Abstract
Background
Urinary bladder cancer is a quite common cancer type in men and women all over the world. Genetic polymorphisms of xenobiotic-metabolizing enzymes could increase individual susceptibility to various cancer types.
Aims
The aim of our study is to evaluate the rate of these polymorphisms in a group of patients from Central Anatolia.
Methods
Our study subjects consist of 65 men with histopathologically confirmed bladder TCC and 70 cancer-free control subjects. Restriction fragment length polymorphism (RFLP) method was used for the detection of polymorphisms of GSTT1 and GSTM1.
Results
There was no association between bladder cancer and GSTM1 polymorphism (ORs = 0.64, 95 % CI = 0.32–1.29), but the probability of bladder cancer in patients with GSTT1 null genotype (67.9 %), was significantly higher from the probability of bladder cancer with GSTT1 normal genotype (43.0 %) statistically (ORs = 2.8, 95 % CI = 1.16–6.75).
Conclusion
Polymorphisms of these genes have been assessed to evaluate the relative risk of various cancers. Our intention is to continue this study with larger series of bladder cancer patients in a group of Turkish population from Central Anatolia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary bladder cancer is the fourth most common type of cancer in men and the seventh in women in the world [1]. According to KIDEM (Turkish cancer follow-up and control centre), the incidence of bladder cancer in Turkey is the second after lung cancer, among the genitourinary cancers, it is the mostly seen cancer type in men [2]. It is mostly seen in 6th and 7th decades. Although the rate of men to women is 3/1, survey is shorter in women (31 %) [3, 4]. Nearly 90–95 % of bladder cancers are transitional cell carcinomas (TCC). Environmental carcinogens are to be the most seen causes of bladder cancer. Besides these, tobacco smoking and exposure to aromatic amines and polycyclic hydrocarbons have been related with risk of bladder cancer [5]. It is suggested that genetic polymorphisms of xenobiotic-metabolizing enzymes could increase individual susceptibility to various environmental and clinical conditions. Deoxyribonucleic acid (DNA) damage that occurs in cells is affected from the imbalance between activation and detoxification of carcinogens [6]. Glutathione-S-transferases (GSTs) constitute a superfamily of phase II enzymes, these enzymes are involved in the detoxification of exogenous substrates such as xenobiotics, environmental substances and carcinogenic compounds. To date, human cytosolic GST superfamily contains at least 16 genes subdivided into 8 distinct classes [7]. Functional polymorphisms have been identified in the GSTM1, GSTT1, and GSTP1 genes which are coding for GSTs enzymes [8].
Polymorphisms of GSTM1, GSTT1, and GSTP1 genes have been assessed to evaluate the relative risk of various cancers. Among them the most extensively studied are the GSTM1 and the GSTT1 null polymorphisms, and the GSTP1 313 A/G substitution. The functional consequences of the GSTM1 and GSTT1 null genotypes are no enzymatic activity. Many investigators agree that the GSTM1 “null” genotype as the risk factor for the bladder cancer especially among smokers [9]. It had been shown that individuals with the null genotype for GSTT1 were at higher risk for developing bladder cancer [10]. The purpose of this study was to determine the frequencies of polymorphisms of GSTT1 and GSTM1 genes and their association with bladder TCC among the patients with bladder cancer.
Materials and methods
The study subjects consisted of 65 patients with histopathologically confirmed bladder TCC (61.7 ± 9.9) and 70 cancer-free control subjects (59.6 ± 10.0), recruited from urology clinic between October 2009 and October 2011. No patient received chemotherapy or radiotherapy before recruitment. All cancer patients were newly diagnosed with TCC of the bladder. The control group consisted of non-related healthy men and women without history of malignant disease who were matched to those in the case group for geographic origin, smoking status, and age range. The number of cigarettes per day was 15 on average. The smoking status is shown in Table 1. All study subjects provided informed consent prior to participating in the study, which was conducted according to Helsinki declaration (2004).
