Abstract
Background
The incidence of thyroid cancer (TC) is increasing rapidly worldwide. The target therapy for papillary TC (PTC) is limited, and the studies of PTC prognostic biomarkers are not common. As a new member of annexin A (ANXA) family, the function and clinical significance of ANXA10 in PTC have not been well investigated.
Methods
Expressions of all the 12 ANXA members were detected with qPCR in 12 PTC tissues, and the ANXA10 mRNAs in PTCs and their adjacent normal thyroid tissues were compared. The subcellular location and expression of ANXA10 in 121 PTC patients were investigated with immunohistochemistry, which further classified the patients into subgroups with low or high ANXA10. The clinical significance and prognostic value of ANXA10 were estimated by analyzing its correlation with clinical factors and overall survival rates by the chi-squared test, univariate analyses, and multivariate analyses.
Results
ANXA10 had the highest expression in PTCs among all the ANXA members. Moreover, ANXA10 was significantly upregulated in PTC compared with normal thyroid tissues. The PTC patients with low and high expression of ANXA10 took up 70.25% (85/121) and 29.75% (36/121), respectively. ANXA10 expression was associated with tumor size, differentiation, and overall survival rates of PTC. ANXA10 was an independent prognostic biomarker predicting the poor outcome of PTC.
Conclusions
ANXA10 expression was upregulated in PTC, and it was an independent prognostic biomarker of PTC, suggesting that ANXA10 may be a promising target for individual treatment of ANXA10.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of thyroid cancer (TC) is increasing rapidly in the past decades, and becomes the fourth common cancer type worldwide [1]. TC was histologically categorized into differentiated TC (DTC) and the poorly differentiated TC. Papillary TC (PTC) is the main histological type of DTC, accounting for more than 85% of all TC types [2]. The overall 5-year survival for PTC patients in early stage is more than 90% [3]. Although the patients with PTC usually have a good outcome, a portion of PTC patients suffer with disease progression. Approximately 10% of PTC patients have distant metastases at presentation or during follow-up. In those cases, systemic treatment, especially targeted therapy, would be applied. However, the targeted drugs are limited for advanced progressive PTC, and more efforts should be made to explore more biomarkers and potential target therapy.
Annexin family is the largest category of eukaryotic calcium and phospholipid-binding proteins, consisted of more than 160 members which are categorized into 65 different species [4]. The Ca2+-dependent binding to phospholipid-containing membranes is the feature of annexin family. Annexins play essential roles in various cellular and physiological processes, including vesicle trafficking and organization, exocytosis, endocytosis, and calcium ion channel formation [5]. Annexin A (ANXA) is a subgroup of annexin family and comprised of 12 members according to the official nomenclature proposed in 1999 [6]. The functions and oncogenic roles of ANXA members were hinted in several types of cancers, especially ANXA1 and ANXA2 [7, 8]. However, the oncogenic function of ANXA in tumor progression is still controversial, and the expressions of ANXAs in normal tissues and cancers are context- and time-specific; therefore, different ANXA may play different role in different cancer types. The expressions of ANXAs in PTC have not been well studied, so we detected the expression and clinical significance of ANXA family of PTC in this study.
In our study, we screened the expressions of all the 12 ANXA members in 12 PTC tissues using quantitative real-time PCR (qPCR), and ANXA10 expressions in PTCs and tumor-adjacent tissues were compared. The ANXA10 expressions were investigated in 121 cases of PTCs with immunhistochemistry (IHC). The clinical significance of ANXA10 was evaluated by analyzing its correlation with clinical factors, and the prognostic value of ANXA10 was estimated by univariate analyses and multivariate analyses.
Materials and methods
Patients and tissue samples
In our study, the inception cohort was consisted of 355 patients diagnosed as PTC and underwent the radical surgery in the Central Hospital affiliated to Shandong First Medical University and the YIDU Central Hospital from 2005 to 2018. The inception cohort was constituted of 224 female patients and 131 male patients, with an average age of 45.2 years old. The validation cohort comprised of 121 patients (75 female patients and 46 male patients) was further selected out from the inception cohort if they had complete medical record and follow-ups. Another 12 pairs of PTC tissues and tumor-adjacent thyroid tissues were collected during operation for RNA extraction without interfering the pathological diagnosis. All patients were followed up for at least 10 months, and the overall survival (OS) time was defined as the time between operation and the patient’s death or last follow-up. The pathological stage of PTC was classified using seventh TNM staging system of AJCC/UICC. All the specimens were obtained with the written consent of patients. The study was approved by the Institutional Ethics Committee of the Central Hospital affiliated to Shandong First Medical University and the YIDU Central Hospital.
