Abstract
Due to stringent environmental requirements and the complex occurrence of valuable metals, traditional pyrometallurgical methods are unsuitable for treating low-grade nickel-copper matte. A clean and sustainable two-stage sulfating roasting and water-leaching process was used to simultaneously extract valuable metals from low-grade nickel-copper matte. Ammonium and sodium sulfate were used as sulfating agents. The first roasting temperature, mass ratio of ammonium sulfate to matte, roasting time, dosage of sodium sulfate, second roasting temperature and leaching temperature were studied. Under optimal conditions, 98.89% of Ni, 97.48% of Cu and 95.82% of Co, but only 1.34% of Fe, were extracted. X-ray diffraction (XRD) and scanning electron microscopy (SEM) were used to reveal the sulfating mechanism during the roasting process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Low-grade Ni-Cu matte, which is processed using the flash smelting method, is an intermediate in the conversion of concentrate into high-grade Ni-Cu matte.
Traditionally, pyrometallurgical methods involve placing low-grade Ni-Cu matte into a converter to blow it into high-grade Ni-Cu matte. The Jinchuan group then uses a floatation-magnetic method to separate Ni and Cu.1 However, because valuable metals occur in multiple states in concentrate, the smelting low-grade Ni-Cu matte contains a quantified amount of Fe. In the subsequent conversion process, more than 70% of Co and a portion of Ni and Cu are lost. Furthermore, the blowing conversion method yields environmentally hazardous gases (SO2 or SO3), which are typically converted into sulfuric acid. However, Jinchuan is located in the western Gobi Desert of China, and due to high transportation costs, there is a significant overstock of sulfuric acid.
Hydrometallurgical methods are adopted to treat low-grade Ni-Cu matte; these methods have the advantages of low temperature, environmental friendliness and economical equipment investment. These methods include oxidative ammonia/ammonium sulfate leaching, acid-oxygen pressure leaching, atmospheric acid-oxygen leaching, FeCl3-HCl leaching, Cu(II)–Cl—HCl–Cl2 system leaching and CuCl2–NaCl–HCl system leaching.2,3,4,5,6,7,–8 However, the above-mentioned methods have a moderate recovery of valuable metals and limited selectivity for precious metals compared with their selectivity for Fe. In some methods, like FeCl3-HCl and Cu(II)–Cl—HCl–Cl2, the existence of Cl− is harmful for electrolytic deposition.
In this work, a two-stage ammonium sulfating roasting and water-leaching process was used to convert metals into their respective water-soluble metal sulfates in a controllable manner, and the sulfating roasting mechanism was revealed.
Experimental
Materials
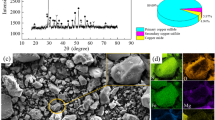
Low-grade Ni-Cu matte was obtained from a flash smelter in Jinchuan, China. Figure 1 shows the x-ray diffraction (XRD) patterns and a micrograph of low-grade Ni-Cu matte (for EDS scanning mapping images of matte, see supplementary Fig. S1; for SEM images of crushed matte, see supplementary Fig. S2). The low-grade Ni-Cu matte primarily consisted of magnetite (Fe3O4), pentlandite (Fe4.005Ni4.995S8), iron nickel alloy (FeNi3) and bornite (Cu5FeS4). Table I shows the the chemical composition by using the XRF method and each phase’s content by using the Highscore Plus 3.0 Rietveld method in matte.
Methods
The low-grade Ni-Cu matte was first crushed in a jaw crusher, pulverized in a planetary ball mill and sifted into different particle sizes. Then, the sifted powder was mixed. Na2SO4 (CAS#: 7757-82-6, AR), (NH4)2SO4 (CAS#: 7783-20-2, AR), dimethylglyoxime (CAS#: 95-45-4, AR), BCO (CAS#: 370-81-0, AR) and other common reagents were purchased from Kermel, China.
This article describes a sulfating roasting water-leaching process. Before the roasting test, the matte sample was crushed, ground and screened into fractions with a predetermined particle size. Then, the fraction was mixed with ammonium sulfate in a particular mass ratio, pelletized with a diameter range of 5–10 mm and dried at 80°C for 24 h. The pellets were transferred to a corundum crucible, which was roasted in a vertical furnace with an intelligent temperature control instrument (maximum, 1200°C). The leaching process was executed in a water bath with an intelligent temperature control instrument and magnetic stirring equipment.
