Abstract
Background
Numerous studies have demonstrated that elevated serum uric acid concentration (SUA) is correlated with lipid profile in hypertensive or obese patients. However, the relationship between serum uric acid levels and lipid profile in non-obese late adolescent population was not examined before.
Aims
In this study we decided to assess the potential relationship between SUA and lipid profile, according to gender in adolescents with HU.
Methods
The study group comprises 607 Polish adolescents (474 males, 133 females) with HU. Retrospective analysis included demographic, clinical, and laboratory data. Lipid profile was assessed including total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), total cholesterol to HDL-C ratio (TC/HDL-C).
Results
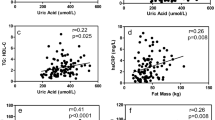
In the examined group 187/607 (31 %) of teenagers were diagnosed with metabolic syndrome (MetS). Median BMI-Z-score was 1.11 Q1–Q3: (−0.02–2.03) and both females and males in the upper tertile of SUA had statistically significant higher BMI-Z-score. The males in the upper tertile of serum uric acid levels also had higher values of TG and lower of HDL-C. In females, we have not found significant differences in lipid profile. Multiple regression analyses indicated that male gender, BMI-Z-score, and presence of hypertension correlated significantly with serum uric acid concentration.
Conclusions
In summary, the results of our study confirm higher prevalence of hyperuricemia in males when compared to females and a stronger association of HU with BMI-Z-score and lipid profile in male adolescents. Nevertheless, multiple regression does not confirm the independent association of SUA with lipid profile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The relationship between serum uric acid levels (SUA) and cardiovascular conditions has been reported since the early 1960s [1]. Since then, a number of epidemiologic studies on relation between SUA levels and a wide variety of cardiovascular conditions, including hypertension, metabolic syndrome, and kidney disease have been published [2–4]. The prevalence and incidence of hyperuricemia (HU) in the world population have increased over the past 40 years and the SUA levels have increased in both men and women of various demographic groups [5]. Data from recent epidemiologic studies revealed that between 10 and 20 % of most western populations are hyperuricemic [6]. The prevalence of HU is varying in different populations and areas. The highest was observed in Taiwan’s population 43.7 % in men and 27.4 % in women aged ≥15 years [7]. Among the population of the United States the prevalence of HU in adult males is 21.2 and 21.6 % in females, as in adolescents 6 and 9 % for males and females, respectively [8]. Data from the German population showed that only 2.6 % of females but 28.6 % of males were hyperuricemic [9]. In Italy Ruggiero et al. [10] reported prevalence of HU in the whole population of 9.3 %. There is no data about the prevalence of HU in the Polish healthy population, however in untreated hypertensive patients it is estimated for 15.4 % [11].
Feig et al. [12] reported that the relation between uric acid and cardiovascular disease is observed not only in patients with severe HU (defined as more than 6 mg/dl in women and more than 7 mg/dl in men) but also with serum uric acid levels >5.5 mg/dl. The study reported presence of HU in 89 % of children with essential hypertension and only in 30 % of secondary hypertension.
Based on experimental study HU is considered as a mediator of proinflammatory endocrine imbalance in adipose tissue which may be responsible for dyslipidemia and the inflammatory process leading to atherogenesis [13]. In 1993, Zavaroni et al. [14] suggested that HU is commonly part of the cluster of metabolic and hemodynamic abnormalities along with abdominal obesity, glucose intolerance, insulin resistance, dyslipidemia, and hypertension. In another review Tsouli et al. [15] summarized the pathophysiologic association between SUA and metabolic syndrome (MetS) and focused on the prevention of HU and its cardiovascular consequences. Recent study by Feig et al. [16] suggested that HU might have an important contributory role in MetS. Hyperuricemia is considered a mediator of endothelial dysfunction, leading to reduced insulin-stimulated nitric oxide-induced vasodilatation in skeletal muscle, and as a consequence reduced glucose uptake in skeletal muscle; and it leads to inflammation and oxidative changes in adipocytes.
