Abstract
The recovery of iron from chromium vanadium-bearing titanomagnetite concentrate was investigated by direct reduction, followed by magnetic separation. The results indicated that the metallization rate of iron can reach 98.9% at a temperature of 1200°C for a reduction duration of 60 min with the addition of 16% graphite powder and 0.5% sodium oxalate. Although the addition of borax, sodium carbonate and sodium oxalate to the chromium vanadium-bearing titanomagnetite concentrate can all improve the metallization rate of iron, the effect of sodium oxalate was the best. Sodium oxalate not only increases the metallization rate of iron but also promotes the growth of metallic iron. After magnetic separating, the recovery of iron was 92.8% and the iron content of magnetic concentrate was 88.4%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Panzhihua-Xichang Area of China is widely recognized as having large reserves of vanadium-bearing titanomagnetite,1 with the Hongge mineral deposit the largest.2 It is also the largest chromium-bearing deposit in China.3 Vanadium, chromium and titanium are all important strategic metals and industrial raw materials, and the comprehensive recovery of vanadium, chromium and titanium from the Hongge mineral deposit is therefore required for reasonable use.
At present, the use of vanadium-bearing titanomagnetite concentrate is to smelt it in a blast furnace. Vanadium can be recovered in the form of vanadium slag by molten iron selective oxidation. Most of the Ti component is separated from the iron and concentrated into the slag in the iron blast furnace process.4 Due to the dispersed distribution of the Ti components in various fine-grained mineral phases with complex interfacial combinations, it is difficult to recover the Ti components from this slag.5
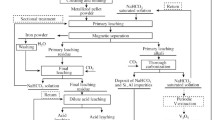
To recover iron, titanium, vanadium and chromium, a process of direct reduction and magnetic separation has been proposed.6–9 After that, metallic iron and the concentration material containing the oxides of titanium, vanadium and chromium are obtained. The key to exploit and use the chromium vanadium-bearing titanomagnetite lies in separating the iron, chromium and vanadium as well as the titanium. In the magnetic separation process, the higher the iron metallization rate as well as the larger the particle sizes of the iron, the better the iron separation effect becomes. To improve the metallization rate of the iron, additive agents have often been used in the direct reduction process, such as fluorite, sodium carbonate, sodium sulfate and borax.10–14 These additives can to some extent increase the metallization rate of the iron, but the effect is not ideal.
To further increase the metallization rate and particle size of the iron, a new kind of additive agent, sodium oxalate, has been used. The present work focuses on the effect of sodium oxalate in the direct reduction process.
Experimental
Materials and Analysis
The chromium vanadium-bearing titanomagnetite concentrate used in this study was obtained from the Panzhihua Iron and Steel Company. The sample was ground to 180 mesh (100% through 180 mesh, 80 μm) before direct reduction. The x-ray fluorescence (XRF) analysis of the chromium vanadium-bearing titanomagnetite concentrate (raw concentrate) is listed in Table I. The reducing agent is graphite powder and the additive agent is sodium oxalate, and were of analytical grade.
The content of total iron (TFe) in both the chromium vanadium-bearing titanomagnetite concentrate and the reduction products was measured by sulfuric acid mixed with phosphoric acid dissolving—titanium trichloride–potassium dichromate volumetric analysis.15 The content of metallic iron (MFe) in the reduction products was measured by FeCl3-K2Cr2O7 volumetric analysis.16 The metallization rate of the iron was calculated by the following equation:
where w(MFe) is the mass fraction of metallic iron and w(TFe) is the mass fraction of total iron.
X-ray diffraction (XRD) patterns were recorded by a Rigaku Miniflex Diffractometer with Cu Kα x-ray radiation at 35 kV and 20 mA. TG (thermogravimetric)/DSC (differential scanning calorimetry) curves were obtained on a TG-DSC thermoanalyzer (Netzsch STA 449C) in the temperature range 25–1200°C at a heating rate of 5°C/min under dynamic argon (70 ml/min) atmospheres.
Figure 1 shows the XRD patterns of the chromium vanadium-bearing titanomagnetite concentrate. It can be seen that the mineral compositions of the chromium vanadium-bearing titanomagnetite concentrate are tianomagnetite (Fe2.75Ti0.25O4) and ilmenite (FeTiO3).
Experimental Procedure
The experiments were carried out in a vertical MoSi2 resistance furnace that was controlled by a program controller with an R-type thermocouple. The temperature accuracy was within ±3°C. The protective atmosphere was argon.
Prior to the direct reduction process, 100 g of ground chromium vanadium-bearing titanomagnetite concentrate was placed in a mixing drum, and then uniformly mixed with the desired graphite powder or mixed with the desired graphite powder and the additive agent. The mixed sample was compacted by a hydraulic machine with pressure of 70 KN, and then was transferred to a corundum crucible and placed in a MoSi2 resistance furnace. The sample was heated with a heating rate of 10°C/min to the expected temperature and held at this temperature for a designed time. The sample was then cooled to room temperature. After being crushed and ground, the particle size of the reduced samples was less than 200 mesh (75 µm), and they were then magnetically separated in a water solution by a homemade rotary magnetic separator with magnetic field intensities of 700 GS and 500 GS in sequence.
