Abstract
Nearly 400 million years of evolution and field-testing by the natural world has given humans thousands of wood types, each with unique structure–property relationships to study, exploit, and ideally, to manipulate, but the slow growth of trees makes them a recalcitrant experimental system. Variations in wood features of two genotypes of peach (Prunus persica L.) trees, wild-type and crinkle-leaf, were examined to elucidate the nature of weak wood in crinkle-leaf trees. Crinkle-leaf is a naturally-occurring mutation in which wood strength is altered in conjunction with an easily observed ‘crinkling’ of the leaves’ surface. Trees from three vigor classes (low growth rate, average growth rate, and high growth rate) of each genotype were sampled. No meaningful tendency of dissimilarities among the different vigor classes was found, nor any pattern in features in a genotype-by-vigor analysis. Wild-type trees exhibited longer vessels and fibers, wider rays, and slightly higher specific gravity. Neither cell wall mechanical properties measured with nanoindentation nor cell wall histochemical properties were statistically or observably different between crinkle-leaf and wild-type wood. The crinkle-leaf mutant has the potential to be a useful model system for wood properties investigation and manipulation if it can serve as a field-observable vegetative marker for altered wood properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predicting macroscale wood structure–property relationships on the basis of wood anatomy is one of the most desirable but elusive goals of wood utilization research because it would accelerate researchers’ efforts to both target lesser-known and plantation-grown woods to the most efficient end uses and develop the information necessary to work toward breeding or engineering trees to grow wood with the most desirable traits for specific applications. Traditionally, wood structure–property relationships have been investigated by attempting to correlate material property data collected from “small clear” specimens (in the United States, historically 2″ × 2″ × 30″ long; c.5 cm × 5 cm × 75 cm) with coarse wood anatomical observations or metrics, such as growth ring width or relative percent latewood, or with finer-scaled observations such as cell wall thickness, or S2 cell wall layer cellulose microfibril angle.1–5 Despite decades of interest in descriptive research on structure–property relationships in wood based on wood structure (see Barnett and Jeronomidis6 for an overview), a global predictive model for bulk wood properties based on wood anatomy remains elusive.
One constraint on our ability to predict properties is related to natural variability within a wild-grown tree—the wood is not the same from the pith to the bark, nor from the base of the tree to the top of the crown, and several trees of the same species may vary widely relative to each other based on site characteristics and the interaction between different local environments and the genetics of individual trees, generating a prohibitively large database for analysis.7–11 Plantation forestry seeks to eliminate some of that variability by controlling, either partially or completely, the genetic factors (choosing carefully bred stock for planting, or genetically identical clones of the same plant) and then planting those seedlings in known spacing on known soils.12–14 Adjacent trees will experience the same environmental conditions, and thus local variability is comparatively minimized.15
Another factor constraining our ability to predict macroscale wood properties is the long time-horizons for controlled experimental work in trees. It can take 7–20 or more years for most commercially relevant temperate tree species to reach an age and stature where harvest is at all feasible. Such a generation time is a significant fraction of a scientist’s total career, thus long-term studies of this type are comparatively rare.
One approach to overcome the time-lag associated with tree generation times is to develop tree “model systems” for experimental manipulation. Such systems should be genealogically known, reliably propagable (vegetatively and sexually), fast-growing, not too large in stature, and, ideally, deliver commercial value on short time scales. In this regard, fruit trees are excellent potential model systems because they meet the scientific and economic criteria, and are an existing resource with well-established markets.
A potentially fruitful model system is peach or peach-almond trees (Prunus persica) because they are actively studied for commercial crop production, cultivated across a wide geographic range, and have an extremely well-established commercial market for fruits from a breadth of cultivars. In California alone, a US$233 million freestone peach market is represented by over 50 diverse cultivars grown on 24,000 acres (c. 9700 ha).16 The genome of peach is also known.17 In addition to commercial cultivars and other lineages under breeding or development, there are also naturally occurring mutants, including a Prunus persica mutant known as crinkle-leaf.18
Plants exhibiting the crinkle-leaf (CL) phenotype are characterized by leaves with crinkling and undulation in the laminar surface, thicker and wider lamina, reduced lengths of lamina, fewer serrations of the leaf margin, and shorter petioles and stipules, as compared with leaves in wild-type (WT) trees.18 The CL mutation was found among the progeny of a cross between two normal-leafed peaches.18 Despite these morphological alterations of the leaves, there appears to be no difference in the vigor and fertility of peach and peach-almond hybrids showing the CL phenotype, as compared to normal-leafed siblings. Controlled crosses carried out by Ledbetter18 revealed a pattern consistent with Mendelian inheritance, in which the crinkle-leaf phenotype appears to be controlled by a single recessive gene.
