Abstract
Objective
The aim of this study was to measure any incremental costs or savings within the health system associated with the introduction of the new technology, prucalopride, for the management of chronic constipation.
Methodology
The study design was based on a budget impact analysis conducted by the National Institute of Clinical Excellence (NICE). To validate the findings of the NICE costing template, a case series audit capturing real world data was used to determine the financial impact of adopting prucalopride in 40 women suffering with chronic constipation. This facilitated the application of local unit costs to the resources used and determined whether the use of prucalopride, as an alternative treatment to laxatives, resulted in a reduction in the use of secondary care resources.
Results
Patients were treated with an average of 2.6 laxatives in the baseline (laxatives only) scenario. The total medication costs in the baseline (laxatives only) and the new treatment (prucalopride) scenario amounted to €17,440.84 and €18,417.62, respectively. There was a significant reduction in the number of investigations and procedures in the 12 months after commencing prucalopride, with cost savings of €41,923.28 (€1,048.08 per patient per year) demonstrated. Input cost variables were adjusted as part of sensitivity analysis.
Conclusion
This study validated the findings of the NICE costing template and suggests that the use of prucalopride for the treatment of chronic constipation in women refractory to laxatives has the potential to reduce secondary care resource use and hence led to cost savings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Constipation is a multi-symptom disorder that may be a consequence of a broad range of diseases, ranging from thyroid disease and neurological disorders to such common medications as anti-cholinergic agents. In many instances, no cause is found; primary or idiopathic constipation is also referred to as functional constipation or, more commonly and simply, as chronic constipation. While constipation was traditionally viewed as a single symptom, namely, infrequent defaecation, more recent, detailed analyses of sufferers’ symptoms have made it clear that chronic constipation may include an array of symptoms including straining, incomplete evacuation, bloating and distension [1].
In an attempt to encompass these symptoms, the Rome criteria (ROME III) now define chronic constipation as complaints lasting 12 weeks or more, with onset at least 6 months prior to diagnosis (CC) [1, 2] and featuring two or more of the following symptoms: less than 3 spontaneous complete bowel movements per week, lumpy or hard stools with ≥25 % of bowel movements, sensation of incomplete evacuation with ≥25 % of bowel movements, and straining during 25 % of bowel movements.
The prevalence of CC is estimated to be 10–15 % in developed countries, with some studies, such those from the United States, reporting rates of up to 28 % [3, 4]. In addition, epidemiological surveys report that constipation can be persistent and frequently associated with other gastrointestinal symptoms, in particular upper gastrointestinal symptoms, as well as bloating and cramping.
Health-related quality of life (HR-QoL) is negatively impacted by CC and QoL is consistently lower in the constipated than non-constipated individual [5]. Indeed, the level of impairment in QoL has been shown to be similar to that experienced by patients with diabetes, hypertension, heart disease or depression [5, 6]. In addition, HR-QoL is further impaired as the severity of constipation-related symptoms increases. Social functioning is also negatively impacted by CC, with an associated increase in work absenteeism and presenteeism (underperformance by those at work), resulting in greater indirect costs and economic burden, not only to the patient, but also to healthcare providers [5, 7]. Treatments for constipation have been shown to improve HR-QoL [5].
As well as lifestyle adjustments (which are poorly supported by high quality evidence), laxatives have been a main stay of treatment of CC for many years. However, there is insufficient evidence from randomised controlled trials to confirm the long-term effectiveness of laxatives, and their side effect profile in patients with severe CC has not been well described [8–10]. Many patients’ symptoms are not completely resolved even with a combination of over-the-counter and prescription medications, mainly consisting of laxatives [4, 8, 9], and constipation sufferers complain of poor palatability, lack of predictability of effect and time to onset of effect with various laxatives.
There has, for some time, therefore, been considerable interest in pharmacological approaches to CC and two broad categories of drugs have emerged: promotility agents and drugs that promote intestinal secretion and lubricate the stool. Among these, promotility agents have been more time honoured and date back to the use of cholinergic agonists, such as neostigmine, whose use was severely limited by systemic side effects. Serotonergic agonists, such as cisapride and tegaserod, held promise of greater gut specificity but both had to be withdrawn because of cardiac side effects.
