Abstract
The transformation of selenium-containing phases in copper anode slimes during the leaching process was investigated based on the Eh–pH diagram, leaching efficiencies of metals, and characterization of the residues produced during leaching. The leaching efficiency of selenium increases slowly to 17.7% in the first 50 min and then more rapidly to 98.3% in the next 110 min. The Eh–pH diagram indicates that elemental selenium is an intermediate product of the oxidation of selenide to selenite. The x-ray powder diffraction data and scanning electron microscopy–energy-dispersive x-ray spectroscopy data demonstrate that selenium leaching can be divided into three stages. Ag-Cu selenide first transforms into silver selenide and then converts to elemental selenium. Finally, elemental selenium is dissolved as selenite. The intermediate product, elemental selenium, is the main reason for the slow initial leaching rate of selenium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selenium, an essential element in advanced technologies, is used in various fields, such as in solar cells, in plain paper photocopiers, and as a glass additive.1 , 2 Overall, 90% selenium is obtained from copper anode slimes, which are valuable metallurgical by-products of the copper electrorefining process. In copper anode slimes, selenium exists mostly as intermetallic compounds containing silver and copper, i.e., Ag-Cu selenide, without an exact ratio.3 , 4
Nowadays, hydrometallurgical and pyrometallurgical technologies are used to recover selenium from copper anode slimes.5,6,7 Although pyrometallurgical treatment can recover selenium effectively, it faces difficulties as a result of rising energy costs and strict pollution regulations. Therefore, hydrometallurgical technologies, comparatively environmental-friendly processes, have been proposed to overcome these problems.5 Some examples of hydrometallurgical processes, in which oxidants are always added to the system, are chlorination leaching,8 nitric acid leaching, alkali leaching with sodium nitrate,9 and oxidation pressure leaching with alkali10 and sulfuric acid.11
Several researchers have previously studied the leaching process and the mechanism of selenium oxidation. Chen et al.12 and Hait et al.13 , 14 studied the mineralogical characteristics of copper anode slime and its leached residues and proposed that elemental selenium might be produced by the oxidation of Ag2Se, as follows:
Nevertheless, the mechanism was just an inference, and the transformation of selenium during the leaching process was not discussed further.
Luo et al.15 studied the oxidation dissolution of synthetic copper–silver selenide minerals using the intermittent galvanostatic polarization technique. Hou et al.16 studied the kinetics of leaching selenium from Ni-Mo ore smelter dust. Li et al.17 performed thermodynamic analysis on the leaching process of selenium residues. Kilic et al.18 investigated the recovery of copper and selenium from copper anode slimes; they selected several oxidation additives for the dissolution of selenium. Fan et al.19 investigated the recovery of tellurium from high-tellurium-bearing materials by an alkaline pressure leaching process, with thermodynamic evaluation and experimental study, which is similar to selenium leaching. Mokmeli et al.20 studied the kinetics of selenium removal from a sulfate–sulfuric-acid solution and presented a comprehensive model capable of predicting the rate constants for various Cu2+–H2SO4 compositions and temperatures. It was proposed that a plausible reduction sequence for selenate was HSeO4 − → H2SeO3 → Se → CuSe → Cu2Se, which may be converse to the leaching sequence.21 Taskinen et al.22 , 23 studied the mechanism of selenium vaporization from silver selenide and copper selenide.
According to the results of thermodynamic studies, the selenium oxidation states include selenide (Se2−), elemental selenium (Se0), selenite (Se4+), and selenate (Se6+). Selenides and elemental selenium usually exist as solids. The mechanism for most leaching processes involves oxidation of selenide to either selenite or selenate, which mainly exist as ions in solution. As selenate typically exists at a high electrochemical potential, the most common state found at moderate potentials is selenite.20 Despite extensive research on the thermodynamics, kinetics, and oxidation mechanism of selenium leaching, the exact transformation of selenium-containing phases during the leaching process remains unclear.
In this study, the transformation of selenium-containing phases in copper anode slimes during the leaching process is investigated. As nitric acid is a good oxidant and we have found that selenium can be extracted effectively by a nitric-sulfuric acid mixture, the experiments in this article were conducted in the mixed acid system.