Immunohistochemistry
Expression of ANXA10 in PTC was visualized by IHC using the streptavidin-peroxidase complex method according to our previous studies [9]. Briefly, the tissues were rehydrated with xylene and graded alcohol, and then immersed in 3% H2O2 for inactivating the endogenous peroxidase. After that, the specimens were incubated in boiled citrate buffer (pH = 6.0) for 10 min for antigen retrieval and in 5% bovine serum albumin for 1 h to reduce the unspecific antigen binding. The primary antibody of ANXA10 was used to incubate the tissues at 4 °C overnight, and the corresponding secondary antibody (Sangon, Shanghai, China) was used to incubate slides at room temperature for 1 h. Specimens were then treated by the streptavidin-peroxidase complex reagent and DAB solution (Sangon), and finally administrated with hematoxylin for counterstain.
IHC result evaluation
The results of IHC were estimated by two senior pathologists and cases without consensus would be re-evaluated by another pathologist. The results were semi-quantified by the IHC score referring to the previous study [10]. In brief, the tumor area was first selected by pathologist, and the IHC staining was quantified by Quant center software. Staining intensity was mechanically defined as weak, moderate, and strong staining by the software, and the final IHC score was calculated as follows: IHC score = (percentage of cells of weak intensity × 1) + (percentage of cells of moderate intensity × 2) + (percentage of cells of strong intensity × 3) in the Quant Center software [11, 12]. The validation cohort was classified into low and high ANXA10 by the cutoff calculated by receiver operating characteristic (ROC) curves [13]. The cutoff for ANXA10 was 65.0 in our study.
RNA extraction and qPCR
Total RNAs of the PTC and adjacent tissues were extracted with TRIzol reagent (Invitrogen, Foster City, CA, USA) according to the manual. Reverse transcription and quantification of mRNA were realized by one-step real-time RT-PCR method with SYBR-Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) according to our previous study [9]. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal control. qPCR primers were as follows:
ANXA1 | CTAAGCGAAACAATGCACAGC | CCTCCTCAAGGTGACCTGTAA |
|---|---|---|
ANXA2 | TCTACTGTTCACGAAATCCTGTG | AGTATAGGCTTTGACAGACCCAT |
ANXA3 | TTAGCCCATCAGTGGATGCTG | CTGTGCATTTGACCTCTCAGT |
ANXA4 | GGAGGTACTGTCAAAGCTGCT | GGCAAGGACGCTAATAATGGC |
ANXA5 | GTTTGGCAGGGATCTTCTGGA | TCATAAAGCCGAGAGGGTTTCA |
ANXA6 | AGAGCTACAAGTCCCTCTACG | CCCACAATCAACCGTTCAAAC |
ANXA7 | CCTAGTGGCTTTCCTCCAATG | ACCAGGATAGCCTCCAGGG |
ANXA8 | TCACTTGTGCTGTAACCCTGC | GTTCTTGGTCCGAGAGGCCA |
ANXA9 | CAGCTCATCTCACGAAACTTCC | GGTTCGAGTGGCAAGAATTTCAA |
ANXA10 | TTGTGGAGACTATGTGCAAGGA | GGTATGCCTCTGCAATCATCAT |
ANXA11 | GCCACCAGTGACCTACCCT | CCGAAGCCTTTCATGGCCT |
ANXA13 | GCTAAAGCGAGCAGTCCTCAG | GTCCTGCCCGATAAGATTTCAA |
GAPDH | GAGTCAACGGATTTGGTCGT | GACAAGCTTCCCGTTCTCAG |
Statistical analysis
Software SPSS22.0 (IBM Corporation, USA) was used to analyze all the data and calculate the statistical significance. The correlations between ANXA10 expression and other clinical factors were analyzed with the chi-squared method, and the correlations between ANXA10 and OS curves were evaluated with the Kaplan-Meier method, and the statistical significance was calculated by the log-rank test. The independent prognostic risks were determined by the Cox proportional hazards. P value less than 0.05 was regarded as statistically significant.