Prucalopride is the first selective and specific high-affinity serotonin (5-HT4) receptor agonist [11]. It is a dihydrobenzofurancarboxamide derivative, structurally different from other prokinetics and highly selective exhibiting an at least 290-fold greater affinity for the 5-HT4 receptor than other serotonergic agonists. Prucalopride induces high-amplitude propagating contractions (HAPCs) in the colon, motor events that are known to be distinctly propulsive, and also increases segmenting contractions. The result is an acceleration of proximal colonic emptying as well as in overall colonic transit of faecal matter [11].
The efficacy of prucalopride was established in three multicentre, randomised, double-blind, 12-week duration, placebo-controlled studies among patients with long-standing chronic constipation. Across the three pivotal trials, an average of 23.6 % of patients achieved an increase of at least 3 spontaneous complete bowel movements (SCBM) per week with a daily dose of 2 mg prucalopride compared to 11.3 % for placebo [12–14]. All patients had a long-standing history of chronic constipation and 80 % deemed their previous treatment with laxatives to be inadequate. Indeed, a subsequent analysis which focused exclusively on those who had failed laxatives confirmed the efficacy of prucalopride in laxative failures [15].
The adoption of a new drug and, especially, the introduction of a prescription drug into a disorder where over-the-counter remedies have previously dominated, could impact significantly on a national health budget and the question can reasonably be asked: is this new drug worth the cost?
Net impact (positive or negative) can be analysed by budget impact analysis (BIA) [16]. In the UK, a costing template was designed by the National Institute of Clinical Excellence (NICE) in 2010, to aid decision makers within the National Health Service (NHS) to predict the potential future financial impact of the adoption and diffusion of prucalopride in England, Wales and Northern Ireland. The aim of the costing template was to measure any incremental costs or savings within the health system associated with the introduction of the new technology, prucalopride, for the management of chronic constipation. The NICE costing template was populated by local prevalence data, data from clinical trials and medical expert opinion [17]. This exercise has, to date, not been performed in Ireland where prucalopride was launched in 2011. Our aim, therefore, was to calculate the actual financial impact of prucalopride, by employing an audit of patients with CC treated before and following the availability of prucalopride.
Methods
Study design
The study design was based on a BIA conducted by the NICE. To validate the findings of the NICE costing template, an audit capturing real world data was used to determine the financial impact of adopting prucalopride in women suffering with chronic constipation. A measure of resource use was achieved through the extraction of secondary data from patients’ medical files. This facilitated the application of local unit costs to the resources used and determined whether the use of prucalopride, as an alternative treatment to laxatives, resulted in a reduction in the use of secondary care resources and hence costs.
The study was not a randomised controlled trial but rather a retrospective case series audit, employed to capture real world use of prucalopride in women refractory to laxatives. The baseline scenario corresponded to secondary care resources used in the management of CC 12 months prior to the availability of prucalopride. The second scenario, or new treatment scenario, captured secondary care resource use subsequent to treatment with prucalopride in the same sample of patients. This, therefore, reflected the differences in healthcare resources used in the management of CC before and after the introduction of prucalopride.
Patient population
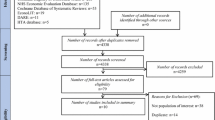
Having obtained ethical approval from the Ethics Committee of the Cork Teaching Hospitals, the case records of consecutive patients with CC attending clinics with a special interest in functional gastrointestinal disorders at Cork University Hospital (CUH) were accessed and those who met Rome III criteria for CC identified. A key word search of medical letters contained within these case records facilitated the identification of patients who had been treated with prucalopride. Fifty seven patients were identified who had a primary diagnosis of CC, satisfied the ROME III criteria for CC and had been treated with prucalopride.
