Abstract
Chromium alloy is a key ingredient in stainless steel production, and slag forms a significant proportion of the production process’s output. To investigate how to avoid the leaching of Cr(VI) from stainless steel slag into the environment, we examined the effect of the FeO content on the stability of chromium in the CaO-SiO2-MgO-Al2O3-Cr2O3 system in laboratory experiments. The solidification process was then studied using FactSage 7.0. The results indicate that the leaching of Cr(VI) can be reduced. As the content of FeO increases in the slag, the content of chromium in the matrix decreases significantly. In our experiments, the concentration of the Cr(VI) leaching was decreased from 0.1434 mg/L to 0.0021 mg/L, and the size of spinel crystals in the slag increased from 5.77 μm to 8.40 μm. The content of calcium and silicon was reduced and the level of ferrum improved in the spinel crystals after the addition of FeO. FeO promotes the formation of a core–shell heterostructure in spinel crystals, with the Fe-enriched shell notably improving the stability of the chromium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium alloy is an essential raw material for making stainless steel. In the first half of 2018, approximately 13.64 million tons of stainless steel was produced in China; 20-30 wt.% of the output for stainless steel was slag,1 which amounted to approximately 3.41 million tons of slag. The stainless steel tailings usually end up in landfills, creating a serious risk of chromium leaching into the surrounding environment and polluting the water and soil.2 Cr(VI) is highly toxic and can enter people’s bodies via the respiratory tract, digestive tract or skin contact, resulting in chromium liver toxicity and kidney damage, chromium-contact dermatitis, bronchial lung cancer, etc.3 Thus, there is an urgent need to solve the problem of the leaching of Cr(VI) from stainless steel slag. So far, instead of removing Cr(VI) from the slag,4 most work has focused on solidifying the chromium to reduce the risk of leaching. Thus, Wu5 found that quality glass ceramics can be obtained by using melted stainless steel slag as the main raw material. Stainless steel slag can also be used for roadbeds, bitumen-producing mortar and the preparation of an eco-friendly cement-based material.6,7 There seem to be many options. However, the leaching of chromium into the surrounding environment is a slow process.8 The long-term impact on the environmental safety of products produced using stainless steel slag is not yet clear, and the relevant technical indicators for stainless steel slag disposal remain unspecified.
Chromium mainly occurs in chromite in nature, with chromium spinel crystals being its main mineral phase. Its chemical formula is (Mg, Fe) (Cr, Al, Fe)2O4,9 which not only inhibits the leaching of chromium, but also enhances its antioxidant capacity.10 The leaching of Cr(VI) from chromite with a grain size < 1 mm is below the detection limit of a spectrophotometer, and minerals containing chromium are safe and stable.11 Regarding stainless steel slag, Strandkvist12 has reported that the leaching of Cr(VI) may be inhibited by an increase in the FeO content. Particularly at a low basicity, FeO behaves as a network modifier; the composition of mineral phases in the EAF slag system are influenced by FeO, which can decrease the melting temperature (1369°C) and promote the precipitation of spinel crystals.13,14 However, the mechanism whereby FeO may have this controlling effect has yet to be fully studied. Our research extends the knowledge regarding the effect of the FeO content on the stability of chromium in CaO-SiO2-MgO-Al2O3-Cr2O3 systems. We also examined the distribution of chromium and the precipitation behavior of spinel crystals in stainless steel slag.
Experiment
Sample Preparation
The chemical composition of the slag samples was synthesized using reagent-grade powders (see Table I). The basicity of the slag was 1.4, and the Cr2O3 content was 6 wt.%. This article focuses on the effect of the FeO content on the stability of chromium in the synthetic slag. The FeO was added as a single component, and seven different samples were prepared where the FeO content was replaced by 0 wt.%, 2 wt.%, 5 wt.%, 8 wt.%, 12 wt.%, 16 wt.% and 20 wt.% of FeC2O4. First, the powders were weighed accurately and thoroughly mixed. Then, the mixture was put into a molybdenum crucible and melted in a high-temperature carbon tube furnace (25 kW, 1650°C). The samples were heated to 1550°C at a rate of 10°C/min in a nitrogen atmosphere. The temperature was then maintained for 30 min. Finally, the samples were taken out of the furnace and cooled in air.