Excessive caloric intake, incorrectly balanced diet and sedentary lifestyle contributes not only to weight gain but also to disturbances in the metabolism of fats and carbohydrates hepatic synthesis of triglycerides. Additionally de novo synthesis of purine compounds accelerates and augments the production of uric acid. Increased amount of visceral adipose tissue causes insulin resistance affecting both glucose and uric acid levels [17], this in turn reduces the antiatherogenic high density lipoprotein cholesterol (HDL-C) particles [5, 18]. Numerous studies have demonstrated that elevated serum concentration of uric acid is strongly correlated with serum levels of total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C) and low values of HDL-C [19–21] in hypertensive or obese patients, however to our best knowledge lipid profile has not been fully evaluated in children and adolescents with HU. The relationship between serum uric acid levels and lipid profile in non-obese late adolescent population was not examined before. Previous studies have primary focused on obese adolescent populations [22, 23]. Similarly previous studies in this field have not reported the gender references.
Therefore, in this study we decided to assess the potential relationship between SUA and lipid profile, according to gender in a large cohort of Polish adolescents with HU.
Methods
The current research is part of a 10-year retrospective cohort study of patients registered with the Pediatric Nephrology Department at Medical University of Bialystok, Poland. The cohort was randomly selected from the medical records generated by Solmed Software. Baseline data were collected in 2002–2012.
The study group comprises 607 Polish adolescents (474 males, 133 females). Retrospective analysis included demographic, clinical, and laboratory data. Laboratory analyses recorded at presentation included complete blood count, serum levels of creatinine, urea, uric acid, thyroid-stimulating hormone, glucose, total cholesterol (TC), low-density lipoprotein cholesterol (LDL), triglycerides (TG), total cholesterol to HDL-C ratio (TC/HDL-C), C-reactive protein, electrolytes, and urinalysis. The inclusion criteria were as follows: adolescents aged 11–21 years, with HU (serum uric acid >5.5 mg/dl), suspected for hypertension or metabolic syndrome. Exclusion criteria were: heart failure, diabetes mellitus, renal or hepatic dysfunction, hematological disease, systemic inflammatory conditions, autoimmune diseases, secondary forms of hypertension, subjects treated with hypertensive agents and medications known to affect SUA levels and BP values, administered with uric acid-lowering agents.
The Bioethics Committee of the Medical University of Bialystok in accordance with the Declaration of Helsinki approved the protocol.
Body weight and height were measured using a balance beam scale and pediatric wall-mounted stadiometer and body mass index (BMI) was calculated as weight (kg) divided by the square of height (m2).
Age- and gender-specific reference values for BMI were generated by the LMS method [24], which characterizes the distribution of a variable by its median (M), the coefficient of variation (S, i.e., the ratio of the SD and mean), and skewness (L) required to transform the data to normality. Evaluation for these parameters is obtained by a maximum-likelihood curve-fitting algorithm to the original data plotted over the independent variable. The formula for calculating Z-score of BMI or height was: LMS−SDS = {[Y/M(t)]L(t)−1}/[L(t) × S(t)], where Y is the individual observation, and L(t), M(t), and S(t) are the specific values of L, M, and S interpolated for the child’s age and gender. The LMS values were taken from OLAF study, published by Kułaga et al. [25].
In our study metabolic syndrome was defined according to the criteria established for young people by Cook et al. [26]. The diagnosis of hypertension was carried out based on recommendations of ESH [27] and HU was defined as serum uric acid level >5.5 mg/dl [12]. Overweight and obesity were defined using criteria developed by Cole et al. [28].
Blood samples were collected in the morning, after night fasting. The venous blood samples were combined with dipotassium EDTA and tested within 30 min of collection. Complete blood counts were measured using an automatic blood counter. Serum creatinine was determined by updated Jaffe reaction, serum uric acid was assessed using the colorimetric method, and serum cholesterol, HDL-cholesterol and triglycerides were determined by the enzymatic method using Hitachi 912 (La Roche Company, Japan). Low-density lipoprotein cholesterol (LDL-C) was calculated by the traditional formula of Friedewald et al. [29]. Serum glucose was measured with the Integra 800 analyzer. Glomerular filtration rate was assessed by updated Schwartz’s formula (GFR), which is recommended in paediatric population [30].