Further inclusion criteria also specified that patients who were included in the audit;
-
1.
had failed on two or more different classes of laxatives,
-
2.
were treated with prucalopride when the technology became available.
Of the 57 patients that were evaluated, 17 patients were excluded for the following reasons; drug-induced constipation, symptoms of IBS, incomplete data, pregnancy and male patients. In total, 40 patients were included for evaluation. All of the patients included in the study were female and over the age of 18 (mean age of 42).
Data collection and analysis
Each of the 40 patient records selected for analysis contained the information required to document the resources used specifically in the management of CC. The medical resources identified and measured were guided by a specialist in gastrointestinal disorders (Table 1).
In the baseline scenario each class and dose of laxative was recorded, which allowed the average daily dose of each laxative to be quantified. Unit costs were applied to the average daily dose allowing average laxative costs per patient to be calculated. Data relating to clinician visits, clinical investigations and procedures, hospitalisations, emergency department admissions were recorded. Baseline symptoms of CC relating to the ROME III criteria were also documented.
Resource use in the management of CC subsequent to treatment with prucalopride was extracted from the same sample of patients and captured in a new treatment scenario. The secondary care resources measured in the new scenario were consistent with the resources identified and measured in the baseline scenario. In addition, the dose and duration of prucalopride use were also documented. Further data documented any change in symptoms of CC.
NICE and HSE guidelines on the use of prucalopride state that each patient initiated on prucalopride must have previously failed to gain symptom relief of CC with two or more different classes of laxatives [18]. Therefore, the cost of 6 months treatment with laxatives was included for each patient in the new treatment scenario.
A number of patients treated with prucalopride were also treated with concomitant laxatives either on the recommendation of the treating physician, their general practitioner or of their own accord. This laxative use was also measured and the average dose per laxative was calculated. The cost of concomitant laxative use was also included in the new treatment scenario. The number of clinician visits, investigations and procedures was recorded per patient in the new treatment scenario. Telephone consultations were not accounted for.
Symptoms of CC were recorded which comprised of the frequency of bowel movements, bloating and distension, as well as sensation of incomplete evacuation. In addition, upper GI symptoms including, acid reflux and heartburn were documented, along with medications used for the treatment of these upper GI symptoms. These medications were not included in the costing of this audit.
On completion of data collection, all data collected was reviewed by a medical expert. The purpose of the review was to identify any medical resources that were generic in nature and not specific to the management of CC.
Data analysis
Analysis was performed on data retrieved from all patients. The cost analysis had two elements: measurement of the quantities of resources and the assignment of unit costs. Unit costs of laxatives and prucalopride were provided by the CUH pharmacy department and were reflective of the prices published in MIMS Ireland 2012 [these prices are the ex-factory costs and not the costs incurred by the Health Services Executive (HSE) in the General Medical Services (GMS) Scheme] [19]. Unit costs for clinician visits, investigations and procedures were provided by the finance department of the CUH and reflected 2012 costs.
Using MS Excel, total and mean market values were calculated for the number of laxatives, prucalopride dose, days of treatment, concomitant laxatives, the number of clinician visits, investigations and procedures, pre and post the availability of prucalopride. Mean values of resource use data from the baseline period and new treatment period were compared.
Any change in the number of bowel movements experienced by this group of patients was noted both in the baseline scenario and new treatment scenario.
Results
Medication costs
Baseline scenario
Analysis revealed that patients with CC were managed on a complex mix of laxatives, with a mean of 2.6 laxatives being prescribed. The average cost of laxatives per patient for 12 months was €436.02. The total laxative cost was €17,440.84 per year.
New Treatment Scenario
An average cost of treating each patient for 6 months with two different classes of laxatives was added to the new treatment scenario costs. This accounted for NHS/HSE guidelines which require the patient to have failed treatment with at least two different classes of laxatives prior to initiating prucalopride and amounted to €3,354.00.
Five (12.5 %) patients were treated with 1 mg prucalopride (based on older age) and 35 (87.5 %) patients were treated with 2 mg prucalopride. To estimate the cost of prucalopride, the dose and the total number of days of treatment (DoT) were considered. The total cost of treatment of forty patients with prucalopride was €14,113.59 per year.