CL trees are also characterized by having brittle branches that prune with approximately 50% of the effort of wild-type wood, suggesting an altered wood phenotype in conjunction with the CL leaf phenotype. This observation is particularly important in the context of a fruit tree because heavy fruit loads can cause branch failures in plants with weak wood. Fruit tree breeding thus requires plants with sufficient wood strength to bear a commercially useful crop, in addition to selecting for desirable fruit characteristics. The apparent correlation between the CL leaf phenotype and observably altered wood properties suggests a unique opportunity to use a foliar character to track the presence of a specific wood property in subsequent generations.
The microscopic wood anatomy of Prunus in general is well known, and that of peach wood has been described,19–21 but not surprisingly the literature shows no comparisons between wood anatomical characteristics of crinkle-leaf and wild-type trees, although there have been characterizations of the wood anatomy of transgenic aspen.22–24
Macroscale wood properties (such as resistance to pruning) will be a result of the interaction of wood component properties at a variety scales, from bulk wood metrics at the growth-ring level, to wood anatomy (cell types, sizes, and relative abundance), to cell wall mechanics, to cell wall chemistry and ultrastructure.1,3,8,9 We hypothesize that differences in bulk wood properties, wood anatomy, cell wall mechanics, and/or cell wall chemistry between CL and WT wood can explain the field observations of brash, weak CL wood. To best assess these differences, we also examine the effect of plant vigor across three vigor classes, and make a provisional examination of genotype-by-vigor effects.
Materials and Methods
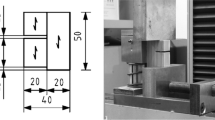
Samples were obtained from 4-year-old Prunus persica trees cultivated under the same silvicultural conditions in Parlier, California, at the San Joaquin Valley Agricultural Sciences Center. Sampled trees were part of progeny 72001, a family developed to examine genetic linkage between red leaves, evergreen and CL characteristics. Individual trees were short (less than 3 m tall) with abundant branches and short internodes. Wild-type and crinkle-leaf individuals were sampled in each of three vigor classes: low, average, and high vigor, based on plant stature observed across the range of the plantings. A tree from each vigor class was harvested and stored at 0°F (−17.8°C). Short stem segments were cut and stored in 75% ethanol prior to sectioning. One individual per vigor class was used to measure wood anatomical characters. Sections 20–30 µm thick were cut with a sliding microtome, compressed between glass slides, boiled in water, then cooled and mounted in glycerin. Sections for phloroglucinol-HCl staining to determine relative lignification between high-vigor individuals of the two genotypes were cut separately and stored in water, without boiling or other chemical modification prior to observation, and the results in CL and WT wood were compared to a xylarium specimen of P. persica, MADw 18883. Sections made for phloroglucinol staining were also stained with Safranin-Astra blue, mounted in water, and photographed on a Leica DM 4000B using a Point Grey Flea3 digital camera. Wood macerations were made using acetic acid and peroxide following Ruzin25 Sections and macerations were observed with a light microscope (Leica DM 2000) and anatomical measurements were taken with the aid of a digital camera (Leica DFC 420). An electronic digitizer (Numonics AccuGrid Digitizer) was used to quantify vessel element length and fiber length (as in Berry and Wiedenhoeft26).
The IAWA List of Microscopic Features for Hardwood Identification27 was used as baseline for description, but the definitions by Wiedenhoeft28 were followed. Characters not mentioned are absent or not applicable.
Measurement of Wood Features
A list of all measured wood features and calculated quantitative indices can be found in Table I. Vessel-related measurements were taken only from the latewood region of the 3rd year (the most external growth ring). The fiber length to vessel length ratio (F/V) represents an approximate measure of the degree of intrusive growth by the fibers during cell maturation and is a means to compare fiber lengths by controlling for fusiform initial length, as represented by the vessel element length.29 A ray area index (RAi) was estimated as a dimensionless quantity to characterize the total area of the ray tissue per unit of tangential area.
