Abstract
In order to meet the demands of humanity and address the global environmental situation, it is imperative that we explore alternative energy sources and advance energy storage technology. The aim of this study was to investigate the impact of Nd-doping on the structural and electrochemical performance of FeAl2O4 nanoparticles (NPs). The successful synthesis of Nd-doped FeAl2O4 NPs) was accomplished through a simple sonication process. An evaluation was conducted on the properties of Nd-doping FeAl2O4 NPs) to determine their suitability for supercapacitor (SC) applications. Moreover, the specific capacitance (Cs) of Nd-doped FeAl2O4 NPs) reaches a maximum of 1194.69 F g−1 when subjected to a current density of 1.0 A g−1 compared to FeAl2O4 nanoparticles. Furthermore, Nd-doped FeAl2O4 NPs exhibited excellent cyclic stability and low impedance (Rct = 0.07 Ω), owing to their modified morphology, making a promising material for supercapacitor SC electrodes that offer high capacity, affordability, and environmental friendliness. Our research has validated that the synthesized material can enhance the capacitive properties of transition-metal oxides with spinel structures in new, generated energy storage devices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There has been a rise in global awareness regarding the importance of energy and the significance of preserving a sustainable environment due to the increasing reliance on fossil fuels.1,2,3,4 There is a growing need for energy systems that are both environmentally friendly and economically viable in the long run. There has been a surge in interest in alternative options, like fuel cells, tidal energy, solar energy, and wind energy.5,6,7 However, to guarantee a consistent and dependable power supply from these systems, it is crucial to establish a highly effective storage system. Supercapacitors (SCs) and lithium-ion batteries (LIBs) have been extensively acknowledged as highly promising electrical energy storage technologies. Despite their impressive gravimetric energy density, LIBs still face specific challenges.8,9,10,11 There are certain factors that affect their performance, including a shorter lifespan, lower energy output, and a slower discharge of stored energy. SCs) have garnered significant attention owing to impressive durability, rapid charge and discharge abilities, exceptional power density, and ability to withstand challenging environments. Furthermore, SCs as electrochemical capacitors act as a bridge between LIBs and regular capacitors.12,13,14,15 LIBs have high specific energy but low specific power, and regular capacitors have high power density but poor energy storage. That is why SCs offer a solution that combines both high power and energy storage capabilities. SCs also have a demonstrated remarkable efficiency in providing power for an extensive range of applications, such as power backup systems, hybrid electronic cars, and portable electronics.16,17,18,19,20
In SCs, there are two main groups that may be identified by the way they store charge. They are electrochemical double-layer capacitors that work by capturing electrostatic charges at electrode–electrolyte contact, including graphene and activated carbon materials, and pseudocapacitors (PCs) that employ redox reactions to store charge, such as conducting polymers and transition metal oxide (TMOs).21,22,23,24 However, it was discovered that the energy capacity of SCs is lower compared to LIBs and capacitors. Due to this factor, various electrode materials have been discovered to improve the specific energy of SCs. Recently, there has been much attention paid to the investigation of TMOs with various oxidation states. Because of their low cost, extensive availability, and malleability in terms of both structure and morphology, these oxides are garnering a lot of research interest. Additional characteristics include their high specific capacitance (Cs) and environmental friendliness.25,26,27 For this reason, they are being intensively examined as potential SC electrode materials. There has also been a lot of interest in binary TMOs, such as FeAl2O4,28 CuFe2O4,29 ZnMn2O4,30 NiCo2O4,31 and CoFe2O4,28 as they show promise as electrode materials for PCs. However, FeAl2O4 is a standout choice among the various options available for