Seven (17.5 %) patients were treated with both prucalopride and concomitant laxatives for an average length of 4 months. The average treatment cost was calculated with the dose of concomitant laxatives taken into consideration. It was assumed that these patients could potentially purchase laxatives without prescription and therefore use concomitant laxatives for greater than 4 months. To account for this, the average cost of 12 months concomitant laxative use was applied to these patients. As a result the total cost of treating 40 patients with prucalopride increased to €18,471.62 per year. The average cost per patient in the new treatment scenario including concomitant laxative use was €461.79.
Difference in costs
Medication costs in the baseline scenario and the new treatment scenario amounted to €17,440.84 and €18,417.62, respectively. The difference in costs was €1,030.78.
Other medical costs
There were 104 clinician visits in the 12-month baseline scenario. The total cost of these visits was €10,400, with an average cost of €260 per patient. In the new treatment scenario clinician visits were reduced to 78 visits. This resulted in a reduction of total costs and average costs to €7,800 and €195, respectively; a cost saving of €2,600 per year, on clinician visits.
A total of 84 investigations and procedures were carried out in the pre-prucalopride year (Table 2). The total cost of investigations and procedures for 40 patients was €64,552.06. Average costs amounted to €1,613.80 per patient. Analysis revealed in this baseline period that patients were subjected to on average 2.1 investigations and procedures. Eight (20 %) patients had the same investigation or procedure more than once in this 12-month period.
There was a significant reduction in the number of investigations and procedures in the 12 months after commencing prucalopride. The number of investigations and procedures reduced from 84 in the baseline scenario to 21 in the new treatment scenario. Therefore, total costs were significantly reduced to €24,198 per year in the prucalopride scenario (Table 3). In addition, no patient required a procedure more than once in the new treatment scenario.
This significant reduction in the cost of investigations and procedures was reflected in overall cost savings of €40,354.06 (€1,008.85 per patient per year) in the new treatment scenario (Table 4). A paired t test demonstrated that the difference in costs relating to investigations and procedures was statistically significant.
Clinical outcome
At baseline, 36 (90 %) patients experienced one bowel movement/week, 2 (5 %) patients experienced one bowel movement/2 weeks and a further 2 (5 %) patients experienced one bowel movement/month. In addition, 5 (12.5 %) patients experienced a sensation of incomplete evacuation and 15 (37.5 %) reported bloating and distension. Upper GI symptoms were also common with ten (25 %) patients suffering from heartburn and five (12.5 %) patients experiencing acid regurgitation.
Twenty five (62.5 %) patients had a significant response to prucalopride, defined as an increase of one or more SCBM per week. Of these, 5 (12.5 %) patients had an increase of at least one SCBM per week and 20 (50 %) patients had an increase of three SCBM per week. Thirteen (32.5 %) of the 25 responders these patients also had an improvement in upper gastrointestinal (GI) symptoms; which resulted in the discontinuation of medication for treatment of upper GI symptoms. Three (7.5 %) of patients had some response to prucalopride, with an improvement in bloating and distension. Twelve (30 %) of patients did not experience symptom relief of CC with prucalopride; 6 (15 %) because they did not tolerate prucalopride and 6 (15 %) simply obtained no benefit.
Sensitivity analysis
Sensitivity analysis was conducted by adjusting a number of key input variables to the costing, and assessing the impact of this on cost savings output. Variables adjusted included the number of laxatives in the baseline period. The average number of 2.6 laxatives used in the baseline scenario was reduced to 1, 1.5 and 2; analysis demonstrated that the overall cost savings were insensitive to a change in the number of laxatives (Table 5).
