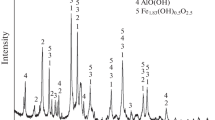
Abstract
Sodium aluminosilicate hydrate is the main equilibrium solid phase of Bayer red mud. Owing to the alkaline insoluble phase, the saltpetering of Bayer red mud tends to occur over time, even after washing procedures. This prevents the large-scale utilization of Bayer red mud for the production of cement, brick, subgrade materials, etc. In this study, a new type of red mud (C-C residue), structured through calcification–carbonization treatment, was used to produce cement clinker. The mineral transformation was studied and the phase, micromorphology, chemical composition, f-CaO content, bending strength and compressive strength of the clinker were characterized. The results show the transformation of C-C residue into the effective components (Ca3SiO5, Ca2SiO4, Ca2FexAl2–xO5 and Ca3Al2O6) of the cement clinker. The chemical composition, f-CaO content and cement strength met the Chinese national standards. Therefore, sintering C-C residue provides a promising solution to the problem of red mud stockpiling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Presently, the Bayer process is the main method of producing alumina. The bauxite residue produced after high-temperature alkali leaching during the Bayer process is called Bayer red mud.1 For each ton of alumina produced, 1.0–1.5 tons of Bayer red mud is produced2 from different sources and different processing parameters.3,4 Figure 1 describes the phase compositions of the typical red mud produced by four alumina plants in China. It can be seen that Bayer red mud is mainly composed of sodium aluminosilicate (hydrate), grossular (sometimes partially replaced by Fe), dawsonite (sometimes partially replaced by Ca) and hematite. Composite oxides containing sodium with different structures are numerous in the equilibrium solid phase of Bayer red mud.
XRD patterns of traditional Bayer red mud from different places. (a, b, c, d) represent four different red mud samples. Their original places, types and chemical compositions are shown in Table I
The strong alkalinity of Bayer red mud hinders its large-scale utilization.5,6 The strong alkalinity is partly caused by the alkaline solution used in the Bayer system and carried by the red mud. This alkali part is soluble and can be removed by enhanced washing. However, the alkali within the solid structure, mainly composed of sodium aluminosilicate hydrate, remains after washing procedures, thus posing a significant disadvantage.7,8 After a long period of time, this sodium aluminosilicate hydrate forms a sodium balance with the external environment. This is known as red mud saltpetering1,9 (Fig. S1 of the supplementary material shows the saltpetering phenomenon of Bayer red mud). This restricts the large-scale and rapid utilization of Bayer red mud in cement, brick and other building materials or pavement base materials.10,11 Although several studies on the preparation of cement or other comprehensive utilization of red mud have been done locally and abroad,12,13,14,15 the alkali content of Bayer red mud still greatly limits the red mud dosage in raw materials. To solve the solid alkali problem in red mud completely, the structural transformation of the equilibrium solid phase is needed.
Many research institutes (including the Northeastern University16,17,18 and Central South University19,20) have done research on the transformation of sodium aluminosilicate hydrate. First, the sodium aluminosilicate hydrate is completely converted into hydrogarnet by adding sufficient amounts of lime, and the solid alkali enters the solution during calcification to implement alkali recycling. Then, the calcified residue, with hydrogarnet as the main phase, is decomposed into calcium silicate, calcium carbonate and aluminum hydroxide by high-pressure CO2 in the carbonization process. The aluminum hydroxide was recovered from the carbonized residue by the leaching of low-concentration lye. The final residue after washing is a new type of red mud with calcium silicate and calcium carbonate as its main components, theoretically. Because of the mineral complexity of the original red mud, the actual phases and the basic chemical compositions of the final residue (C-C residue) are shown in Fig. 2 and Table I (rest materials, such as H2O, are not included in the percentages). It can be seen that the total Na2O content of the red mud can be reduced to < 1% through the structural transformation of calcification–carbonization treatment. The C-C residue almost met the alkali content requirement in the production of Portland cement. Figure S1 of the supplementary material compares the aspect of the Bayer red mud with the C-C residue after a period of time. The Bayer red mud suffers a serious saltpetering phenomenon, while the C-C residue does not.