TMOs. However, the TMO FeAl2O4’s wide band gap and poor electric conductivity restrict its potential for electrochemical applications. Effective control of the electrical characteristics is crucial for optimizing the electrochemical properties, which can be accomplished through proper doping procedures. The presence of doped metal ions in TMO composites has led to the creation of multiple electrochemical active sites.29,30 In addition, the deliberate inclusion of impurities has a notable effect on the number of charge carriers by producing free charge carriers. Recent investigation has shown that the inclusion of the specific impurity of rate-earth ions can significantly improve the electrical, magnetic, and electrochemical characteristics of TMOs.31,32 This research aims to enhance the electrochemical behavior of these composites for high-performance SCs. This is a result of the strong synergistic behavior exhibited by the metal ions, which ultimately leads to an increase in capacitance values compared to TMOs.33 Extensive studies have demonstrated that doped TMOs and rare-earth elements possess enhanced electrical and supercapacitor properties. For example, Bhujun et al.,’s studied the effect of Al-doped spinel ferrite, which exhibited high electrical conduction with a Cs of 548 Fg−1 at 100 mV s−1 with an Ed of 3.84 Wh kg−1 and a specific power of 270 Wh kg−1.34 Monohar et al., synthesized nickel-doped spinel which exhibited a Cs of 119.04 F g−1 at 1.1 A g−1.35 Alahmari et al., prepared Zn-doped CrVO4 which exhibited a Cs of 448 F g−1 at 1 mA and an Ed of 54.5 Wh kg−1 at a Pd of 1350 W kg−1 and a notable efficiency of 99% at the 10,000th cycles.36
The synthesis process utilizes Nd as a dopant to modify the morphology and enhance the electrochemical properties. A sonication method was used to prepare binder-free electrodes with Nd-doped FeAl2O4 active materials grown on conductive Ni foam (NF) substrate. An investigation was conducted to observe the effect of the Nd doping on the performance of electrodes. It is fascinating to observe that the doping of Nd during the sonication preparation alters the morphology of the electrode and incorporates it into the FeAl2O4 lattice. This leads to the formation of a Nd-doped FeAl2O4 electrode, which exhibits a significant enhancement in capacitive behavior.
Materials and Methods
Reagents
Iron nitrate (Fe(NO3)2·9H2O, Merk, ≥ 98.99%).), aluminum nitrates (Al(NO3)3·9H2O, Merk, ≥ 98.997%).), neodymium nitrate Nd(NO3)3·9H2O, Merk, ≥ 99.9%).), sodium hydroxide (NaOH), deionized water (DI H2O), and ethanol were utilized as the initial materials. All chemical reagents used in the study were of high quality and used as received.
Preparation of FeAl2O4 and Nd-Doped FeAl2O4 Nanoparticles (NPs)
The synthesis of FeAl2O4 and Nd-doped FeAl2O4 NPs was carried out using a probe sonicator through sonochemical methods. For the experiment, a 0.1-M solution of Fe(NO3)2·9H2O and 0.2 M Al(NO3)3·9H2O were synthesized by dissolving in DI H2O. To achieve a pH level of 11, a 3.0-M NaOH solution was gradually introduced as a precipitation reagent. An ultrasonic probe, controlled by a microprocessor, was used to subject the contents of the beaker to high-intensity ultrasound for a continuous duration of 60 min. As a result of increased molecular collisions, the temperature gradually reached 80°C throughout the process. The resulting precipitate underwent filtration and was subsequently washed with DI H20. Afterwards, it went through a drying process for 4 h at 60°C in an electric oven. The final solid product was carefully heated in a furnace for a period of 5 h at a temperature of 450°C.
Figure 1 shows the synthesis of Nd-doped FeAl2O4 NPs for which the procedure was carried out in the same manner, with the addition of 0.1 M Nd(NO3)3·9H20 as a dopant.
Material Characterizations
X-ray diffraction (XRD; D/max 2500 PC) diffractograms were attained for structural analysis. Raman spectrometry (JY-HR800) was used to analyze the spectral range of 100–900 cm−1 for vibration modes in the crystal. Scanning electron microscopic (SEM; SUPRA-55, JEM-2100) images were recorded with energy-dispersive X-ray analysis (EDX) for elemental composition. Brunauer–Emmett–Teller (BET) and specific surface area analyses were carried out with a TriStar3020 N2 adsorption/desorption analyzer.