The concentrations of Ni and Cu were analyzed by spectrophotometry using an ultraviolet spectrophotometer (PUXI, Beijing); Co was analyzed using atomic absorption spectrometry (Shimadzu-AA6680, Japan), and Fe was analyzed using chemical titration of the filtrate. The initial and post-roasting phase compositions of the samples were determined using XRD (Rigaku-SmartLab, Cu/Kα, Japan). Microtopographies of the sample were determined using scanning electron microscopy (SEM) (Zeiss-Sigma 300, German).
Results and Discussion
First Roasting Process
In the first roasting process, the leaching conditions were fixed with a leaching temperature of 80°C, leaching time of 90 min, liquid–solid ratio of 6:1 and stirring speed of 600 r/min.
Effect of the Roasting Temperature
Figure 2a illustrates the effect of roasting temperature on the metal extraction under the following conditions: an (NH4)2SO4 to matte mass ratio of 5:1, Na2SO4 dosage of 60% (relative to the weight of the matte), roasting time of 4 h and matte particle size of 80–75 μm.
Effect of the following parameters on the metal extractions: (a) effect of roasting temperature; (b) effect of ratio of ammonium sulfate on matte; (c) effect of time; (d) effect of dosage of sodium sulfate; (e) effect of particle size; (f) effect of leaching temperature; and (g) effect of second roasting temperature
As shown in Fig. 2a, the extraction of Ni, Cu, Co and Fe first increases over the roasting temperature range of 350–500°C, reaching their highest values of 92.09%, 93.87% and 89.18% at 500°C and 57.42% at 450°C, respectively, but then they decrease as the roasting temperature increases. The decomposition of (NH4)2SO4 can be divided into two stages,9 as shown in Eqs. 1 and 2 [DTA-TG curves of (NH4)2SO4, see supplementary Fig. S3]. The sulfating activation becomes violent as the roasting temperature increases, accompanied by the decomposition of (NH4)2SO4. However, when the roasting temperature exceeds 550°C, the decomposition rate of (NH4)2SO4 is too rapid, which leaves insufficient time for the reactions between NH4HSO4 or SO3 with the mineral phases in the matte to occur. Therefore, the extraction of Ni, Cu and Co decreases when the roasting temperature exceeds 550°C. The significant decrease in Fe from 450°C to 500°C can be attributed to the decomposition of iron sulfates.10,11 Hence, the roasting temperature of 500°C was recommended for the following experiments. (For the collected volatiles, see supplementary Fig. S4.)
Effect of the Mass Ratio of Ammonium Sulfate to Matte
Figure 2b shows the effect of the mass ratio of ammonium sulfate to matte under the following conditions: a roasting temperature of 500°C, Na2SO4 dosage of 60%, roasting time of 4 h and matte particle size of 80–75 μm.
Figure 2b shows that the extractions of Ni, Cu and Co increase with the enhanced mass ratio of (NH4)2SO4 to matte. However, a mass ratio of (NH4)2SO4 to matte exceeding 5:1 has little effect on the extractions of Ni, Cu and Co. The extraction of Fe first increases with the higher mass ratio of (NH4)2SO4 to matte and then decreases when the mass ratio exceeds 6:1, which can be attributed to the formation of NH4Fe3(OH)6(SO4)2, as shown in Eq. 3. Therefore, the mass ratio of 5:1 was used in the following experiments.
Effect of Roasting Time
The roasting temperature was maintained at 500°C, the dosage of Na2SO4 at 60%, the roasting time at 4 h and the matte particle size at 80–75 μm. The results are presented in Fig. 2c.
Figure 2c shows that as the roasting time increased to 2–3.5 h, the extraction of Ni, Cu, Co and Fe increased, reaching values of 90.18%, 91.78%, 91.65% and 70.32%, respectively. Then, the extraction of Ni, Cu and Co remained almost constant after 3.5 h; the decreased extraction of Fe could be attributed to the decomposition of iron sulfates.
