Abstract
Background
Proton pump inhibitors (PPIs) are widely used expensive medications.
Aims
We performed a cross-sectional study to determine the extent and indication of PPI use in Irish acute medical wards.
Methods
Fifty-five medical charts were reviewed at the beginning and end of 1 month.
Results and conclusions
Thirty-three patients were prescribed PPIs; 26 prior to admission. The prescribing of PPIs was concordant with guideline recommendations in only 30% of cases. Two-thirds of PPI use was unlicensed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proton pump inhibitors (PPIs) are expensive and are among the most widely sold drugs in the world. PPIs featured in three places in the top ten products of highest cost in the general medical service (GMS) reimbursement scheme in 2006. Spending on lansoprazole and esomeprazole accounted for €18.6 and €16.4 million, respectively [1].
PPIs are indicated in the short term for treatment of gastric and duodenal ulcers, gastro-oesophageal reflux disease, endoscopically confirmed oesophagitis, NSAID-associated ulcers and Zollinger–Ellison syndrome. They are also used in combination with antibacterials for the eradication of Helicobacter pylori. Patients with endoscopically confirmed erosive, ulcerative, or stricturing oesophagitis or with Barrett’s oesophagus usually need to be maintained on PPI therapy. PPIs act by blocking the hydrogen–potassium adenosine tri-phosphatase enzyme system (the ‘proton pump’) of gastric parietal cells. Though widely used, PPI therapy is not without risk. Common short-term side effects of PPIs include gastrointestinal disturbances, headache and dizziness. More serious, less common side effects include liver dysfunction, interstitial nephritis, blood disorders, and by decreasing gastric acidity a higher risk of gastrointestinal infections [2].
PPI use has also been linked to increased risk of community acquired pneumonia (CAP) and Clostridium difficile [3, 4]. Furthermore, long-term use of PPIs is associated with increased risk of hip fracture, increasing with duration of therapy [5]. They have also been linked to decreased effect of clopidogrel on platelets [6].
The aim of this study was to examine the use of PPIs in acute medical wards and to assess whether this use was appropriate and licensed.
Methods
We conducted a cross-sectional chart review on patients at a major Dublin teaching hospital. We evaluated drug charts and case notes of all patients in the acute medical admissions unit on 27th May 2008. The acute medical admissions unit comprises two wards and there are 60 beds in total. The same case notes and prescription charts were again reviewed 1 month later in order to evaluate changes in patients’ medications. This unit was chosen because it is where most patients are transferred to from the emergency department. It was felt that analysis in this setting would give us a snapshot of a typical patient coming in from the community. In turn, we chose to conduct a second analysis 1 month later in order to gauge the effects of prescribing practices in a tertiary hospital setting. There were no exclusion criteria. Information regarding age, sex, alcohol consumption, investigation with gastroscopy, reason for PPI use and prescription on admission and discharge from hospital was recorded. Excess alcohol was defined as more than 14 units for females and more than 21 units for males. National Institute for Clinical Excellence (NICE) guidelines and the British National Formulary were used to determine if use of PPIs was in line with approved indications [2, 7]. We considered the following as licensed indication for PPI use:
-
Uninvestigated dyspepsia (awaiting OGD);
-
H. Pylori associated peptic ulcer disease;
-
Non-H. Pylori associated gastric and duodenal ulcers;
-
Gastroesophageal reflux disease (OGD proven);
-
Prevention of NSAID-induced ulcers;
-
Upper GI bleeds.
Results
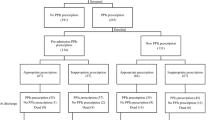
A total of 55 patients participated in the study. The mean age was 68 years and 65% of patients were male (n = 36). Thirty-three (60%) patients were taking a PPI. Esomeprazole accounted for the most frequently prescribed PPI (56%) and the remainder was taking either lansoprazole (33%) or pantoprazole (9%).
GPs had commenced PPI therapy in the case of 26 (79%) patients, while the remainder had the medication commenced during their hospital stay. PPI prescribing was according to accepted BNF guidelines in just 10 (30%) of the patients. The remaining 23 (70%) patients had no clearly documented reason for PPI therapy and had not had endoscopy performed. Sixteen (29%) patients consumed excess alcohol and 13 (81%) of these patients were on a PPI. Use was unlicensed in almost half of this patient group.
Prescription charts and discharge summaries were analysed 1 month later. At this point 27 (82%) of the 33 patients who had been on a PPI had been discharged. Seventy-six per cent of this patient group (n = 25) patients had a PPI included on their list of discharge medications. Five (15%) patients remained as inpatients and one patient had died. All of the inpatients were still taking a PPI. In total, PPI therapy had been discontinued in just 6% of cases.
Discussion
We found that prescription of PPIs was according to current guidelines in only 30% of patients. Our results are similar to previously published studies. Naunton et al. [8] published a paper which reported that 63% of patients receiving a PPI did not fulfil the Australian Schedule of Pharmaceutical Benefits criteria. An Irish study conducted by Sebastian et al. [9] had an identical finding whereby an appropriate indication for PPI therapy was not apparent in 63% of cases. Work carried out by Van Vliet et al. [10] in the Netherlands stated that 40% of patients on PPI therapy did not have a registered indication for use. Research performed in an Irish Midlands hospital by Mat Saad et al. [11] found that one-third of patients were taking PPI medication for an unapproved indication. Considering the increased risk of CAP and C. difficile infection and the proposed decreased effect of other medications and delayed diagnosis of malignancy associated with PPI use, our study shows the need for increased pharmacovigilance when prescribing PPIs. Though small in patient numbers, our results reveal concerning figures.