Electrode Assembly and Electrochemical Test
To prepare the electrodes, the material (0.005 g) was sonicated in DI water to create a paste, which was applied onto NF measuring 1.5 × 1.5 cm2. Subsequently, the electrodes were dried at 60°C to remove any remaining solvents. The electrochemical characteristics were measured utilizing an AUTOLAB PGSTAT-204 workstation in a three-electrode system. The electrodes were composed of platinum wire, Ag/AgCl, and the synthesized material. Numerous electrochemical tests, including cyclic voltammetry (CV) and galvanostatic charge–discharge (more), were measured in 2.0 M KOH electrolyte in an alkaline environment. The CV was conducted at different scan rates (5, 10, 20, and 30 mV s−1) while maintaining a potential of 0.0–0.75 V. However, the Cs was measured using:
where m is the mass placed (0.005 g) on the NF, potential (∆V) is the voltage, and s is the sweeping rate, which indicates the speed at which the experiment is being conducted.
The measurement of Cs at current density (1–3 A g−1) by GCD involved using:
where I is the applied current, ∆t the discharge time, m the loaded mass, and ΔV a change in potential.
Furthermore, Eqs. 3 and 4 can be used to measure the specific energy (Ed) (Wh kg−1) and specific power (Pd) (W kg−1), respectively.
The impedance characteristics were conducted using electrochemical impedance spectroscopy (EIS) in the range of 0.1–100 kHz.
Results and Discussion
Evaluation of Structural Analysis
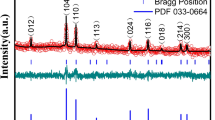
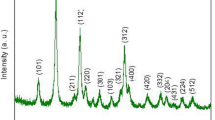
An investigation of crystal structure of the synthesized material was carried out by XRD, Fig. 2a shows the XRD patterns of FeAl2O4 and Nd-doped FeAl2O4 NPs. The diffraction peaks of FeAl2O4 matched with the standard cubic structure (Fd-3m space group), indicated by JCPDS: 01-086-2320. The peaks observed at specific values of 18.73°, 30.98°, 36.17°, 44.34°, 48.61°, 55.07°, 58.79°, and 64.30° correspond to the lattice planes (111), (2 2 0), (3 1 1), (4 0 0), (331), (4 2 2), (5 1 1), and (4 4 0), respectively. In the XRD pattern of Nd-doped FeAl2O4 NPs, a slight shifting of peaks was observed which indicates that the Nd3+ ion was likely doped into the Fe2+ and Al3+ sites with strong and distinct peaks, indicating a single-phase and highly crystalline nature. The average crystallite size (D) of the FeAl2O4 and Nd-doped FeAl2O4 NPs was measured as 28.55 Å and 16.56 Å, respectively, utilizing the Deby–Scherrer equation:
where k is a constant, 0.9, λ the wavelength, β (the fullwidth at half-maximum, and θ the Bragg diffraction angle.
The variation in the results can be described by the disparity in ionic radii of Nd3+ (1.12 Å), Fe2+ (0.83 Å), and Al3+ (0.57 Å). It was noted that the average crystallite size decreased and that there was a slight shift in peaks, indicating the successful formation of Nd-doped FeAl2O4 NPs.37 Figure 2b displays Raman spectra of the FeAl2O4 and Nd-doped FeAl2O4 NPs acquired at room temperature. In the FeAl2O4 material, distinct peaks at approximately 91.44 cm−1, 217.53 cm−1, 346.66 cm−1, 564.64 cm−1, 669.18 cm−1, and 736.13 cm−1 can be observed, indicating the presence of acoustic combinations. The peak at 91.44 cm−1 corresponds to the transverse acoustic mode (2TA). Further, a peak emerges at 217.53 cm−1 with A1 symmetry, complementing the existing 2TA mode, and a peak is observed at 346.66 cm−1 (E1 symmetry), indicating the existence of the E1 (TO) mode. Further peaks at 564.64 cm−1, 669.18 cm−1, and 736.13 cm−1 correspond to specific symmetries and modes. These include A1 symmetry with M − K points for the first peak and combinations of transverse acoustic and transverse optical (TA + TO) with H and M points and longitudinal acoustic and transverse optical (LA + TO) with an M point for the second and third peaks, respectively.38 In addition, including the Nd dopant caused a slight decrease in the wavenumbers of the vibrational modes of FeAl2O4. It is worth noting that there was an observed increase in peak intensity in the Nd-doped FeAl2O4 NPs, which suggests that Nd ions have been successfully incorporated into the FeAl2O4 structures.
