Abstract
A new process of producing synthetic rutile from molten titanium slag with the addition B2O3 is proposed. The process includes a molten modification process and a leaching process. The molten modification process was conducted by adding B2O3 into molten slag. The leaching process was conducted by adding hydrochloric acid and subsequent NaOH. The results show that CaO and MgO are leached out by hydrochloric acid and that synthetic rutile is further improved by NaOH. The optimized conditions are 2% B2O3 amount, 5% hydrochloric concentration, 80°C leaching temperature, and 30 min leaching time. The synthetic rutile with 86.77% TiO2 and 1.23% (CaO + MgO) was prepared. From x-ray diffraction results, thermodynamic calculation and the theory of bond parameter function, with the addition of B2O3, calcium silicate is transformed into calcium borate and anosovite is transformed into magnesium borate. Calcium borate and magnesium borate are leached out by hydrochloric acid, leading to the enrichment of rutile.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The products of titanium metallurgy mainly contain two types: titanium white and metallic titanium. Titanium white from the fluidized bed chlorination and metallic titanium are both produced from TiCl4.1 , 2 The fluidized bed chlorination is the main process of producing TiCl4 when compared with the molten salt process.3,4, – 5 TiO2 and gangues react with chlorine gas at 1000°C to form corresponding volatile chlorides during the fluidized bed chlorination.6 The melting points of CaCl2 and MgCl2 are 775°C and 714°C, respectively. CaCl2 and MgCl2 are liquid under the temperature of chlorinating titanium slag.7 If the CaO and MgO content in the titanium-rich material is high, CaCl2 and MgCl2 will make reactant particles cohere and accumulate in fluidized bed easily, deteriorating the steady state of the fluidized bed. Therefore, the CaO and MgO content in titanium-rich material for fluidized bed chlorination is required to be as low as possible, usually less than 1.5% to avoid deteriorating the steady state of the fluidized bed.8
Researchers have tended to upgrade ilmenite to qualified titanium-rich material due to the natural rutile shortage. Several industrial processes have been developed to upgrade ilmenite to qualified titanium-rich material, including the smelting process,9,10,11, – 12 the Bencher process,12 and the acid leaching process.12 The electric furnace smelting is regarded as the most significant process in China due to its high efficiency and low waste emission. Panzhihua, located in the Sichuan province of China, holds ilmenite reserves of 870 million tons, accounting for 35% of total reserves in the world.13 Nevertheless, the CaO and MgO content in titanium slag from Panzhihua ilmenite is too high to be directly employed for fluidized bed chlorination. In this case, it is imperative to develop a new process of producing synthetic rutile from high CaO and MgO titanium slag.
In recent decades, solid and gas additives have been applied for producing synthetic rutile from high CaO and MgO titanium slag. Process features are summarized in Table I and discussed subsequently.
Although qualified synthetic rutile was obtained by these processes, they are highly additive and require significant energy consumption. It is necessary to explore a novel and efficient additive to modify the titanium slag to obtain synthetic rutile. B2O3 is a kind of fluxing agent popularly applied in the metallurgy industry. The addition of B2O3 greatly increases the acidity and influences the stability of the slags.20 , 21 Therefore, B2O3 has been used to modify the Ti-bearing blast furnace slags to obtain the rutile. It has been found that small amounts of B2O3 can remarkably restrict the linkage between Ca2+ and \( {\text{TiO}}_{3}^{2 - } \), enhancing the precipitation of rutile in Ti-bearing blast furnace slags.22 , 23 Therefore, it is reasonable to expect that small amounts of B2O3 can also restrict the precipitation of anosovite and calcium silicate in titanium slag to obtain the rutile efficiently. Furthermore, to use the sensible heat of titanium slag and avoid the reheating for the modification in Table I, B2O3 can be added into molten titanium slag during production of titanium slag from ilmenite, more specifically when ilmenite has been reduced to be molten iron and titanium slag in an electric furnace.
In this article, the new process consists of the molten modification process and the leaching process. The molten modification process was implemented by adding B2O3 into the molten titanium slag in an induction furnace to simulate the molten modification process, and subsequent leaching process was carried out to obtain synthetic rutile. The leaching process consists of hydrochloric leaching and NaOH leaching. Molten modification conditions and hydrochloric leaching conditions were optimized. NaOH leaching was selected as the necessary process to improve the synthetic rutile under predetermined conditions. Furthermore, the molten modification mechanism and hydrochloric leaching mechanism were investigated by x-ray diffraction (XRD), thermodynamic calculation, and the theory of bond parameter function.