As shown in Fig. 3, the diffraction peaks of (NH4)2Fe2(SO4)3 appear in sample 1, indicating that Fe is initially converted into ammonium ferric sulfate. With a prolonged roasting time, the diffraction peaks of the phases in sample 1 disappear, while the diffraction peaks of Fe2(SO4)3, Na3Fe(SO4)3 and Na2Cu(SO4)2 appear in sample 2, indicating that (NH4)2Fe2(SO4)3 was completely decomposed and that sodium-metal (Cu, Fe) sulfate was formed. As the roasting time increases, the diffraction peaks of metal sulfides disappear, while the diffraction peaks of Fe2(SO4)3, Na3Fe(SO4)3 and Na2Cu(SO4)2 intensify. Therefore, initially, the metal sulfides are converted to ammonium-metal sulfates. Then, the ammonium-metal sulfates decompose and are converted to metal sulfates or sodium-metal sulfates. Hence, a roasting time of 3.5 h was recommended in the subsequent experiments.
Effect of the Sodium Sulfate Dosage
Sodium sulfate is a common active agent in the sulfating roasting process, as it can promote the sulfation of metals, especially Ni, Cu and Co.12,13,14,–15 Hence, Na2SO4 was added to activate the sulfating process to metal sulfides. The roasting temperature was maintained at 500°C, the mass ratio of (NH4)2SO4 to matte was 5:1, the roasting time was 3.5 h, and the particle size of the matte was 80–75 μm. The results are shown in Fig. 2d.
As shown in Fig. 2d, the extractions of Ni, Cu and Co increase as the Na2SO4 dosage increases. Specifically, the extractions of Ni, Cu and Co initially increase rapidly by 20.75% (from 69.53% at 0% to 90.28% at 40%), 40.85% (from 50.53% at 0% to 91.38% at 40%) and 22.40% (from 62.38% at 0% to 84.78% at 40%), respectively, and then they increase slowly with the continued increase in dosage of Na2SO4; moreover, the extraction of Fe increases by 47.75% (from 23.48% at 0% to 71.23% at 120%).
As shown in Fig. 4, the main phases in sample 1 are Fe2(SO4)3 and CuSO4. After adding 20% of Na2SO4, the intensities of the diffraction peaks of CuSO4 in sample 2 become stronger than those in 1, which corresponds with the increase of the Cu extraction. When 20% of Na2SO4 is continuously added, the diffraction peaks of NiSO4(6H2O) appear in sample 3, indicating that the extent to which Ni is sulfated depends on the amount of Na2SO4. The diffraction peaks of Na3Fe(SO4)3 and Na2Cu(SO4)2(2H2O) appear in sample 4, and after adding 100% of Na2SO4, the main phases in sample 6 are Fe2(SO4)3, Na3Fe(SO4)3 and Na2Cu(SO4)2. The SEM images of 1 and 6 show that after adding Na2SO4, the polyporous particles transform into adhesive particles. The accepted activation mechanism of Na2SO4 in the sulfating roasting process is the formation of N2S2O7, as shown in Eq. 4, with the extensive erosion effect and the cycle phase transformation of the liquid phase to the solid phase.12,16,17 This mechanism explains the increase in the metal extraction with the enhanced dosage of Na2SO4. Therefore, a Na2SO4 dosage of 40% was used in the subsequent experiment.
Effect of Particle Size
Figure 2e shows the effect of particle size on the extraction of Ni, Cu, Co and Fe by keeping the roasting temperature at 500°C, the mass ratio of (NH4)2SO4 to matte at 5:1, the dosage of sodium sulfate at 40% and the roasting time at 3.5 h.
As shown in Fig. 2e, the extractions of Ni, Cu, Co and Fe increase as the matte particle size decreases from 250–178 μm to 80–75 μm, although the extractions of Cu and Ni show a small decrease with particle sizes less than 75 μm. For solid–solid–gas reactions, smaller particles induce more contiguous opportunities for each of the reactants. However, a too small particle size narrows the gap and gets a tight contact between particles; this leaves insufficient space to exchange gases, which explains why the extractions of Cu and Ni slightly decrease when the particle size is smaller than 75 μm. Hence, the particle size of 80–75 μm was adopted in the later experiments.
Effect of Leaching Temperature
Figure 2f shows the effect of leaching temperature when the roasting temperature is 500°C, the mass ratio of (NH4)2SO4 to matte is 5:1, the dosage of sodium sulfate is 40%, the roasting time is 3.5 h and the particle size is 80–75 μm.