The clinical information about tumor size, number, stage, and grade, radiotherapy, and chemotherapy were obtained from medical records. In all of the bladder cancer patients, the tumors were diagnosed histologically as TCC. The tumors were classified as superficial (pTa–pT1) or invasive (pT2–pT4) according to the 1997 TNM staging system of the American joint committee on cancer. The histologic grades were subdivided into low grade and high grade using the grading systems of the world health organization (WHO) and the international society of urological pathology (ISUP). The ethical committee has approved the study. All patients were asked to read and sign the informed consent form prior to the genetic testing.
Blood sampling and extraction of DNA
Blood samples (5 ml of blood was drawn into tubes containing EDTA from each patient) were obtained from patients and control groups. Immediately after collection, whole blood was stored at −20 °C until use. Genomic DNA for polymerase chain reaction (PCR) was isolated using a commercial kit (QIAamp DNA mini kit; Qiagen, Hilden, Germany).
Genotyping analysis
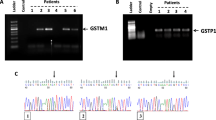
The PCR method for determining GSTM1 and GSTT1 genotypes was the same as that reported previously [8]. The primers for amplifying the GSTM1 gene were 5′-CTG CCC TAC TTG ATT GAT GGG-3′ and 5′-CTG GAT TGT AGC AGA TCA TGC-3′. The PCR was performed in 50 ml reaction buffer containing 200 mM dNTPs, 1.5 mM MgCl2, 1 mm primers, about 1 mg DNA and two units of thermostable Taq DNA polymerase using a programmable thermocycler (Progene, Techne, UK). After 5 min of pretreatment at 94 °C, 35 cycles of 1.5 min denaturation at 94 °C, 1.5 min annealing at 60 °C and 1 min extension at 72 °C were performed. The primers for amplifying the GSTT1 gene were 5′-TTC CTT ACT GGT CCT CAC ATC TC-3′ and 5′-TCA CCG GAT CAT GGC CAG CA-3′. The PCR was performed in 50 ml reaction buffer containing 200 mM dNTPs, 1.5 mM MgCl2, 10 pmol of each primer, about 1 mg DNA and two units of thermostable Taq DNA polymerase using a programmable thermocycler. After 5 min of pretreatment at 94 °C, 35 cycles of 1 min denaturation at 94 °C, 1.15 min annealing at 60 °C, and 1 min extension at 72 °C were performed. For evaluating the GSTM1 and GSTT1 polymorphism the amplification products were analyzed by gel electrophoresis (2 % agarose). A 900 bp fragment was amplified by PCR with the GSTM1 primers and a 480 bp fragment was amplified by PCR with the GSTT1 primers. The absence of amplified product was consistent with the null genotypes. The genotypes were confirmed by direct sequencing (Amersham, GE Healthcare life sciences, Sweden).
Statistical analysis
The analysis of the results was carried out using SPSS for Windows (version 18.0). The homogeneity between patients and controls was calculated using by Chi-square and Student’s t test. The association between bladder cancer and GSTT1 and/or GSTM1 null genotypes was reported as odds ratios (ORs) and 95 % confidence intervals (CI). All p values were two-tailed and the statistical significance was set at p < 0.05.
Results
Sixty-five patients with histopathologically confirmed bladder TCC (61.7 ± 9.9) and 70 cancer-free control subjects (59.6 ± 10.0) were consisted in the study from urology clinics in Turkey Yuksek Ihtisas Education and Training Hospital. All cancer patients were newly diagnosed with TCC of the bladder. The control group consisted of non-related healthy men and women without history of malignant disease.
There is homogeneity between outcome and age (p = 0.214, Levene’s test = 0.705). The statistical analysis showed that smoking status was not associated with outcome (p = 0.676) (Table 1).
Although the odds ratio of bladder cancer risk associated with GSTT1 null genotype was 2.8 (95 % CI = 1.16–6.75), there is no statistical association between GSTM1 null genotype and bladder cancer. According to the our results, an individual who had either null GSTT1 and GSTM1 genotypes or both null GSTT1 and GSTM1 genotypes was 1.24 and 1.88 times more likely to have bladder cancer, but statistically not significant (p = 0.569 and p = 0.757, respectively) (Table 2).