Statistical analysis
Data analysis was performed using a computer program Statistica 10.0 PL. Discrete variables were expressed as counts (percentage), whereas continuous variables as median with the interquartile range (Q 1–Q 3), unless stated otherwise. The comparison between the two groups was done with the Chi-square and Fisher exact tests for categorical variables and t test for continuous variables for normally distributed data or Mann–Whitney test for the data distributed not normally. Pearson single-regression analyses and Spearman single-regression analyses were used for parametric and nonparametric data, respectively. Standard least-squares method was used for multiple regression analyses. Statistical significance was set at p < 0.05.
Results
The demographics and baseline laboratory data of patients are summarized in Table 1. The study included 607 patients aged 15.8 ± 1.84 years (M: 474, F: 133).
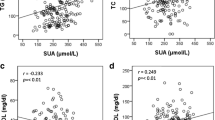
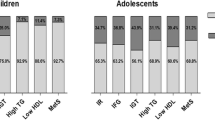
In the examined group 187/607 (31 %) of teenagers were diagnosed with metabolic syndrome. The prevalence of MetS in males and females was similar: 29.5 and 31.5 %, respectively. In teenagers with HU not diagnosed with metabolic syndrome, isolated primary hypertension was found in 202/420 (48 %) of patients and obesity in 83/420 (19.7 %) of patients. In the whole examined group the median BMI-Z-score was 1.11 Q1–Q3: (−0.02–2.03) and was similar in both genders (p > 0.05). On the other hand SUA concentration was significantly higher in males (median was 6.41 mg/dl) than in females (6.01 mg/dl) (p < 0.001). No gender differences were found in serum TC and TG, but HDL cholesterol was significantly higher in females (p < 0.05). Serum creatinine was higher and GFR lower in males than in females (p < 0.01). The baseline biochemical characteristics of participants grouped by sex-specific serum uric acid tertiles are shown in Table 1. Compared with those in the lower tertile, both females and males in the upper tertile of serum uric acid levels had statistically significant higher BMI-Z-score. Males in the upper tertile of serum uric acid levels also had higher values of triglycerides and lower of HDL cholesterol. However, in females we have not found significant differences in lipid profile.
In our study we found statistically significant relation between SUA and BMI-Z-score as well as lipid parameters in adolescents with HU. We found statistically significant positive correlations between SUA and BMI-Z-score (r = 0.42, p < 0.001), TG (r = 0.26, p < 0.001), TG/HDL-C (r = 0.25, p < 0.001), and statistically significant negative correlation between UA level and HDL-C (r = −0.16, p < 0.05). This relationship was stronger among males than females (Table 2).
Multiple regression analysis indicated that male gender (p < 0.01), BMI-Z-score (p < 0.01), and presence of hypertension (p < 0.01) correlated significantly with serum uric acid concentration.
Discussion
The most interesting finding of this retrospective study was much higher prevalence of HU in male than in female teenagers. This is in agreement with many reports from literature. The same results were obtained in US and Chinese adults [8, 31] and adolescents [32], as well. Another important finding of this study was that males had higher serum level of uric acid, and serum triglyceride levels were significantly higher and HDL cholesterol significantly lower in males in the upper tertile of serum uric acid levels. This observation was not confirmed in girls. It is interesting to note that serum uric acid correlated significantly with BMI-Z-score in both males and females. These findings confirmed the results obtained in the population study in Mexico [33], Taiwan [34] and China [35]. Statistically significant gender differences between males and females were confirmed in Bogalusa Heart Study [36]. Similar results in children aged 6–18 years were published by Jones et al. [37] (Table 3).
The prevalence of metabolic abnormalities in teenagers is increasing. The results of Third National Health and Nutrition Examination Survey US children aged 12–19 years reported MetS in about 10 %. In overweight/obese children, over 33 % had MetS and two-thirds of all adolescents had at least one metabolic abnormality. Additional results showed high prevalence of low HDL-C, hypertriglyceridemia, and central obesity both for boys and girls and for older and younger adolescents. In the population of young adults the incidence of MetS is 8–12 % in non-obese and 34–41 % in the obese patients [38].