Experimental
Materials
The copper anode slimes were purchased from Jinchuan Group Ltd. All chemical reagents used in this work were of analytical grade. The mineralogical process was investigated in a previous work,24 which indicated that the main phases of the copper anode slimes include lead sulfate, barium sulfate, copper sulfate, copper oxide, and Ag-Cu selenide. The main elements of the copper anode slimes were analyzed by inductively coupled plasma-atomic emission spectrometry (ICP-AES) and atomic absorption spectroscopy (AAS). The results are shown in Table I.
Procedure
To study the transformation of selenium-containing phases during the leaching process without the influence of copper sulfate or copper oxide, the raw copper anode slimes were first washed with a 0.5-M sulfuric acid solution. Table I shows the chemical composition of the decopperized anode slimes.
First, 560-mL of a 0.5-M HNO3 and 1.25-M H2SO4 acid mixture was placed in a 1000-mL flask (d = 14 cm), which was put into a water bath and heated to 90°C with a stirring speed of 300 rpm (double blade, d = 6 cm). At temperature stabilization, 8-g decopperized anode slimes were added to initiate the reaction. Then, 2-mL samples were withdrawn periodically from the reactor and the lixivium was separated from the residues. Finally, the filtrate was diluted to 100 mL and analyzed by ICP-AES to determine the copper, silver, and selenium contents.
Characterization
The residues obtained during the leaching process were characterized by x-ray powder diffraction (XRD) and a scanning electron microscope equipped with an energy-dispersive detector (SEM–EDS) to determine the transformation of the selenium-containing phases.
Results and Discussion
Leaching Experiments
The leaching efficiencies of copper, silver, and selenium with time are presented in Fig. 1. The leaching rate of copper is faster than that of silver and selenium, and the efficiency reached 98.4% in 5 min. The leaching efficiency of silver increases gradually up to 80.0% in the first 60 min, and it maintains this value until 160 min. The leaching efficiency of selenium increases slowly in the first 50 min up to 17.7% and then more rapidly up to 98.3% in the next 110 min.
Eh–pH Diagram
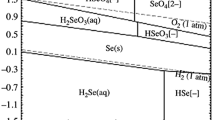
The stability regions for selenium oxidation states with copper and silver at pH = −0.5 are shown in Fig. 2 (based on the Eh–pH of the Se-Cu-H2O and Se-Ag-H2O systems using FactSage 6.1). It is obvious that a large stability field of elemental selenium is located between those of selenide and selenite. From a thermodynamic viewpoint, the elemental selenium may therefore be an intermediate product in the oxidation from selenide to selenite. Similar to the Se-Cu-H2O system, the oxidation potentials for transforming selenide to elemental selenium and for transforming elemental selenium to selenite are 0.03 V and 0.74 V in the Se-Ag-H2O system, respectively. Nevertheless, the potential for transforming CuSe2 into Cu2+ (0.5 V) is lower than that for transforming Ag2Se into Ag+ (0.79 V; Ag2SeO3 is soluble in nitric acid). It can be inferred that it is more thermodynamically favorable to extract copper from Au-Cu selenide than silver.15
According to the thermodynamic analysis, the chemical reaction can be expressed as follows:21
Transformation of Selenium-Containing Phases
The phase transformation of copper anode slimes during leaching was studied by XRD and SEM–EDS. The decopperized anode slimes, i.e., the residues obtained after leaching for 5 min, 50 min (the inflection in the curve), and 160 min, were selected as samples. Figure 3 shows the XRD patterns of the samples. Figure 4 shows the SEM images and EDS spectra of the samples. As this investigation is focused on Cu, Ag, and particularly Se, the peaks for Pb, S, O, C, and Au in EDS spectra are not discussed herein. Table II shows the chemical analysis of the samples and the EDS results of selenium-containing phases (the total contents of Cu, Ag, and Se were normalized to 100 wt.%).
Ag-Cu Selenide → Ag2Se
The XRD pattern of the decopperized anode slimes is shown in Fig. 3a. It indicates that selenium occurs as Ag-Cu selenide. Figure 4a shows a particle in this phase with the corresponding spectra in Fig. 4 EDS 1. According to Table II, the composition is Cu x Ag y Se (x = 0.24–0.64, y = 1.21–2.31), with an average of Cu0.4Ag1.88Se. Apparently, the relative ratio of copper, silver, and selenium is variable in the Ag-Cu selenide.