Figure 3a, b exhibits SEM images of FeAl2O4 and Nd-doped FeAl2O4 NPs. The FeAl2O4 images (Fig. 3a) reveal NPs with an irregular shape and a certain degree of aggregation. In addition, the Nd-doped FeAl2O4 sample (Fig. 3b) demonstrates enhanced NP morphology with reduced aggregation, leading to improved electrochemical activities. In addition, the elemental composition was evaluated by EDX investigation in Fig. 3c, d. This analysis of FeAl2O4 (Fig. 3c) revealed the existence of Fe, Al, C, and O elements with no impurity peaks, indicating the high purity of the material. Further, the Nd-doped FeAl2O4 NPs displayed in Fig. 3d show the presence of Nd, Fe, Al, C, and O elements in appropriate amounts. This observation validates the inclusion of Nd within the FeAl2O4 lattice structures.
To analyze the surface area (SA) of the FeAl2O4 and Nd-doped FeAl2O4 NP material, the BET measurement technique was employed. Figure 4 illustrates isotherms of FeAl2O4 and Nd-doped FeAl2O4 NPs which shows a type III isotherm with a mesoporous structure.31 By analyzing the N2 adsorption/desorption isotherm, it was discovered that the SAs of FeAl2O4 and Nd-doped FeAl2O4 NPs were 79.47 m2 g−1 and 111.15 m2 g−1, respectively. The unique structure of Nd-doped FeAl2O4 NPs with a large SA allows for enhanced accessibility of electrolyte ions and facilitates efficient intercalation and de-intercalation processes, resulting in enhanced electrochemical capacities of Nd-doped FeAl2O4 NPs.
Electrochemical Measurements
The electrochemical properties of the FeAl2O4 and Nd-doped FeAl2O4 NPs electrodes analysis were involved, using CV in a conventional three-electrode system. The CV curves for the FeAl2O4 and Nd-doped FeAl2O4 NPs electrode materials with a potential of 0.0–0.75 V at scan rates of 5 mV s−1, 10 mV s−1, 20 mV s−1, and 30 mV s−1 are shown in Fig. 5a, b. From the CV curves, it can be observed that the current steadily increases as the scan rate rises. This suggests a direct relationship between voltametric current and scan rate. In addition, distinctive peaks of the cyclic voltammograms of the FeAl2O4 and Nd-doped FeAl2O4 NPs electrodes were the result of the redox reaction contributing to the pseudocapacitive nature suggesting a straightforward and reversible oxidation and reduction process.33 Surprisingly, the Nd-doped FeAl2O4 NPs electrode material exhibited robust redox peaks with a larger loop area, potentially indicating a stronger synergistic consequence between the Nd, Fe, and Al ions. These features have the potential to expand the performance of spinel-structured electrodes in electrochemical analysis.