We confirmed metabolic syndrome in about 30 % of hyperuricemic teenagers. In the whole examined group serum uric acid correlated positively with serum TG and TG/HDL-C ratio and negatively with HDL-C in both genders. We did not confirm the results of Bogalusa Heart Study [39] which demonstrated correlation between HU and total cholesterol. Correlation between SUA and TG found in our study is consistent with the results of longitudinal prospective study conducted by Rathmann et al. [40] who found regularity in changes of serum triglycerides strictly related to changes in uric acid, independent of changes in BMI. According to Takahashi et al. [41] the higher concentration of TG may be related to lower HDL-C in hyperuricemic individuals. Ford et al. [42] showed a close relation between HU and dyslipidemia as well as BMI-Z-score in nationally representative sample of US children and adolescents. The authors reported statistically significant, strong positive correlation between serum UA and lipid components of MetS and body parameters. The findings of the current study are also consistent with Wang et al. [43], who found significantly correlations between SUA and triglyceride and HDL-C in longitudinal study of Chinese male adolescents. Similar results were observed by other authors [22, 44]. Strong relationship between SUA to serum TG and HDL-C both men and women also was observed by Conen et al. [45]. On the other hand, Denzer et al. [46] in obese children and adolescents showed strong positive correlation between SUA and total cholesterol but not triglycerides. In our study we found different relationships between serum uric acid and lipid profile in late adolescents according to age. In females we only noticed the relationship between serum uric acid and BMI-Z-score. It might be caused by lower cut-off levels recommended for girls by Feig et al. [12]. In males we found statistically significant correlations between SUA and both lipid profile and BMI-Z-score. Our results are in agreement with data obtained by Lippi et al. [19], who showed that the relationships between serum UA and lipid parameters remained elusive in men. As emphasized by other researchers reduction of serum UA in males should be considered, since this intervention might act synergistically with lipid lowering therapies to reduce cardiovascular risk [47].
Multiple regression analysis indicated that male gender, BMI-Z-score, and presence of hypertension (p < 0.01) correlated significantly with serum uric acid concentration.
In summary, the results of our study confirm higher prevalence of hyperuricemia in males when compared to females and stronger association of HU with BMI-Z-score and lipid profile in male adolescents. However, the results of multiple regression does not confirm the independent association of SUA with lipid profile. Further studies are necessary to assess the potential role of complex treatment of HU and hyperlipidemia.
There are several limitations in this study including its retrospective design, single-center site and inability to calculate population-based rates. Another limitation is the small number of females compared with men. Thirdly, as the study is retrospective we did not get the data of waist circumference, and we were not evaluating the possible correlations.
References
Cannon PJ, Stason WB, Demartini FE et al (1966) Hyperuricemia in primary and renal hypertension. N Engl J Med 275:457–464. doi:10.1056/NEJM196609012750902
Sui X, Church TS, Meriwether RA et al (2008) Uric acid and the development of metabolic syndrome in women and men. Metabolism 57:845–852. doi:10.1016/j.metabol.2008.01.030
Grayson PC, Kim SY, LaValley M, Choi HK (2010) Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 63:102–110. doi:10.1002/acr.20344
Noone DG, Marks SD (2013) Hyperuricemia is associated with hypertension, obesity, and albuminuria in children with chronic kidney disease. J Pediatr 162:128–132. doi:10.1016/j.jpeds.2012.06.008