Results
Expression of ANXA family in PTC
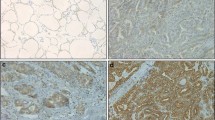
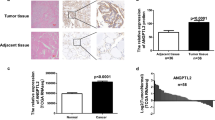
The mRNA levels of all the 12 ANXA proteins were detected in 12 cases of fresh PTC tumors. The mRNA of ANXA1 in PTC was set as the base, and mRNA of other members was calculated as the ratio to ANXA1. As the result, expression of different ANXAs varied substantially in PTCs, and interestingly, ANXA10 had the highest expression in PTC among all the ANXA proteins (Fig. 1a). Furthermore, we compared the mRNA of ANXA10 in PTCs and tumor-adjacent normal thyroid tissues and demonstrated that ANXA10 was significantly overexpressed in PTCs (Fig. 1b). This result suggested a potential role of ANXA10 in tumorigenesis of PTC. Moreover, the expression of AXNA10 was further investigated in a retrospective cohort as large as 121 PTCs via IHC. In our study, ANXA10 was mainly expressed in the cell membrane. These 121 patients were divided into subgroups with low- and high-ANXA10 expression by the cutoff of IHC score (Fig. 1c). The PTC patients with low and high expression of ANXA10 took up 70.25% (85/121) and 29.75% (36/121), respectively (Table 1).
Expression of ANXA family in PTC. a The mRNAs of all ANXAs in 12 fresh PTC tissues were detected with qPCR. All data were standardized with ANXA1 mRNA as baseline. ANXA10 had the highest expression among these members. b The mRNAs of ANXA10 in 12 pairs of PTCs and normal thyroid tissues were detected with qPCR. ANXA10 mRNA in PTCs was significantly higher than that in normal thyroid tissues. P < 0.001 with paired t test. c ANXA10 expression in 121 PTCs were detected with IHC, which divided PTCs into groups with low and high ANXA10 expression. The IHC scores of these two cases were 14.0 and 133.5, respectively
ANXA10 is associated with tumor size and differentiation of PTC
Since ANXA10 was predominantly expressed in PTC among all ANXA proteins, we estimated the clinical significance of ANXA10 by analyzing the correlation between ANXA10 and the clinical factors including TNM stage, tumor number, size, site, and differentiation, as well as the gender and age of the patients (Table 2). In our study, the expression of ANXA10 was notably relevant to the tumor size and differentiation, indicating that ANXA10 may play an important role in tumor differentiation and proliferation.
ANXA10 is a prognostic biomarker of PTC
To better depict the clinical significance of ANXA10 in PTC, we accessed the relevance between ANXA10, the clinical variables, and the OS rate of PTC. High expression of ANXA10 was substantially correlated with low OS rate of PTC (P = 0.014) (Fig. 2a). The 5-year OS rate of low and high ANXA10 was 96.2% and 86.0%, respectively (Table 3). In addition, advanced N stage (P = 0.005) and TNM stage (P = 0.041) also indicated the unfavorable prognosis of PTC, with the 5-year OS rates as 97.7% vs. 84.8% and 94.5% vs. 88.0%, respectively (Fig. 2b, c). Moreover, tumor differentiation and T stage also predicted the poor outcome, but the statistical significance was not potent enough (P = 0.094 and 0.134, respectively).
ANXA10 has independent prognostic significance of PTC
All the potential prognostic variables were further enrolled into the Cox regression hazard model for multivariate analyses (Table 3). Variables with a P value less than 0.20 were selected into the multivariate analysis, including ANXA10 expression, T stage, N stage, and tumor differentiation. TNM stage was naturally excluded from the multivariate analysis because of its interaction with other factors. In these factors, ANXA10 was confirmed as an independent prognostic biomarker of PTC (P = 0.007). High ANXA10 expression was capable to indicate the poor outcome of PTC independently (hazard ratio = 9.19, 95% confidential incidence = 1.84–45.86). Additionally, advanced N stage was also confirmed as the independent prognostic variable of PTC (P = 0.047).
Discussion
Annexins participate in many physiological and pathological processes such as providing a membrane scaffold for changes in the cell shape and migration [5]. As one of the most well-studied ANXA proteins, the functions of ANXA1 were reported to promote tumor progression such as tumor growth, migration, invasion, drug resistance in breast cancer, gastric cancer, and so on, via multiple molecular mechanisms such as regulating transforming growth factor-β (TGF-β) signaling pathway, activating formyl peptide receptor/extracellular signal-regulated kinase/integrin β-1-binding protein 1 pathway, or promoting alternative macrophage polarization [14,15,16]. As a new member of ANXA, the relevance of ANXA10 and cancer was rarely reported. Ectopic expression of ANXA10 was reported in gastric cancer, hepatocellular carcinoma, cholangiocarcinoma, and so on [17,18,19,20]. A recent study demonstrated that ANXA10 was capable to promote invasion by activating Stat3 and facilitating epithelial-mesenchymal interaction (EMT) in cholangiocarcinoma [21]. Here in our study, we observed that ANXA10 expression was mainly relevant with tumor size and differentiation, prompting that ANXA10 was also involved in the tumor progression of PTC, which is the reason of poor prognosis.