The Cs of the FeAl2O4 and Nd-doped FeAl2O4 NPs electrodes was determined utilizing Eq. 1, which exhibited 588.78 F g−1 and 908.59 F g−1, respectively, at 5 mV s−1. The enhanced Cs of the Nd-doped FeAl2O4 NPs electrode can be explained by the strong redox peaks observed, suggesting a larger active area and extra electroactive sites, which allow for better contact and accessibility to aqueous electrolyte ions during the analysis. When the scan rate was increased, the redox peaks moved towards extreme (high and low) potentials (Fig. 5c), suggesting that there was a higher diffusion resistance for ions inside the electrode.39 In addition, a graph between the Cs of the FeAl2O4 and Nd-doped FeAl2O4 NPs electrodes versus scan rate i shown in Fig. 5d. The Cs value declined as the scan rate increased. Interestingly, when the scan rate was increased, diffusion of the electrolyte ions was hindered by internal resistance and polarization. In contrast, as the scan rate was reduced, active sites on both the inner and outer interfaces of the material contributed to providing a comparatively developed Cs.40
To gain a deeper insight into the behavior of the FeAl2O4 and Nd-doped FeAl2O4 NPs electrodes for supercapacitor (SC) applications, we conducted GCD measurements. Fig. 6a, b displays GCD curves at 1–3 A g−1 compared to Ag/AgCl for the FeAl2O4 and Nd-doped FeAl2O4 NPs electrodes. It is worth mentioning that the Nd-doped FeAl2O4 NPs electrode has a longer discharge time compared to FeAl2O4 electrodes. These findings indicate that the enhanced capacitive behavior was due to the electrode’s greater surface area and the presence of a mesoporous structure. Additionally, the Cs of the FeAl2O4 and Nd-doped FeAl2O4 NPs electrodes was analyzed using Eq. 2. It is worth noting that the Nd-doped FeAl2O4 NPs electrode reveals a Cs of 1194.69 F g−1, significantly surpassing that of FeAl2O4 (697.73 F g−1) at 1 A g−1. The GCD profile showed that, as the current density rises, the discharge time decreases with a decreasing trend of Cs (Fig. 6c). This suggests that, at 3 A g−1 current density, entirely active sites were not available for electrolyte ions to carry out electrochemical reactions.41 The specific power (Pd) and specific energy (Ed) were derived from the GCD curve by Eqs. 3 and 4, as shown in Fig. 6d. However, Nd-doped FeAl2O4 NPs exhibit a maximum specific power of 313 W kg−1 and specific energy of 65 Wh kg−1 at 1 A g−1. The Cs at different current densities with the electrolytes achieved in this study and reported for previous doped spinel-based electrodes are displayed in Table I.
The synthesized material underwent analysis using EIS and Nyquist plots (Fig. 7a) of the FeAl2O4 and Nd-doped FeAl2O4 NPs electrodes clearly demonstrating a comprehensive examination of the internal resistive characteristics between the electrolyte and the electrode. At higher frequencies, the intercept reveals the presence of electrolyte resistance (Rs) that emerges from the interaction of ionic and electrical components. The size of the semi-circle reflects the level of resistance that occurs when a charge is transferred (Rct) between the electrode and the electrolyte. Rct includes the resistance that occurs within particles themselves, as well as the resistance that occurs between the particles and the current collector. The Rs value for the FeAl2O4 (1.63 Ω) and Nd-doped FeAl2O4 NPs electrodes was found to be 0.9 Ω. The Nd-doped FeAl2O4 NPs electrode exhibited a low Rct value of 0.07 Ω, while the FeAl2O4 electrode had 0.12 Ω. These results suggest that the Nd-doped FeAl2O4 NPs electrode exhibited very low levels of charge transfer and electrolyte resistance. This feature enhanced the conductivity between the electrode and electrolyte, making it easier to insert and remove ions from the electrode surface.47 The chronoamperometric method was employed to assess the stability and electrochemical performance of the Nd-doped FeAl2O4 NPs electrode. From Fig. 7b, it is obvious that the current density of the Nd-doped FeAl2O4 NPs electrode initially decreased, with the drop rate being significant in the earlier period, but, later on, it exhibited a stable behavior for 60 h. This indicates that the Nd-doped FeAl2O4 NPs electrode had a highly responsive electrochemical behavior and stable electrochemical properties. In order to ascertain the longevity of the supercapacitor, it was crucial to examine its cyclic stability and reversibility. The cyclic stability of the electrode made of Nd-doped FeAl2O4 NPs was evaluated (Fig. 7c) by subjecting it to 5000 cycles at 5 mVs−1.The image clearly demonstrates that, even after the 5000th CV cycle, no significant changes were observed. The results demonstrate exceptional durability throughout extended cycling. Additionally, it was clear from the XRD pattern (Fig. 7d) that the phase and structure of the Nd-doped FeAl2O4 NPs electrode material remained intact, which suggests that there was an increased level of electrolyte ion transport within the active electrode material.8
Conclusion
An efficient technique involving sonication was employed to successfully synthesize Nd-doped FeAl2O4 nanoparticles (NPs), which can be employed in energy storage applications. The electrochemical measurements of the NPs was investigated and found to exhibit a substantial enhancement in the specific capacitance (Cs) of 1194.69 F g−1 at 1 A g−1 and greater specific energy (65 Wh kg−1) and specific power (313 W kg−1). The Nd-doped FeAl2O4 NPs electrode showed a remarkable reduction in impedance characteristics (Rs = 0.9 Ω, Rct = 0.07 Ω) while maintaining an exceptional cycle stability of 60 h after 5000 cycles. Therefore, the Nd-doped FeAl2O4 NPs electrode exhibits immense potential as material for supercapacitor (SC) applications due to its cost-effective precursor, energy-efficient synthesis process, and outstanding electrochemical performance.