Experimental
Titanium Slag and Reagents
Titanium slag from Panzhihua ilmenite was used in this work. The slag was analyzed by x-ray fluorescence (XRF). The chemical composition of the slag was as follows (wt.%): TiO2 = 73.81, FeO = 12.21, SiO2 = 5.89, MnO = 1.53, MgO = 2.51, Al2O3 = 2.83, and CaO = 1.00. The CaO and MgO content are 3.51%, which are too high to be used for fluidized bed chlorination. All the other reagents are analytical grades in this article.
Experimental Procedure
The experiment includes a molten modification process and a leaching process. In the molten modification process, B2O3 was added into molten titanium slag when ilmenite had been reduced to be molten iron and titanium slag. In this article, a mixture of B2O3 and titanium slag was placed in a cylindrical molybdenum crucible and then heated by an induction furnace to simulate the molten modification process. The modified slag was obtained after the molten modification process. The leaching process includes hydrochloric leaching and NaOH leaching. The molten modification conditions and hydrochloric leaching conditions were optimized. NaOH leaching was implemented under predetermined conditions because the process of removing SiO2 by NaOH leaching was matured.
Molten Modification Process
The molten modification process was simulated in an induction furnace, where the temperature was controlled by the input power. When the power of the induction furnace was set at 11.00 kW, the temperature of melts in crucibles was 1700°C. A mixture sample, which was charged into a cylindrical molybdenum crucible, was heated and melted in an induction furnace with the power of 11.00 kW for 30 min to simulate the molten state of titanium slag in an electric furnace. After 30 min, the molybdenum crucible was taken out and cooled naturally in air. After being cooled to room temperature, the slag samples were grounded for the leaching process use.
Leaching Process
The leaching process includes hydrochloric leaching and NaOH leaching. The modified slag was leached with hydrochloric acid under a predetermined stirring speed of 300 rpm, liquid/solid ratio of 10.0 mL/g, and particle size range of 74–125 μm. In the hydrochloric leaching process, removing efficiencies for the impurities were evaluated by the leaching rate of CaO and MgO. The NaOH leaching process was implemented with predetermined conditions: 8% NaOH concentration, 110°C leaching temperature, 60 min leaching time, and 5-mL/g liquid/solid ratio. After leaching, the slurry was filtered by vacuum. The residues were dried in an oven at 105°C for 12 h and calcined in air at 900°C for 30 min.
Results and Discussion
Mechanism of the Molten Modification Process
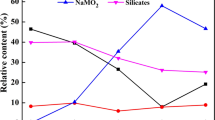
The mechanism of the molten modification process was investigated by XRD, thermodynamic calculation, and the theory of bond parameter function. The phases in modified slag with different B2O3 amounts were characterized by XRD (Fig. 1). Compared with the peaks in untreated slag, the peaks of calcium silicate weaken while the peaks of calcium borate appear in modified slag. These results indicate that calcium silicate is partly transformed into calcium borate. The peak intensities of anosovite become smaller, whereas the peak intensities of rutile are sharper with the B2O3 amount increasing. It implies that anosovite is transformed into magnesium borate in the molten modification process, enriching rutile.
With the XRD results, possible reactions in the molten modification process can be expressed as Eqs. 1–4. Figure 2 presents the relationship between Gibbs free energy changes of reactions and temperature calculated by FactSage6.3 software.24 The more negative value of the Gibbs free energy change means greater reaction tendency. It can be seen that the Gibbs free energy change of Eq. 3 is more negative than that of Eq. 1, indicating that MgO reacts with B2O3 more easily than with TiO2. The Gibbs free energy change of Eq. 4 is more negative than that of Eq. 2, indicating that CaO reacts with B2O3 more easily than with SiO2. With the addition of B2O3, the activity of CaO and MgO becomes small based on the molecular theory for slag structure proposed by H. Schenck,25 , 26 inhibiting the precipitation of CaSiO3 and MgTi2O5 and facilitating the formation of Ca2B2O5 and Mg3B2O6. It is in accord with the experiment mechanism shown by the results from XRD:
The reaction mechanism in the molten modification process was also demonstrated by the theory of bond parameter function. The \( {\text{X}}_{\text{P}}^{*} {\text{Z}}/{\text{R}}_{\text{K}} \) value of the ions in the modified slag are Ca2+ = 2.02, Mg2+ = 3.7, Fe2+ = 4.45, Ti3+ = 6.1, Fe3+ = 8.42, Al3+ = 9.0, Ti4+ = 9.44, Si4+ = 18.56, and B3+ = 22.67.27 , 28 The parameter \( {\text{X}}_{\text{P}}^{*} {\text{Z}}/{\text{R}}_{\text{K}} \) represents the acidity and basicity capacity of the ions in the melt, where \( {\text{X}}_{\text{P}}^{*} \) is electronegativity of the element and Z and RK are the valence and actual radius of the ion, respectively. When the \( {\text{X}}_{\text{P}}^{*} {\text{Z}}/{\text{R}}_{\text{K}} \) value increases, basicity decreases and acidity increases. Therefore, B3+ has the strongest acidity, while Ca2+ has the strongest basicity and Mg2+ has the second strongest basicity after Ca2+. Therefore, CaO reacts with B2O3 preferentially, generating calcium borate. MgO reacts with B2O3 subsequently, generating magnesium borate. These results are consistent with the phase transformations in Fig. 1 and the thermodynamic calculation in Fig. 2.