To account for any potential front loading of healthcare resources in the management of CC, the costs of both computerized tomography (CT) scans of the abdomen and CT scans of the abdomen and pelvis were removed in both the baseline and new treatment scenarios. It was determined that it was less likely that such procedures would be repeated after initial investigation for structural bowel abnormalities. Both CT abdomen and CT abdomen and pelvis are costly procedures; however, sensitivity analysis established that cost savings of €26,073.22 (€651.83 per patient) were maintained when the costs of these procedures were removed (Table 6).
Discussion
Our results suggest that total medication costs in the baseline scenario were €17,440.84 for the 12-month period with an average cost of €436.02 per patient. Total medication costs in the new treatment scenario were €18,471.62, with an average cost of €461.79 per patient. This audit revealed a reduction in clinician visits, from 104 visits to 78 visits which could, potentially, result in cost savings of €2,600 per year. The most significant impact on resources was demonstrated in the reduction of investigations and procedures from 84 to 21. A reduction of 75 % in investigations and procedures, led to cost savings of €40,354.06. The overall cost savings in the 12 months subsequent to the introduction of prucalopride in this patient sample were €41,923.28, an average of €1,048.08 cost savings per patient.
It could be argued that in the normal course of events, most investigations would have taken place during initial encounters with the patient and were less likely to occur during subsequent follow-up; however, even if one discounts all investigation costs, there was still a cost advantage to prucalopride. For example, with the removal of all CT scans a total cost saving of €26,073 (average €651 per patient) still occurred. Our findings also suggest that the overall cost savings were insensitive to a reduction in the number of laxatives. The difference in the cost savings is presented in Table 5.
Previous to these findings, a costing template developed by NICE was used to aid the prediction of potential cost savings when treating patients refractory to laxatives with the new technology prucalopride. The costing template measured resource use in patients treated with laxatives, followed by a measure of resource use when prucalopride was introduced. This allowed the comparison of resource use in the baseline scenario and a new treatment scenario. The NICE costing template demonstrated the potential for cost savings in patients’ refractory to laxatives subsequent to the introduction of prucalopride. This costing template was hypothetical in nature, however, the clinical management of CC was guided by clinical expert opinion and the efficacy and tolerability of prucalopride was informed by clinical data [17].
A key objective of this audit was to use real world data to validate the findings of the NICE costing template to reflect real world clinical practice. Hence, the collection of secondary data revealed there were variances in the type and number of resources used in the management of CC. The audit highlighted that a complex mix of laxatives was used in the baseline scenario to manage symptoms of CC in this sample. Patients were treated with a mean of 2.6 laxatives which is significantly greater than the 1 laxative that is assumed in the baseline scenario of the NICE costing template. This suggests that the overall treatment cost with laxatives could potentially be greater in real life clinical practice.
The cost of 6 months laxative use per patient was included in the new treatment scenario to reflect costs considered in the NICE costing template, and to account for NICE and HSE guidelines. However, the audit also revealed additional concomitant laxative use in 7 (17.5 %) patients post initiation of treatment with prucalopride. This laxative use was at low doses for an average period of 4 months. However, to account for the potential of patients to self-medicate with OTC laxatives, the cost of 12 months concomitant laxative use was included.
The findings of the medical audit suggest that, at baseline, the type and number of investigations and procedures used in the management of CC were greater than assumed by the NICE costing template. The NICE template assumed that each patient used only one secondary care resource in the management of CC. However, the audit demonstrated that, at baseline, 84 investigations or procedures were used in the management of CC. Therefore, an average 2.1 secondary care resources per patient were used in the baseline scenario, with some patients having repeat procedures. There was also greater variation in the types of investigations and procedures. This difference most likely reflected the refractory nature of this population; the very population for whom prucalopride is intended.
The NICE costing template suggested the potential for cost savings in the management of CC in women refractory to laxatives, subsequent to the introduction of prucalopride. These findings were validated by conducting a case series audit. Methodologies such as case series audits have been employed by previous research to facilitate the assessment of the health economic implications with the introduction of a new medical technology.