With radical surgery and radiation therapy, patients with localized and differentiated PTC usually have good OS rates. However, lymphatic metastasis of PTC is common, and more than half of patients with anaplastic carcinoma present with metastasis [22]. In the era of genomics, emerging gene mutations and biomarkers are discovered, such as BRAF, RAS, eukaryotic translation initiation factor 1A (EIF1AX), and RET, but few of them hasten the drug development till now [23]. Moreover, most studies focused on the undifferentiated tumors because of its poor outcome, and very few investigated the prognostic biomarker of well-differentiated PTC. Here, we showed the prognostic value of ANXA10 after a long time follow-up more than 150 months. This result was a great supplement to the biomarker study of well-differentiated PTC and may help elucidate the molecular mechanism of PTC progression.
Several serologic and histologic markers for thyroid cancer have been examined over the last decades, but the efforts are not enough for the precise treatment of thyroid cancer. The targeted drugs of TC are very limited when patients suffered from recurrence or metastasis. The understanding of the pathogenesis and genetics and the identification of new biomarkers can facilitate the development of new drugs. The approved drugs in the USA and in the European Union are mainly sorafenib and lenvatinib [24], and new attempts are still needed to develop the precise treatment of PTC. Here, we identified ANXA10 as a prognostic biomarker by screening all the ANXA members, and confirmed its independent prognostic value, hinting that therapies targeting ANXA10 may be a promising approach to treat PTC. Unfortunately, the exact molecular function of ANXA10 in PTC is still unknown, and there is no available small molecular inhibitor of ANXA10 till now. As the understanding of ANXA10 deepens, the role of ANXA10 as a potential drug target will appear.
In conclusion, we screened the expressions of all the 12 ANXA members in PTCs for the first time and proved that ANXA10 was upregulated in PTC. Furthermore, we demonstrated that ANXA10 was a prognostic biomarker of PTC and indicated poor prognosis independently. Our results suggested that ANXA10 may be a promising target for individual treatment of ANXA10 and that ANXA10 high expression was a characterization of high risk after radical surgery.
References
Singh Ospina N, Iniguez-Ariza NM, Castro MR (2020) Thyroid nodules: diagnostic evaluation based on thyroid cancer risk assessment. BMJ 368:l6670. https://doi.org/10.1136/bmj.l6670
Kitahara CM, Sosa JA (2016) The changing incidence of thyroid cancer. Nat Rev Endocrinol 12:646–653. https://doi.org/10.1038/nrendo.2016.110
Vuong HG, Odate T, Duong UNP, Mochizuki K, Nakazawa T, Katoh R, Kondo T (2018) Prognostic importance of solid variant papillary thyroid carcinoma: a systematic review and meta-analysis. Head Neck 40:1588–1597. https://doi.org/10.1002/hed.25123
Gerke V, Moss SE (2002) Annexins: from structure to function. Physiol Rev 82:331–371. https://doi.org/10.1152/physrev.00030.2001
Gerke V, Creutz CE, Moss SE (2005) Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol 6:449–461. https://doi.org/10.1038/nrm1661
Schloer S, Pajonczyk D, Rescher U (2018) Annexins in translational research: hidden treasures to be found. Int J Mol Sci 19. https://doi.org/10.3390/ijms19061781
Sharma M, Annexin C (2019) A2 (ANX A2): an emerging biomarker and potential therapeutic target for aggressive cancers. Int J Cancer 144:2074–2081. https://doi.org/10.1002/ijc.31817
Foo SL, Yap G, Cui J, Lim LHK (2019) Annexin-A1—a blessing or a curse in cancer? Trends Mol Med 25:315–327. https://doi.org/10.1016/j.molmed.2019.02.004
Liu XJ, Liu WL, Yang FM, Yang XQ, Lu XF (2015) Hepatoma-derived growth factor predicts unfavorable prognosis of epithelial ovarian cancer. Onco Targets Ther 8:2101–2109. https://doi.org/10.2147/OTT.S85660