Presently, the main raw materials for the production of Portland cement include calcareous raw materials (providing CaO) and aluminosiliceous raw materials (mainly providing alumina, silica and partly ferric oxide). Limestone is a major calcareous raw material being mined and used in great quantities. Faced with the resource crisis of limestone, China has increased the taxation of limestone resources and incorporated it into the management of the land department. The cement industry must find new sources of calcium to achieve sustainable development. In C-C residue, the CaO content is generally high, reaching > 30% irrespective of the ignition loss. After high-temperature sintering, the carbonate in the C-C residue is decomposed, and CO2 and H2O are removed. As a result, the CaO content can reach > 60%, which satisfies the requirement of the common Portland cement clinker (62–67%). The C-C residue also contains SiO2, Al2O3 and Fe2O3, which meets the basic composition conditions for sintering cement.21 Moreover, red mud exhibits certain agglutinability.22,23,24,25 If the minerals in the C-C residue can transform into the effective minerals of the cement clinker (such as Ca3SiO5, Ca2SiO4, Ca3Al2O6, Ca2FexAl2–xO5) through the sintering process and ensure a certain strength, the red mud will be reused as mineral resources, and the pollution caused by red mud storage will be avoided.26
Experimental
Materials
The mineral and chemical compositions of the types of red mud are shown in Fig. 2c and Table I. Because of the high iron content in the Bayer red muds (a), (b) and (d), they are not suitable for heavy usage in the batching of the cement raw meal. The diasporic red mud (c) was then employed to study the sintering process of the new structured red mud (C-C residue).
To obtain cement that meets the strength requirements, the content of various oxides in the clinker was controlled according to three blending schemes (Table SI). To achieve a certain proportion of the chemical composition, chemical agents (CaO, SiO2, Fe2O3) with analytical purity were added to the raw meal.
Preparation of C-C Residue
To prepare the C-C residue (calcification-carbonization residue) used for sintering cement clinker, the sodium aluminate solution (Na2O 240 g l−1, Al2O3 127 g l−1), low-grade bauxite and CaO (mass ratio CaO/SiO2 = 4) were mixed, heated and kept at 240°C for 1 h to complete the calcification transformation. The calcified solid product was obtained through slurry filtration, which was to be used for the subsequent carbonization transformation. In the carbonization process, the calcified solid product and distilled water were mixed (liquid–solid ratio 5:1) and together put into the autoclave. The CO2 (0.8 MPa) was bubbled below the liquid level and kept at 120°C for 2 h to complete the carbonization transformation. Then, filtration was conducted to obtain the carbonized product for the subsequent Al(OH)3 leaching process. NaOH (100 g l−1) solution was used to extract the Al(OH)3 from the carbonized residue (liquid–solid ratio 5:1), and the reactants were kept at 60°C for 1 h. After that, the experimental procedures carbonization and Al(OH)3 leaching were repeated to improve the transformation effect. The second leaching product after washing and drying was the C-C residue used for sintering cement clinker.
Experimental Methods
The C-C residue was first dried in an electro-thermostatic blast oven (DHG-914385-III, CIMO, China) at 100°C for 1 day. Then, the C-C residue and pure reagents were blended according to Scheme A of Table SI and ground with a vibrating mill (RK/ZM, Rock, China). The fineness of the raw meal was controlled to 1–4% residue content with a 0.2-mm square hole sieve. The ground raw meal was put into a mixing tank (RK/SHJ, Rock, China) to homogenize the mixture for 1 h and then sintered in a tubular resistance furnace (8817EK, Sigma, China), holding at different temperatures (1250°C, 1300°C, 1350°C, 1400°C, 1450°C) for distinct amounts of time (5 min, 15 min, 30 min, 1 h, 2 h). The clinker was cooled by air. This clinker prepared from C-C residue was referred to as C-C clinker. The phase, chemical composition, f-CaO content, micromorphology and cement strength of the C-C clinker were analyzed.
