on baseline host responses with ELISPOT. There was a trend towards a survival advantage with vaccination, with median OS time of 17.8 months in docetaxel-resistant patients who received PPV versus 10.8 months in docetaxel-resistant controls. Lower levels of IL-6 in prevaccine samples was associated with more favorable OS [20]. Another phase II study randomized 57 patients in a 1:1 ratio to receive PPV plus low-dose estramustine phosphate (EMP) compared with EMP alone. A significant benefit in the primary endpoint progression-free survival (PFS) was seen, with a median PFS of 8.5 months in the treatment group and 2.8 months in the control group [21].
DNA vaccines
-
A phase I/IIa study evaluated pTVG-HP, which consists of cell-free plasmid DNA encoding PAP, coadministered with GM-CSF in 22 patients with biochemically recurrent disease (stage D0). The therapy was well tolerated, and increase in PSA doubling time compared with pretreatment was observed [22]. Long-term antigen-specific T-cell responses have been observed with this DNA vaccine, and are associated with greater increase in PSA doubling time [23]. Phase II studies are currently ongoing. A two-armed study evaluating the therapy with GM-CSF in patients with nonmetastatic CRPC compares a predetermined dosing schedule versus an adaptive dosing regimen guided by evidence of T-cell immune response (NCT00849121). Another evaluates metastasis-free survival in biochemically recurrent patients with treatment with vaccine plus GM-CSF against GM-CSF alone (NCT01341652). Modest PSA responses have been seen in treatment with GM-CSF alone [24].
GVAX
-
GVAX is an allogeneic whole tumor cell vaccine, which includes two prostate cancer cell lines, LNCaP and PC3, transduced with GM-CSF. The introduction of multiple antigens, both known and unknown, through a whole cell approach was hypothesized to improve the breadth of immune recognition, and also reduce the chance of immune escape through loss of a single antigen. The therapy was studied in two, large, phase III trials. One trial, which administered GVAX with docetaxel, was terminated prematurely due to an imbalance of deaths, with a survival advantage in the control arm [25]. Another trial, which compared GVAX alone to docetaxel and prednisone, failed to show improvements in OS [26].
TG4010
-
TG4010, a vaccinia-based vector expressing the tumor antigen MUC1 and IL-2, was evaluated in a phase II trial with 40 patients. No difference was seen in the primary endpoint, 50 % reduction in PSA. Thirteen of 40 patients had a greater than twofold increase in PSA doubling time [27].
Emerging therapies (immune checkpoint blockade)
Ipilimumab
-
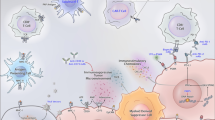
Inhibition of immune checkpoints represents another important major strategy in prostate cancer immunotherapy (Figure 1). Cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) is a molecular “brake” on the immune system. The cell-surface molecule is found on activated T cells and interfaces with the B7 ligands, CD80 and CD86, on antigen-presenting cells (APCs). CTLA-4 binds more avidly to the B7 ligands than CD28 receptors, which are responsible for costimulatory signaling, and leads to negative regulation of T-cell activation and proliferation [28]. CTLA-4 also is expressed constitutively on Treg cells, which contribute to the induction of peripheral self-tolerance [29].
Figure 1 Immune Checkpoint Blockade. Inhibition of molecular “brakes” on the immune system with monoclonal antibodies is another important strategy under investigation in prostate cancer. Cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) is a cell-surface molecule on activated T cells and negatively regulates T-cell activation and proliferation. Blockade of CTLA-4 with ipilimumab enhances CD28 binding to B7 ligands and thus costimulatory signaling. Programmed death 1 (PD-1) is another T-cell cell-surface molecule that interfaces with PD-1 ligands (including PD-L1 and PD-L2) found on tumor cells as well as stromal cells in the tumor microenvironment, leading to inhibition. Blockade of this interaction with antibodies directed against PD-1 or PD-1 ligands enhances T-cell activation.
-
Ipilimumab is a fully human IgG1 monoclonal antibody which blocks CTLA-4, leading to augmentation of antitumor immunity and suppression of tolerance. The therapy is commercially approved for the treatment of metastatic or unresectable melanoma at a dose of 3 mg/kg weekly for 4 weeks. The pivotal phase III study leading to its approval randomized 676 patients in a 3:1:1 ratio to ipilimumab plus glycoprotein 100 (gp100) peptide vaccine, ipilimumab alone, or gp100 alone. Both treatment groups demonstrated a 6-month advantage in OS, the primary endpoint, compared with gp100 alone. Grade 3 or 4 immune-related adverse events, which were most commonly dermatologic (rash, vitiligo) or gastrointestinal (colitis, diarrhea), occurred in 10-15 % of the ipilimumab groups. Corticosteroids, and rarely infliximab, were used for the treatment of immune-related adverse events [30•]. Durable responses, including complete responses (CR), have been observed in a minority of patients. Importantly, CRs were achieved on average after 30 months [31].