PPIs are widely used and are generally considered to be safe and well tolerated. However, when prescribing PPIs doctors should be mindful of the indication and the suitability of their use. It has been suggested that PPI therapy may confer a risk of developing C. difficile-associated diarrhoea [3]. PPI therapy may also mask features of gastric cancer [12, 13]. PPI use is also associated with higher risk of developing CAP and hip fracture. Based on our results the PPI-treated patients are generally of an older age and more likely to drink alcohol and therefore already at increased risk of and at higher mortality risk from C. difficile, hip fracture and CAP.
PPIs are expensive. Esomeprazole, lansoprazole and omeprazole all featured in the top ten products of highest cost in the GMS for 2006 [1]. Mouly et al. [14] stated that proton pump inhibitors are third among drug classes in the amount they cost the French health care system annually. Weaknesses associated with our study include the fact that it was small. Follow-up of patients who were discharged into the community was not within the remit of the study, therefore the overall length of time that patients were taking a PPI was not recorded. The main strength of the study was its concordance with other national and international findings. Despite the fact that two other Irish studies have already pointed to inappropriate use of gastric antisecretory therapy, it would seem that there has been little change in prescribing practice. It is unclear why adherence to guidelines does not appear to be followed, however, it has been suggested that one factor may be that physicians may be hesitant to discontinue therapy which has been commenced by another practitioner. A recent article published in Forum (Journal of the Irish College of General Practitioners) addressed difficulties faced by GPs in overcoming barriers to rational prescribing. It was suggested that some GPs feel that there is a lack of feedback relating to prescribing practice. This contrasts with general practice in the UK whereby GPs’ prescribing practices are regularly audited, thus providing feedback in relation to number of prescriptions and costs incurred. [15]
In summary, our study shows a high level of unlicensed PPI therapy. Use of these drugs is not benign and is associated with clinical and financial implications. There is potential to improve prescribing practices in primary, secondary and tertiary settings. We recommend that both hospital and community based physicians should restrict PPI use to those patients with an appropriate indication and adhere to duration of treatment guidelines.
References
HSE Primary Care Reimbursements Service. Statistical analysis of claims and payments 2006
British Medical Association and Royal Pharmaceutical Society of Great Britain. British National Formulary, March 2006, p 48
Cunningham R, Dial S (2008) Is over-use of proton pump inhibitors fuelling the current epidemic of Clostridium difficile-associated diarrhoea? J Hosp Infect 70:1–6
Laheij RJ, Sturkenboom MC, Hassing RJ, Dielman J, Stricker BH, Jansen JB (2004) Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA 292:1955–1960
Yang YX, Lewis JD, Epstein S, Metz DC (2006) Long-term PPI therapy and risk of hip fracture. JAMA 296(24):2947–2953
Gilard M, Arnaud B, Cornily JC et al (2008) Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole Clopidogrel Aspirin) study. J Am Coll Cardiol 51:256–260
National Institute for Clinical Excellence (2000) Guidance on the use of proton pump inhibitors for dyspepsia. http://www.nice.org.uk
Naunton M, Peterson GM, Bleasel MD (2000) Overuse of proton pump inhibitors. J Clin Pharm Ther 25:333–340
Sebastian SS, Kernan N, Qasim A, O’Morain CA, Buckley M (2003) Appropriateness of gastric antisecretory therapy in hospital practice. Ir J Med Sci 172(3):115–117
Van Vliet EP, Otten HJ, Rudolphus A et al (2008) Inappropriate prescription of proton pump inhibitors on two pulmonary medicine wards. Eur J Gastroenterol Hepatol 20(7):608–612
Mat Saad AZ, Collins N, Lobo M, O’Connor HJ (2005) Proton pump inhibitors: a survey of prescribing in an Irish General hospital. Int J Clin Pract 59(1):31–34
Reilly JP (1999) Safety profile of the proton-pump inhibitors. Am J Health Syst Pharm 56(23 Suppl 4):S11–S17
Laine L, Ahnen D, McClain C et al (2000) Review article: potential gastrointestinal effects of long-term acid suppression with proton pump inhibitors. Aliment Pharmacol Ther 14:651–668
Mouly S, Charlemagne A, Le Jeunne P, Fagnani F (2008) General practitioners’ management of gastroesophageal reflux in France in 2005: a pharmacoeconomic study. Presse Med 37(10):1397–1406
Ryan G, Glynn L (2009) Overcoming barriers to rational prescribing. Forum J ICGP 26(2):40–42
Acknowledgments
We would like to thank Dr. Murali Sayana, SpR, Department of Orthopaedic Surgery, Waterford Regional Hospital for the valuable suggestions in the writing of this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
D. Molloy and A. Molloy contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Molloy, D., Molloy, A., O’Loughlin, C. et al. Inappropriate use of proton pump inhibitors. Ir J Med Sci 179, 73–75 (2010). https://doi.org/10.1007/s11845-009-0426-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-009-0426-1