The XRD phase of the sintered clinker was analyzed with a D8 Advance diffractometer (Bruker, Germany), operated at 40 kV and 40 mA with a Cu Kα radiation source. The pattern collected and drawn in a 2θ range from 10° to 90° was compared with the patterns in the Joint Committee on Powder Diffraction Standards’ International Centre for Diffraction Data (JCPDS-ICDD) database to identify the crystalline phases. The Na, Al, Si, Ca and Fe contents in the red mud of the sintered clinker were measured quantitatively by x-ray fluorescence (XRF) spectrometry using a ZSXPrimus II XRF spectrometer (Rigaku, Japan). Free calcium oxide (f-CaO) content was determined using an ethylene glycol–ethanol solution. Free calcium in the sample was extracted by heating it to 100°C. Then, the generated ethylene glycol calcium was titrated with benzoic acid anhydrous ethanol standard solution with a CFC-5 cement-free calcium rapid tester. An ultra-high-resolution scanning electron microscope (SU-8010, Hitachi, Japan) was used to analyze the micromorphology of the sintered clinker. The measurements of the bending strength and compressive strength of the cement were entrusted to the Liaoning Hengwei Cement Group Co., Ltd. (China). These tests were carried out based on the relevant national standard of China (Method of testing cements—Determination of strength GB/T 17671-1999).
Results and Discussion
Phase Transformation of C-C Clinker
Figure 3 shows the phase transformation of the C-C clinker under different heat holding times at 1400°C from 5 min to 2 h. After 5 min of the sintering process, the main phases of the clinker are Ca2SiO4, Ca2FexAl2–xO5, Ca3SiO5 and some unreacted CaO. At 15 min, CaO completely reacts and produces CaFeO3 and Ca3Al2O6. The experimental results prove that sintering can transform the C-C residue (which is produced by the hydrometallurgical process) into the effective components (Ca3SiO5, Ca2SiO4, Ca2FexAl2–xO5 and Ca3Al2O6) of cement clinker. For holding times > 30 min, the peaks of CaFeO3 and Ca3Al2O6 gradually weaken until total disappearance.
Figure 4 describes the phase transformation of the sintered clinker holding at different temperatures for 1 h. From 1250°C to 1300°C, the main phases of the clinker are Ca2SiO4 and Ca2FexAl2–xO5. When the sintering temperature increases to 1350°C, Ca3SiO5 generation commences, and as the temperature continues to increase, the diffraction peaks of Ca3SiO5 become more evident. At 1450°C, the disappearance of the Ca2SiO4 peaks indicates that it has been completely transformed into Ca3SiO5.
According to Figs. 3 and 4, the formation of different phases is affected by different sintering parameters. The transformation Ca2SiO4 → Ca3SiO5 mainly depends on the sintering temperature (Fig. 4). When the required temperature is met, Ca3SiO5 is formed within 5 min (Fig. 3). Therefore, higher temperature can promote the transformation from Ca2SiO4 to Ca3SiO5. The experimental results show that the transformations CaO → CaFeO3 and Ca3Al2O6 → Ca2FexAl2–xO5 can happen at a lower sintering temperature (1250°C for 1 h); however, it is not completed at 1400°C after 5 min. This transformation is more likely to rely on multiple factors rather than a single factor.
f-CaO Content of C-C Clinker
During the sintering of cement clinker, most CaO reacts with acid oxides to synthesize Ca3SiO5, Ca2SiO4, Ca3Al2O6 and other minerals. However, due to the influence of composition, fineness, uniformity of raw meal and sintering temperature, a small amount of CaO cannot combine with acid oxides to form minerals and still exist as free CaO (f-CaO). The f-CaO content in the clinker is an important control feature that affects the volume stability and strength of cement. Figure 5 illustrates the f-CaO content of the C-C clinker under different blending schemes and temperatures. The raw meal blending and compositions of their clinker for schemes A–C in Fig. 5 can be found in Table SI and Table SII in the supplementary material. With the increase of temperature from 1300°C to 1500°C, the f-CaO content decreases remarkably from 8.50% to 0.64%. These values are calculated as the average of the three schemes. This C-C clinker meets the Chinese national standard requirement (Portland cement clinker GB/T 21372-2008, ≤ 1.5%) for f-CaO content by changing the sintering temperature and raw meal blending.