Leaching Test
The synthetic slag samples were subjected to a leaching test, according to the leaching standard for solid waste HJ/T299-2007. Ten grams of each sample was placed in a 100-mL plastic bottle, and a 100-mL sulfuric acid-nitric acid solution (2:1, pH = 3.20) was added. This was placed in a flip oscillator and rotated at 20°C for 20 h at 30 r/min; 50 mL (50 nm for the filter membrane) of the completed samples was extracted as a test solution in a 100-mL volumetric flask using a suction filter. An indicator was configured with 50 mL of acetone (analytic reagent), 0.5 g of diphenylcarbazide and a drop of acetic acid (analytic reagent); 2 mL of the indicator, 2 mL of hydrochloric acid (1 mol/L) and 50 mL of the test solution were mixed with distilled water to take the amount to 100 mL. A spectrophotometer was used to measure the transmission properties of the solution at 540 nm, and the concentration of Cr(VI) in the extract was calculated from the standard curve.
Analytic Method
The slag samples were inlayed in resin (HMR4) by a machine (XQ-2B) for SEM/EDS analysis. The phase transition and precipitation behavior of the spinel phase during solidification of the slag was studied using FactSage 7.0, based on the Scheil–Gulliver equation. The specific calculation conditions were as follows:15
-
(1)
Databases: FactPS, FToxide, FSstel;
-
(2)
Compounds: DEA gas, pure solid;
-
(3)
Liquid phase: FToxid-SLAGA, FToxid-SPINA, FToxid-MeO_A, FToxid-bC2SA, FToxid-aC2SA and FToxid- Mel_A, where the FToxid-SLAGA was set as the Scheil–Gulliver Cooling target phase.
Results
Leaching Toxicity
The leaching test curve of Cr(VI), according to the various levels of the FeO content in the samples, is shown in Fig. 1. As the FeO content in the slag increased from 0 wt.% to 20 wt.%, the concentration of the Cr(VI) decreased from 0.1434 mg/L to 0.0021 mg/L, which suggests that the addition of the FeO effectively inhibited the leaching of Cr(VI). The Environmental Protection Industry Standard HJ/T301-2007 of the People’s Republic of China fully specifies the utilization of chromium-containing solid wastes. When chromium-containing steel slag is used as a roadbed material, concrete aggregate or cement mixture, according to HJ/T299-2007 the concentration of Cr(VI) must be no more than 0.5 mg/L. When used for making bricks and blocks, the Cr(VI) concentration must be no more than 0.1 mg/L. The leaching of Cr(VI) in this test was much lower than the standard limits.
Microscopic Properties
As shown in Fig. 2, two mineral phases were observed by the SEM. The white phase is the spinel, and the gray phase is the matrix. Rapid cooling in the air resulted in the low-temperature phase crystallizing too late, so it entered directly into an amorphous matrix phase or silicate phase. It can also be observed that, as the FeO content increased, the grain size of the spinel crystals gradually increased as well.
The chemical composition of the mineral phases in Fig. 2 was detected using EDS. Ten random points were chosen in the matrix and spinel crystals, respectively, to detect the composition. On the basis of this, an average was established. The chemical composition of the matrix phase is shown in Table II. The detectable chromium content in the matrix gradually decreased as the FeO content in the slag increased, and, when the FeO content was at its highest level, the chromium content in the matrix was below the detectable limit using EDS. In addition, the content of ferrum in the matrix positively correlated with the FeO content of the sample. The varying chemical composition of the spinel crystals is shown in Table III. As the FeO content increased, the Ca, Si, Al, Mg and Cr content in the spinel crystals all decreased, while the Fe content increased.