Liu Z, Sun R, Zhang X, Qiu B, Chen T, Li Z, Xu Y, Zhang Z (2019) Transcription factor 7 promotes the progression of perihilar cholangiocarcinoma by inducing the transcription of c-Myc and FOS-like antigen 1. EBioMedicine 45:181–191. https://doi.org/10.1016/j.ebiom.2019.06.023
Azim HA Jr, Peccatori FA, Brohée S, Branstetter D, Loi S, Viale G, Piccart M, Dougall WC, Pruneri G, Sotiriou C (2015) RANK-ligand (RANKL) expression in young breast cancer patients and during pregnancy. Breast Cancer Res 17:24. https://doi.org/10.1186/s13058-015-0538-7
Yeo W, Chan SL, Mo FKF, Chu CM, Hui JWY, Tong JHM, Chan AWH, Koh J, Hui EP, Loong H, Lee K, Li L, Ma B, To KF, Yu SCH (2015) Phase I/II study of temsirolimus for patients with unresectable hepatocellular carcinoma (HCC)—a correlative study to explore potential biomarkers for response. BMC Cancer 15:395. https://doi.org/10.1186/s12885-015-1334-6
Xu YF, Liu ZL, Pan C, Yang XQ, Ning SL, Liu HD, Guo S, Yu JM, Zhang ZL (2019) HMGB1 correlates with angiogenesis and poor prognosis of perihilar cholangiocarcinoma via elevating VEGFR2 of vessel endothelium. Oncogene 38:868–880. https://doi.org/10.1038/s41388-018-0485-8
Raulf N, Lucarelli P, Thavaraj S, Brown S, Vicencio JM, Sauter T, Tavassoli M (2018) Annexin A1 regulates EGFR activity and alters EGFR-containing tumour-derived exosomes in head and neck cancers. Eur J Cancer 102:52–68. https://doi.org/10.1016/j.ejca.2018.07.123
de Graauw M, van Miltenburg MH, Schmidt MK, Pont C, Lalai R, Kartopawiro J, Pardali E, le Devedec SE, Smit VT, van der Wal A, van't Veer LJ, Cleton-Jansen AM, ten Dijke P, van de Water B (2010) Annexin A1 regulates TGF-beta signaling and promotes metastasis formation of basal-like breast cancer cells. Proc Natl Acad Sci U S A 107:6340–6345. https://doi.org/10.1073/pnas.0913360107
Cheng TY, Wu MS, Lin JT, Lin MT, Shun CT, Huang HY, Hua KT, Kuo ML (2012) Annexin A1 is associated with gastric cancer survival and promotes gastric cancer cell invasiveness through the formyl peptide receptor/extracellular signal-regulated kinase/integrin beta-1-binding protein 1 pathway. Cancer 118:5757–5767. https://doi.org/10.1002/cncr.27565
van der Heijden AG, Mengual L, Lozano JJ, Ingelmo-Torres M, Ribal MJ, Fernández PL, Oosterwijk E, Schalken JA, Alcaraz A, Witjes JA (2016) A five-gene expression signature to predict progression in T1G3 bladder cancer. Eur J Cancer 64:127–136. https://doi.org/10.1016/j.ejca.2016.06.003
Zhu J, Wu J, Pei X, Tan Z, Shi J, Lubman DM (2017) Annexin A10 is a candidate marker associated with the progression of pancreatic precursor lesions to adenocarcinoma. PLoS One 12:e0175039. https://doi.org/10.1371/journal.pone.0175039
Lu SH, Yuan RH, Chen YL, Hsu HC, Jeng YM (2013) Annexin A10 is an immunohistochemical marker for adenocarcinoma of the upper gastrointestinal tract and pancreatobiliary system. Histopathology 63:640–648. https://doi.org/10.1111/his.12229
Macaron C, Lopez R, Pai RK, Burke CA (2016) Expression of annexin A10 in serrated polyps predicts the development of metachronous serrated polyps. Clin Transl Gastroenterol 7:e205. https://doi.org/10.1038/ctg.2016.60
Sun R, Liu Z, Qiu B, Chen T, Li Z, Zhang X, Xu Y, Zhang Z (2019) Annexin10 promotes extrahepatic cholangiocarcinoma metastasis by facilitating EMT via PLA2G4A/PGE2/STAT3 pathway. EBioMedicine 47:142–155. https://doi.org/10.1016/j.ebiom.2019.08.062
Perrier ND, Brierley JD, Tuttle RM (2018) Differentiated and anaplastic thyroid carcinoma: major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 68:55–63. https://doi.org/10.3322/caac.21439
Giordano T, Genomic J (2018) Hallmarks of thyroid neoplasia. Annu Rev Pathol 13:141–162. https://doi.org/10.1146/annurev-pathol-121808-102139
Bible KC, Ryder M (2016) Evolving molecularly targeted therapies for advanced-stage thyroid cancers. Nat Rev Clin Oncol 13:403–416. https://doi.org/10.1038/nrclinonc.2016.19
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, X., Yang, M., Guo, Y. et al. Annexin A10 is a novel prognostic biomarker of papillary thyroid cancer. Ir J Med Sci 190, 59–65 (2021). https://doi.org/10.1007/s11845-020-02263-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-020-02263-x