Figure 2f also shows that the leaching rates of Ni, Cu and Co slightly increase with the enhanced leaching temperature. However, the leaching rate of Fe significantly decreases when the leaching temperature is higher than 90°C because of the rapid formation of NaFe3(SO4)2(OH)6, as shown in Eq. 5. The solution temperature plays a key role in the formation of NaFe3(SO4)2(OH)6; when the temperature is below than 85°C, the reaction rate of Eq. 5 is slow, resulting in a lower generation amount of NaFe3(SO4)2(OH)6 at a fixed leaching time of 90 min. However, when the solution temperature is higher than 90°C, the reaction rate of Eq. 5 accelerates several times more than at lower temperature, while when the solution temperature is higher than 95°C, it has less effect on the reaction.18,19 Therefore, a leaching temperature of 95°C is suggested for the following experiments.
Effect of the Second Roasting Process
During the following separation of metal ions, there is a significant cost for removing iron. Due to the different thermodynamic stabilities of metal sulfates,10,11 a second roasting process was used to achieve an initial separation of the metals. The second roasting process is based on the products after the first roasting process (the conditions are the same as those used in the first roasting process, with a leaching temperature of 95°C), and the roasting time is 2 h. The results are shown in Fig. 2g.
Figure 2g shows the extractions of Ni, Cu and Co increase with an enhanced roasting temperature ranging from 540°C to 680°C. The extractions of Cu and Co reach their peaks of 97.48% and 95.82%, respectively, and the extraction of Ni reaches its maximum of 99.01% at 700°C; all of the extractions decrease as the roasting temperature continues to increase because of the decomposition of their corresponding metal sulfates, as show in Eqs. 6–10.10 The extraction of Fe significantly decreases with the increased roasting temperature; the quick decrease in 600–620°C is attributed to the fast decomposition rate of Fe2(SO4)3 and Na3Fe(SO4)3 at higher temperature.20,21 Compared with the first roasting process, the extractions of Ni, Cu and Co increase by 8.61%, 6.1% and 11.04% at the second roasting temperature of 680°C, respectively. At a higher roasting temperature, Na2SO4 effectively promotes the sulfating process of the metals because of the generated strong sulfation ability intermediate of Na2S2O7, as shown in Eq. 5, and its cycle phase transforms from solid to liquid.12,16,22,23 Overall, the second roasting temperature of 680°C was adopted as the optimal condition. The mechanism diagram of the two-stage selective sulfating roasting process is shown in Fig. 5. [For possible reactions in the two-stage roasting process, see supplementary Eqs. 1–23.]
Leaching Residue
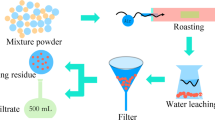
Figure 6 shows the leaching residue of the two-stage roasting product. The main phases in the leaching residue contain Fe2O3 and Fe3O4. In the first stage of the roasting process, metal-bearing phases in matte were converted to corresponding metal sulfates and sodium metal sulfates, while some of Fe2(SO4)3 was decomposed into insoluble Fe2O3 at the first roasting temperature of 500°C, as shown in Eq. 3. In the ensuing second roasting process, Na3Fe(SO4)3 and Fe2(SO4)3 were decomposed rapidly at 680°C. However, when the partial pressure of O2 is lower than a certain value, Fe2O3 will react with SO2 to generate Fe3O4 and FeSO4 as in Eq. 11,24 so the leaching residue contains a small amount of Fe3O4. Figure 7 shows the flow sheet of the selective roasting and water-leaching process.
Conclusion
In this work, a two-stage roasting and water-leaching process was used to simultaneously extract valuable metals from low-grade nickel-copper matte. Ammonium sulfate and sodium sulfate were used as the sulfating agents. During both the first and second roasting processes, sodium sulfate significantly improved the extractions of Ni, Cu and Co. After adding sodium sulfate, metal sulfides were converted to sodium-metal sulfates, not metal sulfates, by using a single sulfating agent, ammonium sulfate. Under optimal conditions (a first roasting temperature of 500°C, mass ratio of (NH4)2SO4 to matte of 5:1, sodium sulfate dosage of 40%, roasting time of 3.5 h, particle size of 80–75 μm, second roasting temperature of 680°C and leaching temperature of 95°C), the extractions of Ni, Cu and Co reached 98.89%, 97.48% and 95.82%, respectively. However, the extraction of Fe was only 1.34%. This process was clean and sustainable for treating low-grade nickel matte.