In the residue leached for 5 min, the copper, silver, and selenium contents are 0.04%, 14.14%, and 6.34%, respectively; in the lixivium, the concentrations of copper, silver, and selenite ions are 341 ppm, 208 ppm, and 22 ppm, respectively. The results demonstrate that copper has been almost completely leached. Figure 3b shows that the diffraction peaks of Ag-Cu selenide (2θ = 36.173°, 63.445°) disappear, whereas new peaks of elemental selenium (2θ = 31.033°, 48.384°, 58.408°, 64.692°) and silver selenide (Ag2Se, 2θ = 34.757°, 48.486°) appear. Figure 4b shows a particle in this residue possessing a porous morphology compared with the particle in Fig. 4a, resulting from the copper having been leached from the Ag-Cu selenide. Furthermore, the copper peaks are absent in the spectra shown in Fig. 4 EDS 2. According to Table II, the composition is Cu x Ag y Se (x = 0–0.09, y = 1.15–2.62), with an average of Cu0.01Ag1.76Se. Therefore, copper was preferentially leached from Ag-Cu selenide rather than silver and Ag-Cu selenide has been transformed into silver selenide, which is consistent with the results shown in the Eh–pH diagram.
Ag2Se → Se0
In the residue leached for 50 min, the silver and selenium contents are 5.68% and 5.71%, respectively. In the lixivium, the concentrations of copper, silver, and selenite ions are 344 ppm, 1402 ppm, and 247 ppm, respectively. Therefore, most of the silver has clearly been leached. Figure 3c shows that the diffraction peaks of silver selenide (2θ = 34.757°) were reduced, whereas those of elemental selenium (2θ = 31.033°, 64.692°) were intensified. Figure 4c shows that the selenium-containing particles become more porous after 50 min leaching than after only 5 min leaching. The spectra in Fig. 4 EDS 3 shows that the peak for silver is significantly reduced compared with Fig. 4 EDS 2. According to Table II, the composition is Ag y Se (x = 0–0.27), with an average of Ag0.13Se. The silver content is significantly reduced, and indeed, sometimes selenium occurs as elemental selenium in this residue. These observations show that the silver selenide was converted into elemental selenium. These findings are consistent with the result shown in Eh–pH diagrams, in which the elemental selenium is the intermediate product in the oxidation from selenide to selenite. Therefore, the slow leaching rate of selenium in the first 50 min during the leaching experiment can be attributed to the formation of the elemental selenium intermediate.
Se0 → SeO3 2−
In the residue leached for 160 min, the silver and selenium contents are 3.45% and 0.15%, respectively. In the lixivium, the concentrations of copper, silver, and selenite ions are 340 ppm, 1776 ppm, and 1378 ppm, respectively, implying that selenium has been almost completely leached. In Fig. 3d, the peaks of silver selenide and elemental selenium almost disappear as 98.3 wt.% selenium has been leached. Figure 4d shows that the selenium-containing phase in the residue is elemental selenium, existing as an attachment to the PbSO4 particle (the main phase of the residue, as shown in XRD pattern). This is further demonstrated in Fig. 4 EDS 4 and Fig. 4 EDS 5. In Table II, elemental selenium is found only in two points. Therefore, almost all the elemental selenium is transformed into selenite ion and dissolved in the solution.
As noted, the transformation of selenium-containing phases during leaching can be described in three stages. First, copper is leached from Ag-Cu selenide, transforming it into silver selenide; second, silver selenide is transformed into elemental selenium; third, elemental selenium is transformed into selenite and dissolved in the solution. The phase transformation of selenium can be expressed as follows:
Conclusion
The transformation of selenium-containing phases in copper anode slimes during the leaching process has been investigated in this article. As selenium occurs as Ag-Cu selenide, the leaching efficiencies of copper, silver, and selenium were studied with time. The stability regions of selenium phases were studied to allow further analysis of the leaching process. Additionally, the intermediate residues formed during the leaching process were investigated to further the understanding of the transformation of selenium-containing phases.