Mechanism of the Hydrochloric Leaching Process
To investigate the mechanism of the hydrochloric leaching process, the modified slags phases before and after leaching were characterized by XRD (Fig. 3). Compared with peaks in modified slag before leaching, the peaks of calcium borate and magnesium borate disappear in the modified slag after leaching. The phase transformations demonstrate that the calcium borate and magnesium borate are leached out by hydrochloric acid. Meanwhile, rutile is the main phase in the final slag. These results suggest that anosovite is almost destroyed in the molten modification process by the addition of B2O3.
Production of Synthetic Rutile by the New Process
Effect of Technical Conditions
The effects of experimental factors on removing CaO and MgO were investigated (Fig. 4). The B2O3 amount has the most significant influence on leaching impurities, followed by hydrochloric concentration. Leaching temperature and leaching time have important effects on leaching impurities. The leaching rate of CaO and MgO increases rapidly with B2O3 amounts from 0% to 2% but slowly from 2% to 6%. The leaching rate of CaO and MgO goes fast with hydrochloric concentration increasing but gradually with a hydrochloric concentration of more than 5%. The leaching rate of CaO and MgO rises with temperature increasing but tardily with temperature greater than 80°C. The leaching rate of CaO and MgO elevates sharply with leaching time increasing but increases gradually with leaching time of more than 30 min.
Therefore, considering the leaching results and energy consumption, the optimized conditions for the new process of producing synthetic rutile are 2% B2O3 amount, 5% hydrochloric concentration, 80°C leaching temperature, and 30 min leaching time. Synthetic rutile containing 80.54% TiO2, 1.12% (CaO + MgO), and 7.87% SiO2 was prepared under optimized conditions. It is obvious that the SiO2 content is too high and the content of TiO2 was less than 85%.
Upgrading of Synthetic Rutile
Considering the high content SiO2 in synthetic rutile after hydrochloric leaching, NaOH leaching was introduced to improve synthetic rutile. Synthetic rutile containing 86.77% TiO2, 1.23% (CaO + MgO), and 0.91% SiO2 was obtained after NaOH leaching. In the new process, the qualified synthetic rutile was prepared for fluidized bed chlorination with lower energy consumption and a smaller amount of additive.
Compared with the previous processes in Table I, the new process has two advantages: the lower energy consumption and smaller amount additive. First, B2O3 was added into molten titanium slag when ilmenite had been reduced to be molten iron and titanium slag in the electric furnace. The modified slag was obtained after the molten modification process. The specific heat capacity for titanium slag is 900 J/(kg °C).29,30,31, – 32 Therefore, the new process uses at least 419 kW h/t sensible heat of molten titanium slag and meanwhile avoids at least 197 kW h/t reheating for the modification in previous processes in Table I. Second, the B2O3 amount in the new process is smaller than in the previous processes in Table I.
Conclusion
-
1.
The XRD results show that with the addition of B2O3, calcium silicate is transformed into a calcium borate and anosovite is transformed into a magnesium borate. Then, calcium borate and magnesium borate are leached out by hydrochloric leaching, enriching the rutile. It is demonstrated by thermodynamic calculation and the molecular theory that with the addition of B2O3, the activity of CaO and MgO become small in modified slag, inhibiting the precipitation of CaSiO3 and MgTi2O5 and facilitating the formation of Ca2B2O5 and Mg3B2O6.
-
2.
The optimized conditions are 2% B2O3 amount, 5% hydrochloric concentration, 80°C leaching temperature, and 30 min leaching time. Synthetic rutile containing 1.12% (CaO + MgO) was prepared under optimized conditions.
-
3.