References
A.E. Warner, C. Diaz, A. Dalvi, P. Mackey, A. Tarasov, and R. Jones, JOM-US 59, 58 (2007).
K.-H. Park, D. Mohapatra, B.R. Reddy, and C.W. Nam, Hydrometallurgy 86, 164 (2007).
J. Provis, J. Van Deventer, J. Rademan, and L. Lorenzen, Hydrometallurgy 70, 83 (2003).
R. Van Schalkwyk, J. Eksteen, J. Petersen, E. Thyse, and G. Akdogan, Miner. Eng. 24, 524 (2011).
K.H. Park, D. Mohapatra, and B.R. Reddy, Sep. Purif. Technol. 51, 332 (2006).
Y. Fu, B.-c. Li, C.-i. Fan, X.-j. Zhai, X.-j. Zhang, and D.-h. Li, Trans. Nonferr. Metal. Soc. 20, s71–s76 (2010).
K.H. Park, D. Mohapatra, K. Hong-In, and G. Xueyi, Sep. Purif. Technol. 56, 303 (2007).
R. Berezowsky, M. Collins, D. Kerfoot, and N. Torres, JOM-US 43, 9 (1991).
I.K. Thege, Thermochim. Acta 60, 149 (1983).
R.V. Siriwardane, J.A. Poston Jr., E.P. Fisher, M.-S. Shen, and A.L. Miltz, Appl. Surf. Sci. 152, 219 (1999).
H. Tagawa, Thermochim. Acta 80, 23 (1984).
M. Jiang, T. Sun, Z. Liu, J. Kou, N. Liu, and S. Zhang, Inter. J. Miner. Process. 123, 32 (2013).
J.E. Hoffmann, JOM-US 41, 33 (1989).
P. Distin, JOM-US 30, 30 (1978).
M. Liu, Z. You, Z. Peng, X. Li, and G. Li, JOM-US 68, 567 (2016).
Q. Li, J.-j. Hu, Y.-b. Yang, B. Xu, T. Jiang, Mechanism of Na2SO4 on Refractory Gold Concentrate at Roasting Pretreatment. In Drying, Roasting, and Calcining of Minerals. Springer; 2015: 59.
K. Luthra, Metall. Mater. Trans. A 13, 1647 (1982).
C. Smeaton (Electronic Theses and Dissertations, 2012), p. 450.
A.J. Monhemius, G. Thorsen, in Proc. Int. Solvent Extr. Conf. ISEC’80, Liege, Belgium, 1980, Vol. 3, paper 80-91.
R. Zboril, M. Mashlan, D. Krausova, The Mechanism of β-Fe2O3 Formation by Solid-State Reaction between NaCl and Fe2(SO4)3, in Mössbauer Spectroscopy in Materials Science (Springer, 1999), p. 49.
G. Kolta and M. Askar, Thermochim. Acta 11, 65 (1975).
D. Lindberg, R. Backman, and P. Chartrand, J. Chem. Thermodyn. 38, 1568 (2006).
D. Yu, T.A. Utigard, and M. Barati, Metall. Mater. Trans. B 45, 662 (2014).
K. Jacob and G. Iyengar, Metall. Trans. B 17, 323 (1986).
Acknowledgements
This research was jointly supported by the National Basic Research Program of China (Grant 2014CB643405), National Natural Science Foundation of China (Grant 51204036), Program for Top Young Talents of Higher Education Institutions of Hebei Province (Grant BJ201604), Fundamental Research Funds for the Central Universities (Grant N152304010) and Hebei Province Natural Science Fund (Grant E2017501073).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cui, F., Mu, W., Wang, S. et al. A Sustainable and Selective Roasting and Water-Leaching Process to Simultaneously Extract Valuable Metals from Low-Grade Ni-Cu Matte. JOM 70, 1977–1984 (2018). https://doi.org/10.1007/s11837-018-2798-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-018-2798-z