A retrospective case series audit was used to establish the health economic implications associated with the introduction of the new technology quetiapine as an alternative to traditional therapeutic regimes, in 21 patients with chronic schizophrenia [20]. The findings indicated that the adoption of the new technology for the treatment of chronic schizophrenia resulted in a reduction in the use of secondary care resources, which were consistent with the outcomes of this study. Interestingly, the study concluded that these cost savings appeared to compensate for the increased acquisition cost of the new technology with potential savings greatest for ‘revolving door’ patients. The reduction in hospitalisation costs would appear to compensate for the increased cost of drug treatment. These findings further support the use of auditing to examine the real world cost savings that can be achieved with the introduction of new medical technology and could inform decisions and aid planning.
Previous studies examining the economic burden relating to the management of CC suggest that it is not an easily treated condition and is a disorder which imposes an economic burden on the individual and society. Studies have also highlighted that resource utilisation associated with the diagnosis and management of constipation is a significant cost driver and it has been highlighted that exhaustive diagnostic evaluation of constipation is costly [21].
A prospective study in the United States underlines that accurate estimates of per-patient health care costs for specific disorders are important in two contexts. First, they are used to make decisions about the allocation of health care resources. Health planners may multiply the per-patient cost of care by the known prevalence of the disorder to arrive at an estimate of total direct health care costs. Second, these costs can be compared to direct costs for other disorders in deciding how to allocate patient care resources and research funds [22].
As demonstrated in this audit, previous research conducted on patients with CC has also revealed a complex mix of laxative use which accords with the findings of this audit. A national audit was conducted of CC in the United Kingdom of 923 patients in 20 centres [23]. It was revealed that 42 % of patients were on combination laxative therapy, with patients on an average of 5 to 6 laxatives per patient. The audit associated complex laxative prescribing with poorly controlled CC, which suggests that patients refractory to laxatives use greater than one laxative to manage CC.
Further studies question the efficacy of laxatives in the management of CC and suggest that better evidence is needed to justify the continued expenditure of funds on laxatives by both the patient and healthcare provider. These studies highlight that continued use of laxatives in patients who do not gain symptom relief of CC can result in increased laxative use, and hence increase costs in the management of CC. The need for an alternative treatment to laxatives in patients who do not experience symptom relief using laxatives is also underlined [24, 25].
This audit had a small sample size of 40 patients. Therefore, in the interpretation of the costing, caution must be applied, as the findings may not be representative of a larger population of patients’ refractory to laxatives.
Individuals in the baseline scenario required a greater amount of secondary care resources than other patients in the sample; resulting in an increase in average resource use and costs in the management of CC. Although resource consumption was greatly reduced in these patients with the introduction of prucalopride; a larger sample would have to be examined to determine if this baseline use of resources is indeed representative of the wider population. Further study is required.
Limitations
While the collection of secondary data allowed for the collection of complete, valid and reliable data, there are limitations to this study. The study was quantitative in nature, with a sample of size of 40 patients selected using purposive sampling. This was to ensure that the patients selected for inclusion in the study provided the necessary data for the audit to be conducted. As is characteristic of a retrospective case series audit, this methodology gives rise to a narrow selection and therefore may be confounded by a selection bias.
Conclusion
Overall, this retrospective study supported the assumption of the NICE cost template in that the failure of patients to experience relief of symptoms of CC with laxatives led to the increased use of secondary care resources accompanied by an increased use of laxatives, investigations and procedures which exceeded the estimates of the NICE costing template. The use of these resources was the main cost driver in the baseline scenario and demonstrates the complex use of healthcare resources in patients who are refractory to laxatives. This study was useful in providing a detailed summation of the potential for reduced resource use and associated cost savings as a consequence of the introduction of a new technology, prucalopride, as an alternative to laxatives. However, these findings are the result of a retrospective case series audit and therefore should be considered with caution. This outcome of this study could be considered as providing the basis for a hypothesis for a future prospective study.