Synthetic rutile containing 86.77% TiO2, 1.23% (CaO + MgO) was obtained with optimized conditions and predetermined NaOH leaching conditions. Compared with the previous processes, the qualified synthetic rutile was prepared for fluidized bed chlorination with lower energy consumption and a smaller amount of additive.
References
W.S. Zhang, Z.W. Zhu, and C.Y. Cheng, Hydrometallurgy 108, 177 (2011).
S.S. Liu, Y.F. Guo, G.Z. Qiu, and T. Jiang, Trans. Nonferrous Met. Soc. China 23, 1174 (2013).
Y. Chang, Non-Ferr. Min. Metall. 25, 37 (2009).
H. Chen, Mod. Mach. 37, 68 (2010).
G. Deng, Gang Tie Fan Tai 32, 1 (2011).
A. Adipuri, Y. Li, G.Q. Zhang, and O. Ostrovski, Int. J. Miner. Process. 100, 166 (2011).
J.G. Speight, Lange’s Handbook of Chemistry, 16th ed. (New York: McGraw-Hill, 2005), pp. 126–163.
G. Deng, Titanium Metallurgy (Beijing: Metallurgical Industry Press, 2010), pp. 56–146.
M. Guéguin and F. Cardarelli, Miner. Process. Extr. Metall. Rev. 28, 1 (2007).
P.C. Pistorius, J. S. Afr. Inst. Min. Metall. 108, 35 (2008).
A.F.S. Schoukens, D.J. Morris, and F.S. McComb, U.S. patent 6,733,561 B2 (2004).
D. Filippou and G. Hudon, JOM 61, 36 (2009).
F.X. Wu, X.H. Li, Z.X. Wang, L. Wu, H.J. Guo, X.H. Xiong, X.P. Zhang, and X.J. Wang, Int. J. Miner. Process. 98, 106 (2011).
B. Jarish and S. Lambert, U.S. patent 4,038,363 (1977).
Y.F. Guo, S.S. Liu, T. Jiang, G.Z. Qiu, and F. Chen, Hydrometallurgy 147, 134 (2014).
H.G. Dong, T. Jiang, Y.F. Guo, J.L. Chen, and X.X. Fan, Hydrometallurgy 113, 119 (2012).
G.W. Elger, R.A. Stadler, and P.E. Sanker, U.S. patent 4,120,694 (1978).
M. Gueguin, U.S. patent 5,063,032 (1990).
K. Borowiec, A.E. Grau, M. Gueguin, and J.F. Turgeon, U.S. patent 6,733,561 B2 (1998).
H.M. Wang, G.R. Li, Q.X. Dai, Y.C. Lei, Y.T. Zhao, B. Li, G.M. Shi, and Z.M. Ren, ISIJ Int. 46, 637 (2006).
X. Yu, G.H. Wen, P. Tang, and H. Wang, Ironmak. Steelmak. 36, 623 (2009).
Y.Q. Sun, J.L. Liao, K. Zheng, X.D. Wang, and Z.T. Zhang, JOM 66, 2168 (2014).
Y.Q. Sun, Z.M. Li, L.L. Liu, X.D. Wang, and Z.T. Zhang, ISIJ Int. 55, 158 (2015).
http://www.factsage.com/. Accessed 26 Aug 2016.
N. Iwamoto, Trans. JWRI 4, 231 (1975).
X.H. Huang, Metallurgy Principle for Iron and Steel, 3rd ed. (Beijing: Metallurgical Industry Press, 2013), pp. 163–193.
N.Y. Chen, Bond Parameter Function and Its Application, 1st ed. (Beijing: Science Press, 1976), pp. 5–28.
S. Ren, J.L. Zhang, X.D. Xing, B.X. Su, Z. Wang, and B.J. Yan, Ironmak. Steelmak. 41, 500 (2014).
H. Kotzé and P.C. Pistorius, J. S. Afr. Inst. Min. Metall. 110, 57 (2010).
H. Kotzé (Ph.D. Dissertation, University of Pretoria, Pretoria, 2007).
J.H. Zietsman and P.C. Pistorius, J. S. Afr. Inst. Min. Metall. 104, 693 (2004).
G. Lin, Iron Steel Scrap China 25, 30 (2007).
Acknowledgements
Special appreciation is extended to master student Lin Bai at the Chongqing University for help on the experiment and sample analyzing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, H., Duan, H., Tan, K. et al. Production of Synthetic Rutile from Molten Titanium Slag with the Addition of B2O3 . JOM 69, 1914–1919 (2017). https://doi.org/10.1007/s11837-017-2288-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-017-2288-8