Discussion
The GSTs play important roles in the protection against many compounds including carcinogens, pesticides, antitumor agents, and environmental pollutants. In humans, the GST family can be divided into different classes as a, m, p, and u enzymes and each class consisting of several isoenzymes with partly overlapping substrate specificity [3]. GSTM1, one member of the GSTM class, is involved in the detoxification of some carcinogens such as benzo[a]pyrene. The null genotype (homozygous for non-functional allele) of GSTM1 has a decreased capability in detoxifying some carcinogens. It has been linked with an increased risk of lung, naso-pharyngeal, breast, urothelial, and prostate cancers, squamous dysplasia of the esophagus and acute lymphocytic leukemia. On the other hand, some investigators failed to find any significant correlation between the GSTM1 genotypes and various types of cancers [11, 12].
GSTT1 also has a functional and a non-functional allele (GSTT1 × 0). The GSTT1 can detoxify smaller reactive hydrocarbons, such as ethylene oxide and diepoxybutane. The null genotype of GSTT1 was reported to be associated with an increased risk of bladder, lung cancer, and myelodysplastic syndrome, whereas other studies did not confirm the associations between GSTT1 null genotype and cancers [13, 14].
Studies investigating the association between GSTs polymorphisms and bladder cancer risk show very conflicting and different results. GSTM1 gene is accepted as polymorphic, and at least four different alleles exist. The deleted genotypes result in the inactive form of the enzyme and have been named as GSTM1 null genotype. The homozygous deletion of GSTM1 has been associated with an increased risk of various types of cancer [15, 16]. There are some studies which report that GSTM1 null genotype is a risk factor for bladder cancer [11, 17, 18]. Many investigators refer the GSTM1 “null” genotype as the risk factor for the bladder cancer, especially among smokers [19]. However, another study suggests that the GSTM1 polymorphism is not associated with risk of bladder cancer [12]. In our study, we also found that there is no association between bladder cancer and GSTM1 polymorphism (ORs = 0.64, 95 % CI = 0.32–1.29).
The polymorphism in the GSTT1 gene loci is caused by a gene deletion; it leads to absence of enzyme activity in individuals with the null genotype. Only a few studies demonstrated nonsignificant diminished risk of bladder cancer with GSTT1 null genotype; besides this, some other studies demonstrated increased risk of bladder cancer with GSTT1 null genotype [17]. Nearly 20 % of Caucasians are homozygotic for a null allele of GSTT1. It was shown that individuals with the null genotype for GSTT1 were at higher risk for developing bladder [17, 20–22]. In our study, the probability of bladder cancer in patients with GSTT1 null genotype (67.9 %), was significantly higher from the probability of bladder cancer with GSTT1 normal genotype (43.0 %) statistically (ORs = 2.8, 95 % CI = 1.16–6.75).
GSTMI and GSTTI null genotypes show their effect as decreased capacity to detoxify certain carcinogens and they are associated with increased risks for developing bladder cancer [8]. In our study, the relationship between the null genotypes of both GSTT1 and GSTM1 and bladder cancer was not statistically significant, although odds ratio was 1.88 for both null genes. We think that this is because of the low number of patients and controls. This is a limitation for our study.
Polymorphisms of GSTM1 and GSTT1 genes have been assessed to evaluate the relative risk of various cancers [18]. In this study, we tried to detect the incidence of these polymorphisms in bladder cancer patients and control group. Although, there was no statistically important association between GSTM1 polymorphism and bladder cancer, it was statistically important for GSTT1 polymorphism and bladder cancer. We intend to continue this study with a larger series of bladder cancer patients and a control group in a group of Turkish population from Central Anatolia.