References
Longstreth GF, Thompson WG, Chey WD et al (2006) Functional bowel disorders. Gastroenterology 130:1480–1491
Higgins PD, Johanson JF (2004) Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol 99:441–449
Stewart WF, Liberman JN, Sandler RS et al (1999) Epidemiology of constipation (EPOC) study in the United States: relation of clinical subtypes to sociodemographic features. Am J Gastroenterol 94:3530–3540
Wald A, Kamm M, Mueller-Lissner S et al (2006) The BI Omnibus study: an international survey of community prevalence of constipation and laxative use in adults. Gastroenterology 130:508
Dennison C, Prasad M, Lloyd A et al (2005) The health-related quality of life and economic burden of constipation. Pharmacoeconomics 23:461–476
Yost KJ, Haan MN, Levine RA et al (2005) Comparing SF-36 scores across three groups of women with different health profiles. Qual Life Res 14:1251–1261
Johanson JF (2007) Review of the treatment options for chronic constipation. Med Gen Med 9:25
Petticrew M, Rodgers M, Booth A (2001) Effectiveness of laxatives in adults. Qual Health Care 10:268–273
Jones MP, Talley NJ, Nuyts G et al (2002) Lack of objective evidence of efficacy of laxatives in chronic constipation. Dig Dis Sci 47:2222–2230
Tramonte SM, Brand MB, Mulrow CD et al (1997) The treatment of chronic constipation in adults: a systematic review. J Gen Intern Med 12:15–24
Quigley EMM (2012) Prucalopride: safety, efficacy and potential applications. Ther Adv Gastroenterol 5:23–30
Camilleri M, Kerstens R, Rykx A (2008) A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med 358:2344–2354
Tack J, van Outryve M, Beyens G et al (2009) Prucalopride (Resolor) in the treatment of severe chronic constipation in patients dissatisfied with laxatives. Gut 58:357–365
Quigley EMM, Vandeplassche L, Kerstens R et al (2008) Clinical trial: the efficacy, impact on quality of life, and safety and tolerability of prucalopride in severe chronic constipation—a 12-week, randomized, double-blind, placebo-controlled study. Aliment Pharmacol Ther 29:315–328
Tack J, Quigley E, Camilleri M et al (2013) Efficacy and safety of oral prucalopride in women with chronic constipation in whom laxatives have failed: an integrated analysis. UEG J 1:48–59
Mauskopf JA, Sullivan SD, Annemans L et al (2007) Principles of good practice for budget impact analysis: report of the ISPOR task force on good research practices—budget impact analysis. Value in Health 10:336–347
(2011) TA211 Prucalopride chronic constipation in women: costing template. Available at: http://guidance.nice.org.uk/TA211/CostingTemplate/xls/English. Accessed 14 May 2012
(2010) Constipation (women)—Prucalopride (TA211). Available at: http://guidance.nice.org.uk/TA211. Accessed 14 May 2012
Monthly Index of Medical Specialties Ireland (MIMS Ireland) (2012) MPI Media Ltd, Dublin, Ireland
Lynch J (2001) The health economic implications of treatment with quetiapine: an audit of long term treatment for patients with chronic schizophrenia. Eur Psychiatry 16(5):307–312
Rantis PC Jr, Vernava AM, Daniel GL et al (1997) Chronic constipation—is the work-up worth the cost? Dis Colon Rectum 40:280–286
Nyrop KA (2007) Costs of health care for irritable bowel syndrome, chronic constipation, functional diarrhoea and functional abdominal pain. Aliment Pharmacol Ther 26(2):237–248
Lynch J (2001) The health economic implications of treatment with quetiapine: an audit of long term treatment for patients with chronic schizophrenia. Eur Psychiatry 16(5):307–312
Addison R (2003) A national audit of chronic constipation in the community. Nurs Times 99(11):34–35
Jones MP (2002) Lack of objective evidence of efficacy of laxatives in chronic constipation. Dig Dis Sci 47(10):2222–2230
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walsh, C., Murphy, J. & Quigley, E.M.M. Pharmacoeconomic study of chronic constipation in a secondary care centre. Ir J Med Sci 184, 863–870 (2015). https://doi.org/10.1007/s11845-014-1204-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-014-1204-2