Wood specific gravity was measured following Williamson and Wiemann,30 with bark and pith removed from samples prior to measuring.
Nanoindentation of Latewood Fibers
Nanoindentation surfaces were prepared such that the indentation direction was perpendicular to the fiber long axis to better replicate the direction of force application during pruning. Specimens were prepared without epoxy embedment to avoid any cell wall alteration by the epoxy.31 One latewood specimen each of CL and WT high-vigor individuals that were not soaked in ethanol or chemically altered were surfaced on a sliding microtome to expose a perfectly tangential surface. That surface was then glued to a stainless steel cylindrical stub. A hand razor was used to carefully trim a pyramid in the exposed wood surface with an apex in a region of fiber cells. Then, the cylindrical stubs were fitted into Sorvall MT-2 ultramicrotome equipped with a diamond knife and nanoindentation surfaces prepared by removing 200-nm-thick sections from the apex until a surface approximately 200 µm on a side was prepared. The resulting surface for nanoindentation was thus perfectly tangential as well.
A Hysitron (Minneapolis, MN, USA) TriboIndenter® equipped with a Berkovich probe was used. The machine compliance, probe area function, and tip roundness effects were determined from a series of 80 load control nanoindents in a fused silica standard as previously described.32 The relative humidity (RH) inside of the nanoindentation enclosure was maintained at 86% RH with an InstruQuest (Coconut Creek, FL, USA) HumiSys™ HF RH generator while the specimens conditioned inside the enclosure for 48 h before and during the testing. The high humidity condition was chosen to again better replicate the moist conditions of the wood inside of a living tree during pruning. On the prepared surface, fibers medially cut through their lumina were chosen for testing. All nanoindent locations were chosen in the middle of an S2 wall layer next to an exposed lumen. The same multiload nanoindents (maximum load of 65 µN) and analyses were used as previously described32 to assess nanoindentation hardness and elastic modulus.
Statistical Analyses
Measurements were grouped to evaluate differences between genotype (wild-type vs. crinkle-leaf), vigor classes (average vs. high vs. low), and genotype-by-vigor. Quantitative ray features were treated separately for each ray width class. Trends of variations were examined using descriptive statistical data analysis33 with Bonferroni corrections in all t tests to account for multiple comparisons. We consider P < 0.05 the minimum threshold to warrant further consideration of a factor. All statistical work was performed in R.34
Results
General qualitative features of the wood regardless of genotype or vigor class are consistent with the description for Prunus persica found in InsideWood.20 Transmitted light micrographs of the latewood of WT and CL wood are shown in Fig. 1. Specific quantitative features of WT and CL wood, as well as differences by vigor class, are reported below, as well as stem diameter, specific gravity, cell wall elastic modulus, and cell wall hardness (Tables II, III).
Transmitted light micrographs of the latewood of wild-type (top) and crinkle-leaf (bottom) wood stained with Safranin-Astra blue and mounted in water. Note the similar relative size, abundance and distribution of vessels and fibers in the two woods. Wild-type wood shows larger rays. Scale bars 250 µm
Comparison of Genotypes
Wood Features
Wild-type wood showed significantly higher wood specific gravity than crinkle-leaf (CL) (SG; P = 0.015). WT wood bore longer vessel elements (VEL; P ≪ 0.001), longer fibers (FL; P ≪ 0.001) and wider rays (RW, when considering all types of rays together; P = 0.013). There were no significant differences between WT and CL trees in mean stem diameter (SD), tangential vessel diameter (TVD), vessels per mm2 (VNM2), or in any of the other measured ray features (RNM2, RH, RHC and TTRH). The fiber to vessel ratio (F/V) and ray area index (RAi) did not differ between genotypes.
Cell Wall Mechanical Properties
Cell wall mechanical properties measured by nanoindentation showed no significant differences between genotypes for either elastic modulus (N-MoE) or Meyer’s hardness (N-H).
Cell Wall Histochemistry
Phloroglucinol-HCl staining of CL and WT wood showed comparatively weak reactions, requiring comparison to the staining reaction with a xylarium specimen of P. persica which also showed a weak staining reaction. There were no observable differences in staining intensity between CL, WT, and the xylarium specimen.