Micromorphology of C-C Clinker and Common Clinker
Figure 6 displays the scanning electron microscopy (SEM) images of the sintered clinker prepared from the C-C residue (left) and the common clinker in the cement plant (right). The raw materials of the common clinker are composed of limestone, quartz stone, bauxite and copper slags. Combining the SEM images (Fig. 6) and the phase transformation (Fig. 4) of the C-C clinker, the micromorphology transformation process of Ca2SiO4 to Ca3SiO5 in the C-C clinker can be clearly observed. At 1250°C, the hexagonal Ca2SiO4 and the irregular mixture of Ca2FexAl2–xO5 can be seen under a scanning electron microscope. When the temperature rises to 1350°C, the six angles (or vertexes) of Ca2SiO4 react preferentially with the surrounding mesophase Ca2FexAl2−xO5. Consequently, the boundary of the crystalline phase spreads outwards and suborbiculate Ca3SiO5 with a larger size starts to form at this temperature. When preparing cement with clinker, the phases of clinker react with water to form their own hydration products. These products interpenetrate and cross into a network structure to finally form a strengthened stone body. As seen in Fig. 6, when the temperature rises to 1450°C, both C-C clinker and common clinker in the cement plant form suborbiculate Ca3SiO5, which solidifies with the surrounding mesophase. This is beneficial for the intersection and penetration of their respective hydration products to produce strength.
Cement Strength of C-C Clinker
To achieve large-scale utilization of red mud, the C-C clinker needs to have good performance and meet certain strength requirements. To this end, a series of exploratory experiments to make cement from C-C residue was carried out under different blending schemes (identical to schemes A-C in Fig. 5) and sintering conditions. To reduce the f-CaO content in the clinker to the national standard limit, the sintering temperature was increased to exceed 1400°C. The bending strength and compressive strength of the cement are shown in Table II. The results show that the temperature mainly affected the early bending strength of the cement. When the other parameters are kept constant while the temperature increases, the 3-day bending strength increases. However, there is no defined pattern for the other three strength values. A possible explanation is that these three strength values are more influenced by the used ingredients. The cement sample prepared by Scheme B at 1500°C for 1 h has superior comprehensive strength performance (refer to the strength grade classification of the national standard in the Table SIII in the supplementary materials). The 3-day and 28-day bending strengths are 4.4 MPa and 7.9 MPa, respectively, higher than the national standard of China, 4.0 MPa and 7.0 MPa (Common Portland cement GB 175-2007) (grade 525). The 3-day and 28-day compressive strengths are respectively 19.2 MPa and 52.3 MPa, higher than the 17.0 MPa and 42.5 MPa of the national standard (grade 425). These results show that C-C clinker can meet the strength requirements of cement production when used as the main ingredient.
Conclusion
The mineral transformation of new structured red mud (C-C residue) through the sintering process was investigated and the properties of the C-C clinker were characterized. The results show that the C-C residue can be transformed into the effective components (Ca3SiO5, Ca2SiO4, Ca2FexAl2–xO5 and Ca3Al2O6) of cement clinker by controlling the sintering temperature and holding time. Increasing the sintering temperature can promote the conversion from Ca2SiO4 to Ca3SiO5 and reduce the f-CaO content in the clinker. The cement strength was determined by using the C-C clinker after blending cement mortar, molding and curing. The 3-day and 28-day bending strengths are 4.4 MPa and 7.9 MPa, respectively, higher than the 4.0 MPa and 7.0 MPa of the national standard of China (Common Portland cement GB 175-2007) (grade 525). The 3-day and 28-day compressive strengths are 19.2 MPa and 52.3 MPa, respectively, higher than the 17.0 MPa and 42.5 MPa of the national standard (grade 425). In conclusion, it is feasible to prepare cement clinker from new structured red mud by sintering. It provides a promising solution to the problem of red mud stockpiling.
References
Y.X. Wang, T.A. Zhang, G.Z. Lyu, F.F. Guo, W.G. Zhang, and Y.H. Zhang, J. Clean. Prod. 188, 456 (2018).