Results and Discussion
Effect of Graphite Powder Addition
In this step, the chromium vanadium-bearing titanomagnetite concentrate was mixed only with the graphite powder. The reduction experiments were conducted at 1200°C for 60 min, and the results are shown in Fig. 2.
As shown, the metallization rate of iron increased rapidly from 56.9% to 93.6% as the graphite powder addition increased from 10% to 16%. Further addition of graphite powder resulted in insignificant increases in the metallization rate of the iron. Hence, 16% graphite powder addition was chosen as the optimized amount.
Effect of Reduction Time
To discover the role of reduction time on the metallization rate of iron, experiments based on alternate reduction times from 6 min to 180 min were carried out, while keeping the reduction temperature at 1200°C and the graphite powder addition at 16%. The results shown in Fig. 3 indicate that the metallization rate of iron increased with the increase of reduction time. At a reduction time of 60 min, the metallization rate of iron reached 93.8%, but a further increase in the reduction time only resulted in a small increase in the iron metallization rate. Therefore, the reduction time was kept at 60 min in the subsequent tests.
Effect of Reduction Temperature
The effect of reduction temperature was investigated over a temperature range from 1100°C to 1250°C. Figure 4 shows a rapid increase in the metallization rate of iron when the reduction temperature increased from 1110°C to 1200°C. After 1200°C, the increase in the metallization rate of iron is no longer obvious. It was concluded that 1200°C was an appropriate reduction temperature.
Effect of Sodium Oxalate Addition
The effect of sodium oxalate addition on the metallization rate of iron was studied while the reduction temperature, the reduction time and the graphite powder addition were kept constant at 1200°C, 60 min and 16%, respectively. According to the results illustrated in Fig. 5, the metallization rate of iron increased with the increase of sodium oxalate addition and reached a peak value of 98.9% when the addition of sodium oxalate was 0.5%, but then decreased with a further increase of sodium oxalate addition. That is to say, a small amount of sodium oxalate addition can improve the metallization rate of iron, but more than 0.5% will hinder the increase.
Figure 6 shows the TG/DSC curves of the mixture of chromium vanadium-bearing titanomagnetite concentrate with 16% graphite powder, and with or without 0.5% sodium oxalate. This is indicated by comparison of the endothermic peak in the DSC curve, showing that the reaction temperature of the mixture with sodium oxalate addition is obviously decreased from 1109°C to 1058°C. Accordingly, it can also be seen from the TG curve that the weightlessness start temperature of the mixture obviously decreased when the sodium oxalate was added.
To further understand the effects of sodium oxalate on direct reduction, XRD analysis of the reduction products was conducted and the results are shown in Fig. 7. Comparing Fig. 7 to Fig. 1, the peaks of the tianomagnetite (Fe2.75Ti0.25O4) and ilmenite (FeTiO3) both disappeared after direct reduction, and a new mineral phase of iron was generated. This is indicated by comparing Fig. 7a and b, showing that the reduction product with sodium oxalate addition consists of two mineral phases: iron (Fe) and magnesium titanium oxide (MgTi2O5), while the other is iron (Fe) and amalcolite (Fe0.5Mg0.5Ti2O5). In the direct reduction process, the addition of sodium oxalate can destroy the magnesium iron solid solution structure, so as to increase the metallization rate of the iron.
To compare the influence of different additives on the iron metallization rate, borax and sodium carbonate were also chosen for testing. They were all of analytical grade. The reduction temperature, reduction time, graphite powder addition and addition of additives were all kept at 1200°C, 60 min, 16% and 0.5%, respectively. The experimental results are shown in Table II. By comparing the different additives, the metallization rate of iron with sodium oxalate addition is obviously higher than those obtained by adding the other additives.
It is generally believed that the addition of sodium ion is beneficial for the improvement of the iron metallization rate in the reduction process. The transposition of Na+ and Fe2+ can not only improve the hole concentration of iron oxide which can increase the adsorption activity of the CO but also cause the crystal lattice distortion of FexO and reduce the activation energy of the interfacial reaction so as to accelerate the reduction reaction of iron oxide.14,17 To further understand the mechanism of sodium oxalate addition, sodium carbonate was chosen as the additive in a contrasting experiment. In the experimental process, the addition of sodium carbonate was 0.395%, in which the content of sodium ion is equal to that of 0.5% sodium oxalate addition. The reduction temperature, reduction time and graphite powder addition were kept at 1200°C, 60 min and 16%, respectively. Under this condition, the metallization rate of iron was 96.4%. It can be seen from Table II that the metallization rate of iron was 98.9% when the addition of sodium oxalate was 0.5%. In the reduction process, with the increase of temperature, sodium oxalate is decomposed into sodium carbonate and carbon monoxide. The production of carbon monoxide is favorable to the generation of metallic iron. At the same time, the sodium carbonate produced by the decomposition has a broader contact with the reactants and higher reactivity, and therefore the effect of sodium oxalate as the additive agent is better than sodium carbonate.