-
Several phase II trials have investigated the role of ipilimumab in prostate cancer. A phase I/II study evaluated ipilimumab at up to 10 mg/kg dose with or without radiotherapy in patients with metastatic CRPC who received no more than one prior chemotherapy. PSA decline and radiographic responses were observed in all dose cohorts. Among 45 patients in the 10-mg/kg group, 8 had PSA declines of greater than 50 %, 1 had a CR lasting greater than 11.3 months, and 6 had stable disease. Immune-related adverse events were similar to those described in previous studies [32••]. A phase II study that randomized 43 chemotherapy-naive CRPC patients to ipilimumab at 3 mg/kg versus ipilimumab and docetaxel did not demonstrate radiologic responses. Two patients in the monotherapy group and one in the combination group had PSA responses [33]. Another phase II study randomized 108 patients with advanced prostate cancer to ipilimumab with androgen ablation versus androgen ablation alone. At 3 months, 55 % of ipilimumab patients had undetectable PSA versus 38 % in the ablation-only group [34].
-
Two phase III trials are ongoing. The first study evaluated the impact of ipilimumab and radiation versus radiation alone in the postdocetaxel setting on OS (NCT00861614). The study's primary endpoint of OS did not reach statistical significance with median OS at 11.2 months with ipilimumab and 10 months with the placebo (hazard ratio [HR] = 0.85; 95 % confidence interval [CI] = 0.72-1.00; p = 0.053). Median progression-free survival favored ipilimumab over placebo (HR = 0.7; 95 % CI = 0.61-0.82) as did prostate-specific antigen (PSA) response rates, as evidenced by declines of ≥50 % in evaluable patients (13.1 % vs. 5.3 %, respectively) [35]. The results of the second study that evaluates ipilimumab versus placebo in metastatic CRPC patients who have not received chemotherapy are pending (NCT01057810).
-
Patient-specific diversity in antibody responses following CTLA-4 blockade seen in one phase I study suggests that the therapy elicits immunity largely against unique, rather than shared, antigens [36•].
-
A phase I trial combining PROSTVAC-VF with ipilimumab did not demonstrate increased immune-related adverse events compared with ipilimumab monotherapy. Fourteen (58 %) of 24 chemotherapy-naïve patients had PSA declines from baseline with treatment [37].
-
A phase I study combining ipilimumab and sipuleucel-T (NCT01832870) is currently enrolling. Primary outcome measures include antigen-specific T-cell responses and proliferation, as well as antibody responses.
Tremelimumab
-
Tremelimumab is a fully human IgG2 monoclonal antibody specific for CTLA-4, which also is undergoing clinical investigation. A phase I trial in PSA-recurrent patients demonstrated dose-limiting toxicities, including diarrhea and skin rash, and prolongation in PSA doubling time was observed in 3 of 11 patients [38].
Anti-PD-1 and anti-PD1L antibodies
-
Programmed death 1 (PD-1) represents another important immune checkpoint. The inhibitory cell-surface molecule expressed on T cells, which binds with PD-1 ligands (including PD-L1 and PD-L2) found on tumor cells and stromal cells in the tumor microenvironment. Anti-PD1 antibodies, including lambrolizumab [39] and nivolumab [40], have recently shown substantial clinical activity in phase I trials for advanced melanoma. Current data suggest that PD-1 and CTLA-4 represent nonredundant, complementary pathways in immune coinhibition. Initial experience also suggests that PD-1 inhibition is associated with a lower incidence of immune-related adverse events than ipilimumab, perhaps corresponding to its more targeted mechanism of action to tumor-specific T cells. Anti-PD-L1 blockade also has been evaluated in several cancers [41].
-
Substantial expression of PD-1 has been identified in prostate infiltrating CD8+ T cells in men with prostate cancer [42]. An earlier phase I study of nivolumab in multiple cancer types enrolled 17 patients with CRPC; however, no objective responses were seen in this group. Also, no objective responses were seen in patients without tumor PD-L1 expression. However, biopsies in this study were optional and obtained in a nonrandom minority of patients, including only two prostate cancer patients [43]. Pidilizumab, another PD-1 antibody, is being evaluated in a phase II trial for the treatment of CRPC in combination with sipuleucel-T and cyclophosphamide (NCT01420965).