Discussion
Effect of the Presence of Different Elements on the Stability of Chromium
Table II shows that the content of chromium in the matrix decreased as the content of FeO in the slag increased. When the content of FeO in the slag was > 12 wt.%, the chromium could no longer be detected by EDS because it had been reduced to just a trace in the matrix. Taking the above results into account, the leaching amount of Cr(VI) was reduced from 0.1434 mg/L to 0.0021 mg/L. The leaching of Cr(VI) chromium is affected by the nature of the mineral phases in the stainless steel slag. According to a FactSage 7.0 simulation, the matrix of stainless steel synthetic slag is mainly composed of three mineral phases: C2S, merwinite and melilite (see Fig. 3). At first sight, these mineral phases could easily be dissolved in water,16 with their entrapped chromium being leached out along with their dissolution. However, new calcium chromate will form when the stainless steel slag is landfilled (see Formulas 1 and 2).8 Additionally, the rate of the leaching of chromium will increase slowly. Calcium chromate is an unstable phase and is easily dissolved in acidic liquids, and its high toxicity can contaminate water.17 Hence, increasing the concentration of chromium in the spinel crystals and reducing the content of chromium in the matrix can effectively inhibit the leaching of Cr(VI) in stainless steel slag.
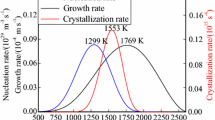
Table III shows that an increase in the FeO content promotes a change in the chemical composition of spinel crystals. A spinel solid solution consists of MgCr2O4, FeCr2O4 and a small amount of MgAl2O4 (see Fig. 4). At the end of the solidification process, the Fe3O4 precipitates from the residual slag and participates in the growth of the spinel by promoting the precipitation of the spinel solid solution because of the spinel structure of Fe3O4. Figure 4 shows the changes in the chemical composition of the spinel during the solidification process. In the initial stage of spinel precipitation (at high temperature), the main component of the spinel solid solution is chromium. As the temperature decreases, the content of Cr, Fe, Mg, Al and other elements gradually increases. On this basis, the FeO replaces the MgO position in the crystal lattice and participates in the formation and growth of the spinel, with the Fe content increasing as the FeO content increases.18 Furthermore, some of the Fe participates in the formation of the spinel solid solution as Fe3O4, which is negatively related to the other elements including Cr, Mg and Al.
The decrease of Ca and Si in the spinel crystals is beneficial to controlling the leaching of chromium. The Ca content in a Cr spinel affects the spinel’s stability. As the Ca content increases, the stability of the chromium spinel will decrease, making it more liable to dissolve in water. Samada et al.,19 reporting on leaching tests using MgO·Cr2O3 and MgO·Cr2O3 with 1 wt.% 2CaO·SiO2, found that the leaching of Cr(VI) in the latter test was much higher than it was in the former. Gelfi et al.20 also found that the C2S phase was more soluble in water than in other silicate mineral phases. As the C2S phase content increased in stainless steel slag from an electric furnace, the leaching of chromium was significantly increased.
Effect of the Spinel Microstructure on the Stability of Chromium
FeO promotes the formation of a three-layered structure with different chemical compositions in spinel crystals, termed a core–shell heterostructure. Fe elements enrich the outer layer, while the Cr elements are mostly present in the inner layer, with a transition layer between them. Figure 5 shows that the brightness at the edge is stronger than it is inside the spinel, with the lowest level being in the center. This means that the distribution of elements within the spinel is not uniform. The Cr, Fe, Mg, O and Al are mostly distributed within the spinel, while the Ca and Si exist mostly in the matrix. Figure 5 shows the bright (Fe-KA) part at the spinel edge contains more Fe, while the darker part in the core is the chromium.
As already mentioned, MgCr2O4 is a high-temperature precipitated phase, while other spinels are formed and precipitated at lower temperatures. Mg1−xFexCryFe2−yO4, as a solid solution, is generated by Fe(II) substituting for Mg(II) in MgCr2O4 on the basis of the principle of isomorphism. As FeCr2O4 and MgCr2O4 have isovalent isomorphism as well as complete isomorphism, Fe(II) can substitute Mg(II) completely under certain conditions. As shown in Fig. 6, kinetic factors determine the crystallization process falling into three stages because of the fast cooling rate. First, there is a high-purity MgCr2O4 spinel (a), with only a minute quantity of Fe substituting Mg in the formation of the substitutional solid solutions. Then, a higher proportion of Fe elements substitutes the Mg and Cr to form an Mg1−xFexCryFe2−yO4 substitutional solid solution, which is the transition layer (b). Finally, as the temperature drops, a protective shell that is an Fe-rich phase is formed, with the concentration of Mg being somewhat decreased and with the main component being Mg1−xFexFe2O4.