Comparison of Vigor Classes
When comparing among vigor classes, there was no consistent statistically significant pattern, other than for SD, which is a direct proxy for vigor: high vigor samples had significantly higher SD than average vigor (P ≪ 0.001), which in turn had higher SD than low vigor (P < 0.01). Similarly, high vigor samples had significantly higher TVD (Ps < 0.01). Average vigor samples had significantly higher VNM2 (Ps < 0.01) and VEL (0.01 < Ps < 0.05), and low vigor samples had significantly lower FL (Ps ≤ 0.01). F/V and RAi were not significantly different between vigor classes.
Genotype-by-Vigor Analysis
Results of genotype-by-vigor analysis are shown in Table III, with a number of features showing statistically significant differences, the relevance of which are discussed below.
Discussion and Conclusions
Field observation of brash, weak wood, easy pruning, and wood failure correlated with the presence of an easily observed vegetative phenotype (crinkled foliar leaves) provided the impetus for this study. Because CL shows Mendelian inheritance and to be recessive,18 it presents a unique opportunity to use the CL leaf phenotype as a marker for altered wood phenotype. To that end, we attempted to elucidate a structure–property relationship in CL and WT wood sufficient to explain the weak, brash wood as a first step toward justifying further research into CL as a model organism for wood properties investigation. Whether CL is a single gene mutation with broad pleiotropic effects, or ideally, if CL is a two-or-more gene mutant, the ability eventually to alter or add genes of interest and use the CL leaf phenotype as an easily visible marker for the presence of the wood alterations warrants further research.
When exploring structure–property relationships in wood, it is logical to look for factors that occur at the same scale as the phenomenon. Pruning resistance is a bulk wood property, as is wood specific gravity (SG). WT wood has 7% higher SG than CL wood (Table II). This difference, while statistically significant, is not of a magnitude sufficient to explain the field observations of weaker wood and reduced pruning resistance, which we estimate to closer to a factor of 50%. In general, approximately 60% of the variation in wood mechanical properties is explained by variations in wood SG.4 In this case, we assume that the wood mechanical property most closely related to pruning resistance is Janka side hardness. Wiemann and Green35 report a general equation to estimate Janka side hardness based on SG. Using the SG reported in Table II and the Wiemann and Green35 equation for hardwoods, we find that predicted bulk hardness of WT wood is estimated to be 15% harder than CL wood, which is still not on the order of the field-observed differences. Clearly, directly measuring pruning resistance would be valuable, and instrumenting pruners for such field measurements is of interest for future research and quantification of field observations.
With the bulk wood metrics insufficient to explain field observations, variations in wood cellular anatomy are the next scale of analysis. A range of wood anatomical features varied according to plant vigor, and many features were also significantly different in the genotype-by-vigor analysis. In both analyses, there were no over-arching patterns other than expected relationships (e.g. higher SG in slower growing individuals, increased F/V in higher vigor plants),thus we feel secure combining results across vigor classes for a genotype-only comparison.
Three wood anatomical features were significantly larger in WT than CL wood: VEL, FL, and RW. In wood anatomy and development, VEL is a proxy for fusiform initial length in the vascular cambium—it is essentially an indication of the length of the mother cells that gave rise to both vessel elements and fibers. Because fibers grow in length from both tips as they mature, the ratio of the fiber length to the vessel element length (the F/V) is a way to look at a relative amount or degree of interdigitation between cells and thus a type of mechanical interlocking of cells. Comparing VEL between genotypes also gives an indication of the relative starting size of cells, so in this way provides a frame of reference to determine if the ultimate FL is a result of larger mother cells (larger VEL), a higher degree of tip growth (higher F/V) or both.
For WT wood, longer fibers are a result of both larger cambial initials (proxied by VEL), and a higher degree of tip growth, although the F/V did not differ significantly between the two genotypes. This is likely a result of the small n associated with genotype level comparisons in a calculated index as compared to a directly measured feature with larger n. The F/V for WT wood ranged from 2.46 to 2.95, whereas in CL it ranged from 2.01 to 2.53, with only a slight overlap between the lowest WT values and the highest CL values. We do not have enough data to assert an effect or a direct relevance, but if with larger sampling a similar difference in F/V were borne out, it could begin to provide a plausible explanation for the field-observations of easier pruning. Even without the mechanical interlocking implied by a higher F/V, the shorter fiber lengths of the CL wood could be a partial explanation for establishing perpendicular-to-grain cleavage paths for pruning forces, because shorter cells must be more abundant per unit length of stem, and thus have more frequent cell ends.