Interventional procedures
Radiation
-
Radiotherapy (RT) also may play an important component of prostate cancer immunotherapy. Recent evidence suggests that while the immune effects of radiation are complex, the therapy has immunostimulatory properties induced by production of cytokines, chemokines, and other signals in the irradiated tumor immune microenvironment, as well by increased capture of tumor antigens. Identification of the optimal dose and fractionation regimens requires further investigation [44].
-
The abscopal effect, a phenomenon in which distant metastatic lesions regress following radiation to an unrelated primary treatment field, has been described in case reports for several cancers [45]. The etiology of this rare scenario is unclear; however, evidence suggests that the effect is tumor-specific and T-cell mediated [46].
-
Antitumor immunity may be augmented in a time-dependent manner following radiotherapy. A study of TRAMP (Transgenic Adenocarcinoma of the Mouse Prostate) mice demonstrated optimal mitigation of tolerance with a tumor vaccine at 3–5 weeks following radiotherapy, when tumor burden is at its lowest. Investigation of immunotherapy timing in the clinical trial setting is planned [47].
Diet and lifestyle
-
Adherence to World Cancer Research Fund (WCRF) lifestyle recommendations recently has been shown to be associated negatively with risk of highly aggressive tumors in newly diagnosed prostate cancer patients. However, to our knowledge, there is not definitive evidence for diet and lifestyle changes in the management of metastatic CRPC [48].
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2013;63:11–30.
Mohler J, Armstrong A, Bahnson R, et al. NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer, V.4.2013. Available at http://www.nccn.org. Accessed September 2013. These guidelines represent national consensus standard of care for the management of prostate cancer.
Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48.
ZYTIGA Prescribing Information. Available at http://www.zytigahcp.com/prescribing-information. Accessed September 2013.
Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97.
JEVTANA Prescribing Information. Available at http://products.sanofi.us/jevtana/jevtana.html. Accessed September 2013.
XOFIGO Prescribing Information. Available at http://labeling.bayerhealthcare.com/html/products/pi/Xofigo_PI.pdf. Accessed September 2013.
Burch PA, Breen JK, Buckner JC, et al. Priming tissue-specific cellular immunity in a phase I trial of autologous dendritic cells for prostate cancer. Clin Cancer Res. 2000;6:2175–82.
Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–94.
Higano CS, Schellhammer PF, Small EJ, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–9.
Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. The Phase III IMPACT trial was the pivotal study leading to the approval of sipuleucel-T.
Sheikh NA, Petrylak D, Kantoff PW, et al. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother. 2013;62:137–47.
Schellhammer PF, Chodak G, Whitmore JB, et al. Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology. 2013;81:1297–302.
Hoos A, Eggermont AMM, Janetzki S, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102:1388–97.
Beer TM, Bernstein GT, Corman JM, et al. Randomized trial of autologous cellular immunotherapy with sipuleucel-T in androgen-dependent prostate cancer. Clin Cancer Res. 2011;17:4558–67.
Fong L, Weinberg V, Chan SE, et al. Neoadjuvant sipuleucel-T in localized prostate cancer: Effects on immune cells within the prostate tumor microenvironment [abstract 2564]. 2012 ASCO Annual Meeting Abstracts. Available at http://meetinglibrary.asco.org. Accessed September 10, 2013.Evaluation of sipuleucel-T in the neoadjuvant setting suggests that the agent can modulate lymphocytes in the prostate tumor microenvironment.
Small E, Lance R, Gardner TA, et al. A Phase 2 trial of sipuleucel-T in combination with concurrent or sequential abiraterone acetate (AA) in patients (pts) with metastatic castrate-resistant prostate cancer (mCRPC) [abstract 2860]. Presented at European Cancer Congress 2013. Amsterdam, The Netherlands; September 27-October 1, 2013. This Phase II study did not demonstrate significant alterations in immune parameters with sipuleucel-T when coadministered with abiraterone.
Gulley JL, Arlen PM, Madan RA, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother. 2010;59:663–74.
Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–105. This is the largest phase II study to date of PROSTVAC-VF.
Noguchi M, Moriya F, Suekane S, et al. Phase II study of personalized peptide vaccination for castration-resistant prostate cancer patients who failed in docetaxel-based chemotherapy. Prostate. 2012;72:834–45.
Noguchi M, Kakuma T, Uemura H, et al. A randomized phase II trial of personalized peptide vaccine plus low dose estramustine phosphate (EMP) versus standard dose EMP in patients with castration resistant prostate cancer. Cancer Immunol Immunother. 2010;59:1001–9.
McNeel DG, Dunphy EJ, Davies JG, et al. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer. J Clin Oncol. 2009;27:4047–54.