H.N. Gu, N. Wang, H.B. Liu, Y.H. Fu, H.F. Tang, and Y.J. Tian, Acta Mineral. Sin. S1, 105 (2010).
D.Y. Liu and C.S. Wu, Materials 5, 1232 (2012).
Y. Liu, C. Lin, and Y. Wu, J. Hazard. Mater. 1–2, 255 (2007).
X.F. Kong, M. Li, S.G. Xue, W. Hartley, C.R. Chen, C. Wu, X.F. Li, and Y.W. Li, J. Hazard. Mater. 324, 382 (2017).
X.F. Kong, Y. Guo, S.G. Xue, W. Hartley, Y.Z. Ye, and Q.Y. Cheng, J. Clean. Prod. 143, 224 (2017).
X.B. Zhu, L. Wang, and X.M. Guan, J. Hazard. Mater. 286, 85 (2015).
A. Xenidis, A.D. Harokopou, E. Mylona, and G. Brofas, JOM 2, 42 (2005).
F. Yang, The Existing Form of Alkali in Red Mud and the Research of Immobilization Methods, master’s thesis (Taiyuan: North University of China, 2015), pp. 2–8.
C. Klauber, M. Graefe, and G. Power, Hydrometallurgy 108, 11 (2011).
C.S. Lv, J.W. Wang, Y.Z. Jia, H.L. Liu, and G.W. Li, J. Saf. Environ. 13, 98 (2013).
P.E. Tsakiridis, S. Agatzini-Leonardou, and P. Oustadakis, J. Hazard. Mater. B116, 103 (2004).
L.Y. Li, Waste Manag. 21, 525 (2001).
W.C. Liu, J.K. Yang, and B. Xiao, J. Hazard. Mater. 161, 474 (2009).
G.H. Li, F.Q. Gu, T. Jiang, J. Luo, B.N. Deng, and Z.W. Peng, JOM 69, 315 (2017).
T.A. Zhang, G.Z. Lv, Y. Liu, Z.M. Zhang, X.F. Zhu, Z.H. Dou. International Patent US15/303,408 (US patent), 2014392419 (Australian patent), 14891022.7 (European Patent).
G.Z. Lu, T.A. Zhang, X.F. Zhu, Y. Liu, Y.X. Wang, F.F. Guo, Q.Y. Zhao, and C.Z. Zheng, JOM 66, 1616 (2014).
Y.X. Wang, T.A. Zhang, G.Z. Lv, W.G. Zhang, X.F. Zhu, and L.Q. Xie, Light Met. 2017, 61 (2017).
J. Zheng, The Basic Research of Recovering Sodium Oxide and Alumina from Sodium Aluminosilicate Hydrate, master’s thesis (Changsha: Central South University, 2012), pp. 1–72.
P.J. Gunning, C.D. Hills, and P.J. Carey, Waste Manag. 30, 1081 (2010).
L.C.A. Venancio, J.A.S. Souza, E.N. Macedo, J.N.N. Quaresma, and A.E.M. Paiva, JOM 9, 41 (2010).
S. Kumar, R. Kumar, and A. Bandopadhyay, Resour. Conserv. Recycl. 4, 301 (2006).
A.R. Hind, S.K. Bhargava, and S.C. Grocott, Colloids Surf. A 1-3, 359 (1999).
D.A. Rubinos, V. Valcarcel, G. Spagnoli, and M.T. Barral, JOM 9, 1607 (2017).
X.M. Liu, N. Zhang, H.H. Sun, J.X. Zhang, and L.T. Li, Cem. Concr. Res. 8, 847 (2011).
K. Hammond, B. Mishra, D. Apelian, and B. Blanpain, JOM 3, 340 (2013).
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (U1710257, U1202274), the State Key Laboratory Fund (YY2016006), the Fundamental Research Fund for the Central Universities of China (N162506003) and the Science and Technology Leading Talents Training Plan (2017HA012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Zhang, Ta., Zhang, Y. et al. Transformation and Characterization of Cement Clinker Prepared from New Structured Red Mud by Sintering. JOM 71, 2505–2512 (2019). https://doi.org/10.1007/s11837-019-03475-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-019-03475-y