Rho YH, Zhu Y, Choi HK (2011) The epidemiology of uric acid and fructose. Semin Nephrol 31:410–419. doi:10.1016/j.semnephrol.2011.08.004
Edwards NL (2009) The role of hyperuricemia in vascular disorders. Curr Opin Rheumatol 21:132–137. doi:10.1097/BOR.0b013e3283257b96
Chuang S-Y, Lee S-C, Hsieh Y-T, Pan W-H (2011) Trends in hyperuricemia and gout prevalence: nutrition and health survey in Taiwan from 1993–1996 to 2005–2008. Asia Pac J Clin Nutr 20:301–308
Zhu Y, Pandya BJ, Choi HK (2011) Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum 63:3136–3141. doi:10.1002/art.30520
Gresser UU, Gathof BB, Zöllner NN (1990) Uric acid levels in southern Germany in 1989. A comparison with studies from 1962, 1971, and 1984. Klin Wochenschr 68:1222–1228
Ruggiero C, Cherubini A, Ble A et al (2006) Uric acid and inflammatory markers. Eur Heart J 27:1174–1181. doi:10.1093/eurheartj/ehi879
Kostka-Jeziorny K, Tykarski A (2007) Wpływ terapii hipotensyjnej na stężenie kwasu moczowego. Nadciśnienie tętnicze
Feig DI (2003) Hyperuricemia in childhood primary hypertension. Hypertension 42:247–252. doi:10.1161/01.HYP.0000085858.66548.59
Baldwin WW, McRae SS, Marek GG et al (2011) Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes 60:1258–1269. doi:10.2337/db10-0916
Zavaroni II, Mazza SS, Fantuzzi MM et al (1993) Changes in insulin and lipid metabolism in males with asymptomatic hyperuricemia. J Intern Med 234:25–30
Tsouli SGS, Liberopoulos ENE, Mikhailidis DPD et al (2006) Elevated serum uric acid levels in metabolic syndrome: an active component or an innocent bystander? Metab Clin Exp 55:1293–1301. doi:10.1016/j.metabol.2006.05.013
Feig DI, Kang D-H, Johnson RJ (2008) Uric acid and cardiovascular risk. N Engl J Med 359:1811–1821. doi:10.1056/NEJMra0800885
Tai ESE, Emmanuel SCS, Chew SKS et al (1999) Isolated low HDL cholesterol: an insulin-resistant state only in the presence of fasting hypertriglyceridemia. Diabetes 48:1088–1092
Chen L-Y, Zhu W-H, Chen Z-W et al (2007) Relationship between hyperuricemia and metabolic syndrome. J Zhejiang Univ Sci B 8:593–598. doi:10.1631/jzus.2007.B0593
Lippi G, Montagnana M, Luca Salvagno G et al (2009) Epidemiological association between uric acid concentration in plasma, lipoprotein(a), and the traditional lipid profile. Clin Cardiol 33:E76–E80. doi:10.1002/clc.20511
Sayin E, Sayin B, Ertugrul DT et al (2013) Is there a link between hyperuricemia, morning blood pressure surge, and non-dipping blood pressure pattern in metabolic syndrome patients? IJNRD 6:71–77. doi:10.2147/IJNRD.S41384
Sarmah D, Sharma B (2013) A correlative study of uric acid with lipid profile. Asian J Med Sci 4:8–14
Ishiro M, Takaya R, Mori Y et al (2013) Association of uric acid with obesity and endothelial dysfunction in children and early adolescents. Ann Nutr Metab 62:169–176. doi:10.1159/000346227
Pacifico L, Cantisani V, Anania C et al (2009) Serum uric acid and its association with metabolic syndrome and carotid atherosclerosis in obese children. Eur J Endocrinol 160(1):45–52. doi:10.1530/EJE-08-0618
Cole TJ, Flegal KM, Nicholls D, Jackson AA (2007) Body mass index cut offs to define thinness in children and adolescents: international survey. BMJ 335:194. doi:10.1136/bmj.39238.399444.55
Kułaga Z, Litwin M, Tkaczyk M et al (2010) Polish 2010 growth references for school-aged children and adolescents. Eur J Pediatr 170:599–609. doi:10.1007/s00431-010-1329-x
Cook SS, Weitzman MM, Auinger PP et al (2003) Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med 157:821–827. doi:10.1001/archpedi.157.8.821
Lurbe E, Cífková R, Cruickshank JK et al (2009) Management of high blood pressure in children and adolescents: recommendations of the European Society of Hypertension. J Hypertens 27:1719–1742. doi:10.1097/HJH.0b013e32832f4f6b
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH (2000) Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320:1240–1243