Becker JT, Olson BM, Johnson LE, et al. DNA vaccine encoding prostatic acid phosphatase (PAP) elicits long-term T-cell responses in patients with recurrent prostate cancer. J Immunother. 2010;33:639–47.
Small EJ, Reese DM, Um B, et al. Therapy of advanced prostate cancer with granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 1999;5:1738–44.
Small EJ, Demkow T, Gerritsen W: A phase III trial of GVAX immunotherapy for prostate cancer in combination with docetaxel versus docetaxel plus prednisone in symptomatic, castration-resistant prostate cancer (CRPC) [abstract 7]. Presented at the Genitounirary Cancers Symposium. Orlando, Florida; February 26–28, 2009.
Higano C, Saad F, Somer B: A phase III trial of GVAX immunotherapy for prostate cancer vs. docetaxel plus prednisone in asymptomatic, castration-resistant prostate cancer (CRPC) [abstract LBA150]. Presented at the Genitourinary Cancers Symposium. Orlando, Florida; February 26–28, 2009.
Dreicer R, Stadler WM, Ahmann FR, et al. MVA-MUC1-IL2 vaccine immunotherapy (TG4010) improves PSA doubling time in patients with prostate cancer with biochemical failure. Invest New Drug. 2009;27:379–86.
Van der Merwe PA, Bodian DL, Daenke S, et al. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J Exp Med. 1997;185:393–403.
Wing K, Yamaguchi T, Sakaguchi S. Cell-autonomous and -non-autonomous roles of CTLA-4 in immune regulation. Trends Immunol. 2011;32:428–33.
Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. This pivotal Phase III trial led to the approval of ipilimumab, a CTLA-4 antibody, in metastatic or unresectable melanoma.
Prieto PA, Yang JC, Sherry RM, et al. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18:2039–47.
Slovin SF, Higano CS, Hamid O, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24:1813–21. This Phase I/II study evaluated ipilimumab with or without radiotherapy in the management of CRPC.
Small E, Higano C, Tchekmedyian N, et al. Randomized phase II study comparing 4 monthly doses of ipilimumab (MDX-010) as a single agent or in combination with a single dose of docetaxel in patients with hormone-refractory prostate cancer. J Clin Oncol (Meeting Abstracts) 2006, 24:suppl 4609.
Tollefson M, Karnes R, Thompson R, et al.. A randomized phase II study of ipilimumab with androgen ablation compared with androgen ablation alone in patients with advanced prostate cancer [abstract 168]. Presented at the Genitounirary Cancers Symposium. San Francisco, California; March 5–7, 2010.
Gerritsen WR, Kwon ED, Fizazi K, et al.. A randomized, multicenter, double-blind phase 3 trial comparing OS in patients with post-docetaxel castration-resistant prostate cancer and bone metastases treated with ipilimumab vs placebo, each following single-dose radiotherapy [abstract 2850]. Presented at European Cancer Congress 2013. Amsterdam, The Netherlands; September 27-October 1, 2013.
Kwek SS, Dao V, Roy R, et al. Diversity of antigen-specific responses induced in vivo with CTLA-4 blockade in prostate cancer patients. J Immunol. 2012;189:3759–66. This Phase I study suggests that CTLA-4 blockade leads to patient-specific immune responses to largely unique antigens.
Madan RA, Mohebtash M, Arlen PM, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:501–8.
McNeel DG, Smith HA, Eickhoff JC, et al. Phase I trial of tremelimumab in combination with short-term androgen deprivation in patients with PSA-recurrent prostate cancer. Cancer Immunol Immunother. 2012;61:1137–47.
Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44.
Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33.
Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65.
Sfanos KS, Bruno TC, Meeker AK, et al. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+. Prostate. 2009;69:1694–703.
Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54.
Dewhirst MW, Burnette B, Weichselbaum RR. Radiation as an Immune Modulator. Semin Radiat Oncol. 2013;23:273–80.
Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–31.
Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–70.
Harris TJ, Hipkiss EL, Borzillary S, et al. Radiotherapy augments the immune response to prostate cancer in a time-dependent manner. Prostate. 2008;68:1319–29.
Arab L, Su J, Steck SE, et al. Adherence to World Cancer Research Fund/American Institute for Cancer Research lifestyle recommendations reduces prostate cancer aggressiveness among African and Caucasian Americans. Nutr Cancer. 2013;65:633–43.
Conflict of Interest
Michael L. Cheng declares that he has no conflict of interest.
Lawrence Fong receives research support from Dendreon Corporation.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheng, M.L., Fong, L. Beyond Sipuleucel-T: Immune Approaches to Treating Prostate Cancer. Curr. Treat. Options in Oncol. 15, 115–126 (2014). https://doi.org/10.1007/s11864-013-0267-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-013-0267-z