Data Availability Statements
The author will make the datasets generated and/or analyzed during the current work available upon reasonable request.
References
M. Hussain, S.D. Alahmari, F.F. Alharbi, S.R. Ejaz, M. Abdullah, S. Aman, A.G. Al-Sehemi, A.M.A. Henaish, A. Sadaf, and H.M.T. Farid, J. Energy Storage 80, 110289 (2024).
S. Khan, M. Usman, M. Abdullah, M. Suleman Waheed, M. Faheem Ashiq, M. Ishfaq Ahmad, A.M. Karami, M. Fahad Ehsan, S. Manzoor, and M. Naeem Ashiq, Fuel 357, 129688 (2024).
A.M. Alenad, N. Ahmad, S. Aman, M. Noman Saeed, H.M.T. Farid, and T.A.M. Taha, J. Taibah Univ. Sci. 17, 2231132 (2023).
F. Qi, X. Lu, Y. Wang, H. Zhang, A. Trukhanov, and Z. Sun, J. Colloid Interface Sci. 607, 1253 (2022).
M. Hayat, M. Abdullah, K. Jabbour, N. Bibi, S. Khan, B. Ali, A.M. Karami, M.F. Ehsan, and M.N. Ashiq, Mater. Chem. Phys. 310, 128436 (2023).
F. Qi, L. Shao, X. Shi, F. Wu, H. Huang, Z. Sun, and A. Trukhanov, J. Colloid Interface Sci. 601, 669 (2021).
M. Hussain, M.M. Alanazi, S.A.M. Abdelmohsen, S.D. Alahmari, M. Abdullah, S. Aman, A. Dahshan, A.M.A. Henaish, Z. Ahmad, and H.M.T. Farid, J. Energy Storage 84, 110920 (2024).
M. Ali, S.D. Alahmari, S.A.M. Abdelmohsen, M.M. Alanazi, A.G. Al-Sehemi, M. Abdullah, S. Aman, and H.M.T. Farid, Ceram. Int. 50, 6931 (2024).
S. Ahmad, K. Jabbour, M. Rafeeq, A. Naz, K.F. Fawy, S. Manzoor, M. Abdullah, N. Ghazouani, A. Mir, and M.N. Ashiq, Ceram. Int. 49, 28036 (2023).
S. Aman, S. Gouadria, F. F. Alharbi, M. N. Saeed, and H. M. T. Farid, JOM 1 (2023).
S. Wang, L. Shao, L. Yu, J. Guan, X. Shi, Z. Sun, J. Cai, H. Huang, and A. Trukhanov, Energy Technol. 9, 2100298 (2021).
X. Zhang, Y. Tang, F. Zhang, and C.-S. Lee, Adv. Energy Mater. 6, 1502588 (2016).
M. Wang, C. Jiang, S. Zhang, X. Song, Y. Tang, and H.M. Cheng, Nat. Chem. 10, 667 (2018).
Y. Zhang, H. Gao, J. Wang, Q. Chi, T. Zhang, C. Zhang, Y. Feng, Y. Zhang, D. Cao, and K. Zhu, Inorg. Chem. Front. 11, 1289 (2024).