The magnetic separation effect is also directly related to the metallic iron particle size in addition to the metallization rate of iron. The larger the metallic iron particle size, the better the iron separation effect. Figure 8 shows the morphology of the reduction products. It can be clearly seen through the morphology that the particles of metallic iron in the reduction products with sodium oxalate addition is much larger than that of the other reduction products. Combined with the result in Table II, a conclusion can be obtained that the additives borax, sodium carbonate and sodium oxalate can all improve the iron metallization rate; however, only sodium oxalate can both increase the metallization rate and promote the growth of metallic iron. As mentioned above, the addition of sodium oxalate can decrease the initial decomposition temperature of the mixture, and, in addition, the presence of sodium ions can easily destroy the phase of chromium vanadium-bearing titanomagnetite concentrate. Under the action of these two aspects, the addition of sodium oxalate can promote the grain growth of iron.
After reduction, the metallic iron and oxide were separated by magnetic separation and the results are shown in Table III. As shown, the recovery of iron was 92.8% and the iron content of the magnetic concentrate was 88.4% when the sodium oxalate was selected as the additive.
The XRF analysis of the magnetic iron concentrate is also shown in Table I, from which it can be seen that most of Ti, Cr and V was separated and entered into the magnetic tailing which can be used as the raw material for extracting the titanium, chromium and vanadium.
Conclusion
Although the addition of borax, sodium carbonate and sodium oxalate can all promote the iron metallization rate of the reduction product, the effect of sodium oxalate is the best. In addition to increasing the metallization rate of the iron, adding sodium oxalate can also promote the growth of metallic iron. The metallization rate of iron can reach 98.9% at a temperature of 1200°C for a reduction duration of 60 min with the addition of 16% graphite powder and 0.5% sodium oxalate. After magnetic separating, the recovery of iron was 92.8% and the iron content of magnetic concentrate was 88.4%.
References
P.R. Taylor, S.A. Shuey, E.E. Vidal, and J.C. Gomez, Miner. Metall. Process. 23, 80 (2006).
J.H. Luo, Z.Y. Wu, E.H. Wu, J.H. Li, X.J. Liao, R. Tang, and S.L. Yang, Iron Steel Vanadium Titan. 36, 73 (2015).
G.Z. He, X.H. Du, K. Zhang, Z.S. Tang, T.P. Lou, and G.F. Tu, J. Mater. Metall. 13, 15 (2014).
M.Y. Wang, X.W. Wang, Y.H. He, T.P. Lou, and Z.T. Sui, Trans. Nonferrous Met. Soc. China 18, 459 (2008).
L. Zhang, L.N. Zhang, M.Y. Wang, G.Q. Li, and Z.T. Sui, J. Non-Cryst. Solids 353, 2214 (2007).
L.S. Zhao, L.N. Wang, T. Qi, D.S. Chen, H.X. Zhao, and Y.H. Liu, Hydrometallurgy 149, 106 (2014).
L.S. Zhao, L.N. Wang, D.S. Chen, H.X. Zhao, Y.H. Liu, and T. Qi, Trans. Nonferrous Met. Soc. China 25, 1325 (2015).
S.S. Liu, Y.F. Guo, G.Z. Qiu, T. Jiang, and F. Chen, Trans. Nonferrous Met. Soc. China 24, 3371 (2014).
X.H. Du, B. Xie, and T.P. Lou, J. Northeast. Univ. (Nat. Sci.) 33, 685 (2012).
D.Q. De, Y.F. Guo, G.Z. Qiu, and T. Jiang, J. Cent. South Univ. Technol. 31, 208 (2000).
D.Q. Zhu, T. Jiang, Y.F. Guo, G.Z. Qiu, and J.C. Xu, Multipurp. Util. Miner. Resour. 2, 16 (1999).
T. Jiang, J. Xu, S.F. Guan, and X.X. Xue, J. Northeast. Univ. (Nat. Sci.) 36, 77 (2015).
L.H. Zhou, D.P. Tao, M.X. Fang, F.H. Zeng, and X. Pu, Chin. J. Rare Met. 33, 406 (2009).
D.S. Chen, B. Song, L.N. Wang, T. Qi, and F.J. Wang, J. Univ. Sci. Technol. Beijing 33, 1331 (2011).
J.F. Liu, Tech. Wind 21, 113 (2013).
Y. Zhang and N.H. Pan, J. Magang Staff Worker’s Univ. 13, 12 (2003).
L.H. Zhou, D.P. Tao, M.X. Fang, F.H. Zeng, and X. Pu, Chin. J. Rare Met. 33, 406 (2009).
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (No. 51104186), Natural Science Foundation of Hunan Province (No. 2016JJ2142), and the Fundamental Research Funds for the Central Universities of Central South University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, M., Zhou, S., Wang, X. et al. Recovery of Iron from Chromium Vanadium-Bearing Titanomagnetite Concentrate by Direct Reduction. JOM 68, 2698–2703 (2016). https://doi.org/10.1007/s11837-016-2083-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-016-2083-y