The leaching experiment indicated that copper was the most easily dissolved and that silver only reached maximum extraction (80%) after 60 min. The leaching efficiency of selenium increased slowly in the first 50 min and then increased faster, to 98.3%, for the remaining time. From a thermodynamic viewpoint, (I) copper is more preferentially leached from Ag-Cu selenide than silver, and (II) elemental selenium may be an intermediate product of the oxidation from selenide to selenite. The XRD and SEM–EDS demonstrated that the transformation from Ag-Cu selenide into selenite during the leaching process could be expressed as follows:
This transformation can explain the different leaching rates of copper, silver, and selenium. Specifically, the intermediate product of elemental selenium is the main reason for the slow leaching rate of selenium in the first 50 min.
References
H. Oosterhof, JOM 67, 398 (2015).
S.C. Spinks, J. Parnell, D. Bellis, and J. Still, Ore Geol. Rev. 72, 114 (2016).
J.D. Scott, Metall. Mater. Trans. B 21, 629 (1990).
H.Y. Yang, X.J. Li, L.L. Tong, and G.B. Chen, Chin. J. Nonferrous Met. 24, 269 (2014).
J. Hait, Trans. Inst. Min. Metall. C 118, 240 (2009).
O. Hyvärinen, L. Lindroos, and E. Yllö, JOM 41, 42 (1989).
D.K. Lu, Y.F. Chang, H.Y. Yang, and F. Xie, Trans. Nonferrous Met. Soc. China 25, 1307 (2015).
A. Chen, Z.W. Peng, J.Y. Hwang, Y.T. Ma, X.H. Liu, and X.Y. Chen, JOM 67, 493 (2015).
D. Li, X. Guo, Z. Xu, Q. Tian, and Q. Feng, Hydrometallurgy 157, 9 (2015).
W.F. Liu, T.Z. Yang, D.C. Zhang, L. Chen, and Y.N. Liu, Int. J. Miner. Process. 128, 48 (2014).
B.Y. Zhang and J.K. Wang, Min. Metall. Eng. 27, 41 (2007).
T.T. Chen and J.E. Dutrizac, Can. Metall. Q. 32, 267 (1993).
J. Hait, R.K. Jana, V. Kumar, and S.K. Sanyal, Ind. Eng. Chem. Res. 41, 6593 (2002).
J. Hait, R.K. Jana, and S.K. Sanyal, Ind. Eng. Chem. Res. 43, 2079 (2004).
R. Luo, N.M. Rice, N. Taylor, and R. Gee, Hydrometallurgy 45, 221 (1997).
X. Hou, L. Xiao, C. Gao, Q. Zhang, and L. Zeng, Hydrometallurgy 104, 76 (2010).
Q. Li, B. Zhang, X.B. Min, W.Q. Shen, and J. Cent, South Univ. 19, 2440 (2012).
Y. Kilic, G. Kartal, and S. Timur, Int. J. Miner. Process. 124, 75 (2013).
Y. Fan, Y. Yang, Y. Xiao, Z. Zhao, and Y. Lei, Hydrometallurgy 139, 95 (2013).
M. Mokmeli, B. Wassink, and D. Dreisinger, Hydrometallurgy 139, 13 (2013).
M. Mokmeli, D. Dreisinger, and B. Wassink, Hydrometallurgy 153, 12 (2015).
P. Taskinen, S. Patana, P. Kobylin, and P. Latostenmaa, Oxid. Met. 81, 503 (2014).
P. Taskinen, S. Patana, P. Kobylin, and P. Latostenmaa, High Temp. Mater. Process. 33, 1 (2014).
X.J. Li, H.Y. Yang, L.L. Tong, and G.B. Chen, J. Northeast Univ. 34, 560 (2013).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. U1608254, 51374066). The authors also appreciate Prof. Wolfgang Sand, University of Duisburg-Essen, Germany, for his assistance in some constructive suggestions and language improvement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X.J., Yang, H.Y., Jin, Z.N. et al. Transformation of Selenium-Containing Phases in Copper Anode Slimes During Leaching. JOM 69, 1932–1938 (2017). https://doi.org/10.1007/s11837-017-2448-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-017-2448-x