X. Zhao, B. Fan, N. Qiao, R.A. Soomro, R. Zhang, and B. Xu, Appl. Surf. Sci. 642, 158639 (2024).
S. Guan, J. Zhou, S. Sun, Q. Peng, X. Guo, B. Liu, X. Zhou, and Y. Tang, Adv. Funct. Mater. 2314890 (2024).
R. Yang, W. Yao, L. Zhou, F. Zhang, Y. Zheng, C. S. Lee, and Y. Tang, Adv. Mater. 2314247 (2024).
M. Abdullah, F.F. Alharbi, R.Y. Khosa, H.A. Alburaih, S. Manzoor, A.G. Abid, H.E. Ali, M.S. Waheed, M.N. Ansari, and H.M.T. Farid, Korean J. Chem. Eng. 40, 1518 (2023).
N.A. Althubiti, M.M. Hassan, M.S. Waheed, S. Aman, R.Y. Khosa, H.M.T. Farid, A. Nazir, M.Z. Ansari, F. Abdulaziz, and T.A.M. Taha, J. Energy Storage 70, 108154 (2023).
S. Manzoor, S.V. Trukhanov, M.N. Ansari, M. Abdullah, A. Alruwaili, A.V. Trukhanov, M.U. Khandaker, A.M. Idris, K.S. El-Nasser, and T.A. Taha, Nanomater. 12, 2209 (2022).
M. Hussain, B.M. Alotaibi, A.W. Alrowaily, H.A. Alyousef, M.F. Alotiby, M. Abdullah, A.G. Al-Sehemi, A.M.A. Henaish, Z. Ahmad, and S. Aman, J. Phys. Chem. Solids 188, 111919 (2024).
S. Mu, Q. Liu, P. Kidkhunthod, X. Zhou, W. Wang, and Y. Tang, Natl. Sci. Rev. 8 (2021).
Z. Huang, P. Luo, S. Jia, H. Zheng, and Z. Lyu, J. Phys. Chem. Solids 167, 110746 (2022).
M. Hussain, M.M. Alanazi, S.A.M. Abdelmohsen, S.D. Alahmari, M. Abdullah, S. Aman, A.G. Al-Sehemi, A.M.A. Henaish, Z. Ahmad, and H.M.T. Farid, Diam. Relat. Mater. 142, 110764 (2024).
D.A. Alshammari, Y.M. Riyad, S. Aman, N. Ahmad, H.M. Tahir Farid, and Z.M. El-Bahy, J. Electroanal. Chem. 945, 117701 (2023).
A.G. Abid, S. Gouadria, S. Manzoor, K.M.S. Katubi, K. Jabbour, M. Abdullah, M. Un Nisa, S. Aman, M.S. Al-Buriahi, and M.N. Ashiq, Fuel 336, 127066 (2023).
S.A. Habib, S.A. Saafan, T.M. Meaz, M.A. Darwish, D. Zhou, M.U. Khandaker, M.A. Islam, H. Mohafez, A.V. Trukhanov, S.V. Trukhanov, and M.K. Omar, Nanomater. 12, 931 (2022).
T. Zahra, M.M. Alanazi, S.D. Alahmari, S.A.M. Abdelmohsen, M. Abdullah, S. Aman, A.G. Al-Sehemi, A.M.A. Henaish, Z. Ahmad, and H.M. Tahir Farid, Int. J. Hydrogen Energy 59, 97 (2024).
H. Donya, S. Aman, N. Ahmad, H. M. Tahir Farid, and T. A. Mohaymen Taha, Int. J. Hydrogen Energy (2023).
M. Abdullah, M.Z. Ansari, Z. Ahamd, P. John, S. Manzoor, A.M. Shawky, H.H. Hegazy, A.H. Chughtai, M.N. Ashiq, and T.A. Taha, Ceram. Int. 49, 6780 (2023).