During the process of crystallization, the segregation of the solute components at the solidification frontier causes a non-uniform distribution of elements in the spinel, so the crystalline structure of the spinel is not a normal octahedron. Our research has shown that the corrosion resistance of chromium-containing spinels increases with an increase in the Fe content under natural conditions.21 The properties of MgCr2O4 in the center and Mg1−xFexCryFe2−yO4 in the transition layer are stable and oxidation resistant, and the finally precipitated Fe-rich shell is even more resistant to corrosion. Furthermore, the leaching of chromium in slag is a liquid–solid interface reaction, so this structure further strengthens the stability of the spinel phase.
Effect of Spinel Size on the Stability of Chromium
The stability of chromium in slag is positively related to the size of the spinel crystals. The average particle size of the spinel phase in the SEM images was assessed using IPP 6.0 (see Fig. 7). Taking into account the leaching test results, when the FeO content increases from 2 wt.% to 20 wt.%, the spinel crystal size increases from 5.77 μm to 8.40 μm. Thus, there is a positive relation between the FeO content and the size of the spinel crystals, while the content and leaching of chromium are inversely related. The spinel phase is a high-temperature precipitated phase and initially precipitates as the slag sample cools. Chromium in natural conditions is mainly in the form of FeCr2O4, so the formation of MgCr2O4 can effectively inhibit the leaching of chromium.22 The spinel phase provides an appropriate mineral phase for the stable curing of chromium because it not only inhibits the oxidation of Cr2O3 but is also insoluble in water. Figure 7 shows that the spinel solid solution precipitated during the slag cooling process contains Al and Fe as well as Mg and Cr. Fe(II) can replace some of the Mg(II) in the formation of the spinel through isomorphism. The enrichment of iron elements and Fe in the system resulting from an increased FeO content promotes the growth of spinel crystals. The spinel crystal structure is stable, making it difficult to produce calcium chromate from the CaO in the matrix. Larger spinel crystals further reduce the probability of chromium coming into contact with alkaline earth metal oxides such as CaO in the slag, and they are generally even more stable.
Proposed On-line Stabilization of Stainless Steel Slag
The on-line stabilization treatment of stainless steel slag produced by different smelting methods is not always the same. There are two presumed ways to treat the slag. One is treatment in the furnace. FeO-containing materials, including iron scale, converter dust, electric furnace dust, blast furnace dust, sintering tailings and so on, are added into the slag after tapping and before de-slagging. Stirring is used to mix and melt the materials. The other way is treatment in the slag tank. Here, FeO-containing materials at the bottom of the slag tank are flushed out by the slag to be melted during the de-slagging process. Additional heating may be required when the heat is insufficient.
Conclusion
The effect of FeO on the stability and distribution of Cr in a stainless steel slag CaO-SiO2-MgO-Al2O3-Cr2O3 system was experimentally investigated using SEM/EDS and FactSage 7.0. The results can be summarized as follows: With an increase in the FeO content, the content of chromium in the matrix is significantly decreased. The spinel crystal size increases from 5.77 μm to 8.40 μm, while the leaching of Cr(VI) is reduced from 0.1434 mg/L to 0.0021 mg/L. Spinel is an appropriate mineral phase for the stable curing of chromium. FeO can improve the enrichment of chromium in spinel crystals while reducing the content of Cr in the matrix and Ca and Si in the spinel, thus inhibiting the leaching of Cr(VI) from the slag. MgCr2O4 is a high-temperature precipitated phase. As a result of isomorphism, Fe(II) can replace some of the Mg(II) when forming the spinel and improve the spinel crystals by encouraging the formation of a core–shell heterostructure. Fe enrichment makes the size of the spinel phase and the FeO content positively related. As the spinel size increases, the chromium in the matrix participates in the formation of the spinel phase. However, while the actual content of chromium in the spinel may increase, its migration capacity is suppressed.