Friedewald WTW, Levy RIR, Fredrickson DSD (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Schwartz GJ, Furth SL (2007) Glomerular filtration rate measurement and estimation in chronic kidney disease. Pediatr Nephrol 22:1839–1848. doi:10.1007/s00467-006-0358-1
Yang J, Liu Z, Zhang C et al (2013) The prevalence of hyperuricemia and its correlates in an inland Chinese adult population, urban and rural of Jinan. Rheumatol Int 33:1511–1517. doi:10.1007/s00296-012-2589-8
Kubota M, Tang L, Nagai A et al (2010) Hyperuricemia in obese children and adolescents: the relationship with metabolic syndrome. Pediatr Rep. doi:10.4081/pr.2010.e12
Perea-Martinez A, Carbajal RL, Rodriguez HR et al (2008) Association of comorbidity with obesity in Mexican children and adolescents. Pediatrics 121:S149–S150. doi:10.1542/peds.2007-2022HHHHHH
Lu JJ-Y, Jiang DD-S, Chou S-M et al (2008) Prevalence of obesity and its association with cardiovascular disease risk factors in adolescent girls from a college in central Taiwan. Kaohsiung J Med Sci 24:144–151. doi:10.1016/S1607-551X(08)70142-6
Wang T, Liu G, Zheng R et al (2009) Serum uric acid and its correlation with cardiovascular risk factors in children with metabolic syndrome. J Clin Pediatr 12:008
Alper ABA, Alper AB, Chen WW et al (2004) Childhood uric acid predicts adult blood pressure: the Bogalusa Heart Study. Hypertension 45:34–38. doi:10.1161/01.HYP.0000150783.79172.bb
Jones DP, Richey PA, Alpert BS, Li R (2008) Serum uric acid and ambulatory blood pressure in children with primary hypertension. Pediatr Res 64:556–561. doi:10.1203/PDR.0b013e318183fd7c
Pereira MA, JACOBS DR, Van Horn L et al (2002) Dairy consumption, obesity, and the insulin resistance syndrome in young adults: the CARDIA study. JAMA 287:2081–2089. doi:10.1001/jama.287.16.2081
Agamah ESE, Webber LSL, Lawrence MM et al (1990) Serum creatinine and its relation to cardiovascular disease risk variables in children and young adults from a biracial community. The Bogalusa Heart Study. J Lab Clin Med 116:327–334
Rathmann W, Funkhouser E, Dyer AR, Roseman JM (1998) Relations of hyperuricemia with the various components of the insulin resistance syndrome in young black and white adults: the CARDIA study. Ann Epidemiol 8:250–261. doi:10.1016/S1047-2797(97)00204-4
Takahashi S, Yamamoto T, Moriwaki Y et al (1995) Increased concentrations of serum Lp(a) lipoprotein in patients with primary gout. Ann Rheum Dis 54:90–93
Ford ES, Li C, Cook S, Choi HK (2007) Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation 115:2526–2532. doi:10.1161/CIRCULATIONAHA.106.657627
Wang J-Y, Chen Y-L, Hsu C-H et al (2012) Predictive value of serum uric acid levels for the diagnosis of metabolic syndrome in adolescents. J Pediatr 161:753–756. doi:10.1016/j.jpeds.2012.03.036
Todorova MK, Ramsheva ZN, Ramshev KN et al (2009) Serum lipids, uric acid and leptin/adiponectin ratio in clinically healthy normal weight and overweight young men, aged 17–20 years. J Mens health 6:63–69. doi:10.1016/j.jomh.2008.09.010
Conen D, Wietlisbach V, Bovet P et al (2004) Prevalence of hyperuricemia and relation of serum uric acid with cardiovascular risk factors in a developing country. BMC Public Health 4:9. doi:10.1186/1471-2458-4-9
Denzer C, Muche R, Mayer H et al (2003) Serum uric acid levels in obese children and adolescents: linkage to testosterone levels and pre-metabolic syndrome. J Pediatr Endocrinol Metab. doi:10.1515/JPEM.2003.16.9.1225
Dawson J, Walters M (2006) Uric acid and xanthine oxidase: future therapeutic targets in the prevention of cardiovascular disease? Br J Clin Pharmacol 62:633–644. doi:10.1111/j.1365-2125.2006.02785.x
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stelmach, M.J., Wasilewska, N., Wicklund-Liland, L.I. et al. Blood lipid profile and BMI-Z-score in adolescents with hyperuricemia. Ir J Med Sci 184, 463–468 (2015). https://doi.org/10.1007/s11845-014-1146-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-014-1146-8