N.A. Althubiti, S. Aman, and T.A.M. Taha, Ceram. Int. 49, 27496 (2023).
T. Tao, J. He, Y. Wang, X. Shi, L. Shao, A. Trukhanov, and Z. Sun, J. Power. Sources 539, 231457 (2022).
X. Li, S. Aftab, S. Hussain, F. Kabir, A.M.A. Henaish, A.G. Al-Sehemi, M.R. Pallavolu, and G. Koyyada, J. Mater. Chem. A 12, 4421 (2024).
Z. Huang, P. Luo, Q. Wu, and H. Zheng, J. Phys. Chem. Solids 161, 110479 (2022).
X. Li, S. Aftab, A. Abbas, S. Hussain, M. Aslam, F. Kabir, H.S.M. Abd-Rabboh, H.H. Hegazy, F. Xu, and M.Z. Ansari, Nano Energy 118, 108979 (2023).
T. Wei, Y. Zhou, C. Sun, X. Guo, S. Xu, D. Chen, and Y. Tang, Nano Res. 1 (2023).
M. K. Tufail, P. Zhai, M. Jia, N. Zhao, and X. Guo, Energy Mater. Adv. 4 (2023).
S. Aman, N. Ahmad, M.B. Tahir, M.M. Alanazi, S.A.M. Abdelmohsen, R.Y. Khosa, and H.M.T. Farid, Surf. Interfaces 38, 102857 (2023).
G. Liu, Y. Yang, X. Lu, F. Qi, Y. Liang, A. Trukhanov, Y. Wu, Z. Sun, X. Lu, and A.C.S. Appl, Mater. Interfaces 14, 31803 (2022).
R.S. Gohar, S. Manzoor, T. Munawar, S. Gouadria, M.F. Ashiq, F. Iqbal, F. Aftab, M. Najam-Ul-Haq, A.V. Trukhanov, and M.N. Ashiq, J. Energy Storage 52, 104930 (2022).
V.S. Kumbhar, A.D. Jagadale, N.M. Shinde, and C.D. Lokhande, Appl. Surf. Sci. 259, 39 (2012).
C. Wang, P. Shi, C. Guo, R. Guo, and J. Qiu, J. Electroanal. Chem. 956, 118072 (2024).
B. Liu, X. Wang, Y. Chen, H. Xie, X. Zhao, A.B. Nassr, and Y. Li, J. Energy Storage 68, 107826 (2023).
Z. Huang, Y. Zhang, H. Wang, and J. Li, Appl. Phys. Lett. 123 (2023).
M. Abdullah, N. Alwadai, M. Al Huwayz, S. Manzoor, P. John, A.G. Abid, M.I. Ghouri, S. Aman, M.S. Al-Buriahi, and M.N. Ashiq, Energy Fuels 37, 1297 (2023).
D. Alhashmialameer, S. Aman, M. Abdullah, R. Y. Khosa, S. Manzor, H. M. Ali, M. H. Helal, H. M. T. Farid, M. S. Waheed, and T. A. Taha, J. Sol-Gel Sci. Technol. 1 (2022).
Z. Liang, F. Du, N. Zhao, and X. Guo, Chin. J. Struct. Chem. 42, 100108 (2023).
Acknowledgements
The Deanship of Scientific Research at King Khalid University is greatly appreciated for funding (R.G.P-1/356/44). The authors express their gratitude to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R132), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. A.M.A. Henaish thanks the the Ministry of Science and Higher Education of the Russian Federation (Ural Federal University Program of Development within the Priority-2030 Program) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
Every author has made an equal contribution.
Corresponding author
Ethics declarations
Competing interests
This work has no conflicts of interest of any kind.
Ethical Approval
The ethical rules of the journal are adhered to by this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hussain, M., Alanazi, M.M., Abdelmohsen, S.A.M. et al. Enhanced Supercapacitive Performance of FeAl2O4 Nanoparticles with Neodymium (Nd) Doping by Sonication Method. JOM 76, 3185–3194 (2024). https://doi.org/10.1007/s11837-024-06518-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-024-06518-1