References
D.M. Proctor, K.A. Fehling, E.C. Shay, J.L. Wittenborn, J.J. Green, C. Avent, R.D. Bigham, M. Connolly, B. Lee, and T.O. Shepker, Environ. Sci. Technol. 34, 1576 (2000).
A. Estokova, L. Palascakova, and M. Kanuchova, Int. J. Environ. Res. Public Health 15, 824 (2018).
A. Fathima and J.R. Rao, Arch. Microbiol. 200, 1 (2017).
Z. Junxue, Z. Zhongyu, S. Ruimeng, L. Xiaoming, and C. Yaru, JOM 70, 2825 (2018).
C.J. Wu, L.E. Fried, L.H. Yang, N. Goldman, and S. Bastea, Nat. Chem. 1, 57 (2009).
F. Moreno-Navarro, G.R. Iglesias, and M.C. Rubio-Gámez, Smart Mater. Struct. 25, 115036 (2016).
S.J. Tae and K. Morita, Met. Mater. Int. 23, 576 (2017).
K. Pillay, B.H. Von, and J. Petersen, Chemosphere 52, 1771 (2003).
S.A.S. Dare, J.A. Pearce, I. Mcdonald, and M.T. Styles, Chem. Geol. 261, 199 (2009).
M.N. Taran, F. Parisi, D. Lenaz, and A.A. Vishnevskyy, Phys. Chem. Miner. 41, 593 (2014).
J.P. Beukes and R.N. Guest, Miner. Eng. 14, 423 (2001).
I. Strandkvist, Å. Sandström, and F. Engström, Steel Res. Int. 88, 1600322 (2017).
J.L. Li, A.J. Xu, D.F. He, Q.X. Yang, and N.Y. Tian, Int. J. Miner. Metall. Mater. 20, 253 (2013).
J.F. Lü, Z.N. Jin, H.Y. Yang, L.L. Tong, G.B. Chen, and F.X. Xiao, Int. J. Miner. Metall. Mater. 32, 07 (2017).
M. Xiong and A.V. Kuznetsov, Flow. Turbul. Combust. 67, 305 (2001).
I. Strandkvist, B. Björkman, and F. Engström, Can. Metall. Q. 54, 446 (2016).
E.V. Sokol, O.L. Gaskova, S.N. Kokh, O.A. Kozmenko, Y.V. Seryotkin, Y. Vapnik, and M.N. Murashko, Am. Mineral. 96, 659 (2011).
G. Chichinadze, D. Shengelia, T. Tsutsunava, N. Maisuradze and G. Beridze, Icges 2017: International Conference on Geological and Earth Sciences, 1 (2017).
Y. Samada, T. Miki, and M. Hino, ISIJ Int. 51, 728 (2011).
G. Cornacchia, S. Agnelli, M. Gelfi, G. Ramorino, and R. Roberti, JOM 67, 1 (2015).
J. Zhang, H.S. Xu, D.H. Wang, Z.H. Zhang, Z.Y. Chen, and R.M. Zhang, Acta. Geosci. Sinica 30, 599 (2009).
K.Y. Lee, J.M. Park, and C.M. Park, VII International Conference on Molten Slags Fluxes and Salts, 601 (2004).
Acknowledgements
The research is supported by National Natural Science Foundation of China (No. 51404173), Hubei Provincial Natural Science Foundation (No. 2016CFB579), China Postdoctoral Science Foundation (No. 2014M562073) and State Key Laboratory of Refractories and Metallurgy. The authors express their sincere gratitude and appreciation to Prof. Mulin Zhang, Wuhan University of Science and Technology, for his valuable advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeng, Q., Li, J., Mou, Q. et al. Effect of FeO on Spinel Crystallization and Chromium Stability in Stainless Steel-Making Slag. JOM 71, 2331–2337 (2019). https://doi.org/10.1007/s11837-019-03465-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-019-03465-0