References
Ferlay J, Bray F, Pisani P, Parkin DM, GLOBOCAN (2002) Cancer incidence, mortality and prevalence worldwide. IARC cancer base no. 5, version 1.0. International agency for research on cancer
Konety BR, Williams RD (2004) Superficial transitional (Ta/T1/CIS) cell carcinoma of the bladder. BJU Int 94:18–21
Jemal A, Thomas A, Murray T, Thun M (2002) Cancer statistics 2002. CA Cancer J Clin 52:23–47
Heney NM (1992) Natural history of superficial bladder cancer. Prognostic features and long-term disease course. Urol Clin NorthAm 19:429–433
Cohen SM, Shirai T, Steineck G (2000) Epidemiology and etiology of premalignant and malignant urothelial changes. Scand J Urol Nephrol (Suppl) 205:105–115
Stern MC, Johnson LR, Bell DA, Taylor JA (2000) XPD Codon 751 polymorphism, metabolism genes, smoking, and bladder cancer risk. Cancer Epidemiol Biomarkers Prev 11:1004–1011
Sanyal S, Festa F, Sakano S et al (2004) Polymorphisms in DNA repair and metabolic genes in bladder cancer. Carcinogenesis 25:729–734
Safarinejad MR, Shafiei N, Safarinejad S (2010) The association of glutathione-S-transferase gene polymorphisms (GSTM1, GSTT1, GSTP1) with idiopathic male infertility. J Hum Genet 55:565–570
Karagas MR, Park S, Warren A, Hamilton J, Nelson HH, Mott LA, Kelsey KT (2005) Gender, smoking, glutathione-S-transferase variants and bladder cancer incidence: a population-based study. Cancer Lett 219:63–69
Srivastava DS, Kumar A, Mittal B, Mittal RD (2004) Polymorphism of GSTM1 and GSTT1 genes in bladder cancer: a study from North India. Arch Toxicol 78:430–434
Shao J, Gu M, Zhang Z, Xu Z, Hu Q, Qian L (2008) Genetic variants of the cytochrome P450 and glutathione S-transferase associated with risk of bladder cancer in a south-eastern Chinese population. Int J Urol 15:216–221
Sobti RC, Al-Badran AI, Sharma S, Sharma SK, Krishan A, Mohan H (2005) Genetic polymorphisms of CYP2D6, GSTM1, and GSTT1 genes and bladder cancer risk in North India. Cancer Genet Cytogenet 156:68–73
McGrath M, Michaud D, De Vivo I (2006) Polymorphisms in GSTT1, GSTM1, NAT1 and NAT2 genes and bladder cancer risk in men and women. BMC Cancer 6:239
Salagovic J, Kalina I, Stubna J, Habalová V, Hrivnák M, Valanský L, Kohút A, Biros E (1998) Genetic polymorphism of glutathione S-transferase M1 and T1 and risk factor in lung and bladder cancer. Neoplasma 45:312–317
Singh M, Shah PP, Singh AP, Ruwali M, Mathur N, Pant MC, Parmar D (2008) Association of genetic polymorphisms in glutathione S-transferases and susceptibility to head and neck cancer. Mutat Res 638:184–194
Smits KM, Gaspari L, Weijenberg MP et al (2003) Interaction between smoking, GSTM1 deletion, and colorectal cancer: results from the GSEC study. Biomarkers 8:299–310
Lee SJ, Cho SH, Park SK et al (2002) Combined effect of glutathione S-transferase M1 and T1 genotypes on bladder cancer risk. Cancer Lett 28(177):173–179
Steinhoff C, Franke KH, Golka K, Thier R, Römer HC, Rötzel C, Ackermann R, Schulz WA (2000) Glutathione transferase isozyme genotypes in patients with prostate and bladder carcinoma. Arch Toxicol 74:521–526
Abdel-Rahman SZ, Anwar WA, Abdel-Aal WE, Mostafa HM, Au WW (1998) GSTM1 and GSTT1 genes are potential risk modifiers for bladder cancer. Cancer Detect Prev 22:129–138
Zeng FF, Liu SY, Wei W et al (2010) Genetic polymorphisms of glutathione S-transferase T1 and bladder cancer risk: a meta-analysis. Clin Exp Med. 10:59–68
Franekova M, Halasova E, Bukovska E, Luptak J, Dobrota D (2008) Gene polymorphisms in bladder cancer. Urol Oncol 26(1):1–8
Katoh T, Inatomi H, Kim H, Yang M, Matsumoto T, Kawamoto T (1998) Effects of glutathione S-transferase (GST) M1 and GSTT1 genotypes on urothelial cancer risk. Cancer Lett 132:147–152
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ceylan, G.G., Ceylan, C., Taşdemir, S. et al. The effect of Glutathione-S-transferases in the susceptibility to bladder cancer. Ir J Med Sci 184, 851–854 (2015). https://doi.org/10.1007/s11845-014-1200-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-014-1200-6