Ray width (RW) also differed significantly between WT and CL, with WT wood having wider rays (Table II). WT wood had slightly less abundant rays (lower RNM2) but not significantly so. A better metric for total ray volume in the wood is the calculated RAi, which was slightly but not significantly higher in WT wood. Rays function in storage, translocation, and biosynthesis in wood, and are not typically implicated in wood mechanical strength, other than in a negative context. That is, it is typically thought that woods with more parenchyma (either in radial system or axially) will, all other factors being equal (vessel size and abundance, fiber wall thickness and length), have lower wood strength, as parenchyma cells are not specialized for mechanical support. In this regard, increased RW in WT wood does not facilitate a logical explanation of greater WT wood strength, based on our understanding of structure–property relationships in wood at this time.
It is interesting that the S2 cell wall mechanical properties of the latewood fibers measured in CL and WT woods were not statistically different. Measured differences in cell wall properties at the nanoscale would be an appealing argument for macroscale wood property differences, but our data do not support such a link.
The similar reaction with phloroglucinol-HCl between WT and CL, and even with a xylarium specimen of Prunus persica, indicates that the relative lignification between these woods as detected with this stain was negligible. The comparative lack of staining suggests that this species, not just the genotypes in question here, have an altered lignin composition compared to many other hardwoods. It is known that phloroglucinol-HCl reacts with 4-O-linked hydroxycinnamyl aldehyde structures in lignin,36 so we infer that the lignin in Prunus persica likely has a low abundance of these reactive sites. Staining with Safranin-Astra blue produced a normal range of color and staining intensity (shown in greyscale, Fig. 1) which indicates that the lack of phloroglucinol staining is not a result of the absence of lignin, but rather is a function of the types of chemical linkages in the lignin. Non-lignified tissues stained with Safranin-Astra blue are blue in color, and lignified cellulose stains red. The sections shown in Fig. 1 developed a strong red color, indicating the presence of lignified cellulose. Because lignin is formed via a free-radical polymerization process, the types and relative abundances of linkages at the time of polymerization contribute to its overall properties, including its reaction with phloroglucinol. Our histochemical data provide a complementary line of evidence supporting the results of the nanonindentation testing, indicating no significant differences in cell wall chemistry properties between the two genotypes, and further show that Prunus persica, whether CL, WT, or a completely different genetic lineage with the species, shows weak staining with phloroglucinol, indicating that a comparative absence of 4-O-linked hydroxycinnamyl aldehyde structures in the lignin is likely a species characteristic.
The potential power of CL as a model system is contingent primarily on it being a two-gene system, so that the wood-related gene can be modified to understand control of wood structure–property relationships and the leaf gene serves as a marker for altered wood properties. If CL is a single-gene mutation with broad pleiotropic effects, that would reduce the value of this system as a model system for exploring control of wood structure–property relationships, but could open another avenue of potentially productive research, namely sourcing CL wood as a feedstock for biofuels. Any change in wood properties that reduces resistance to comminution improves the biofuel conversion energy metrics, so brash, easily pruned CL wood would likely require much less energy to break down into small particles to feed into a biofuels process.37 If this were the case, our data about the chemical composition of the wood cell wall lignin could also be of use in targeting chemical or physical pretreatments to improve conversion efficiency.
Differences in wood specific gravity, vessel length, fiber length, and ray width do not by themselves explain the brash, weak wood characteristic of CL trees. Given the importance of wood specific gravity in structure, storage, and translocation,38 this is certainly an important trait in the CL wood syndrome. Still, we suspect that vessel elements and fibers are good candidates for further study in order to establish if they are actually the result of a pleiotropic effect from the crinkle-leaf expression (CL/cl). The 4-year-old stem material studied here most likely represents juvenile wood, the properties of which may differ from mature stem wood produced by these genotypes. If these genotypes were to be used as a model system, exploration of axial and radial variation in wood properties would be prudent, as the wood properties of CL and WT may change with plant stature and age. Future work to characterize the CL wood phenotype and evaluate its power and potential as a model system should employ quantitative characterization of the wood mechanical properties at the mesoscale, as well as increased numbers of individual trees so that the statistical power of tests is increased.
References
R.E. Booker and J. Sell, Holz Roh Werkst. 56, 1 (1998).
J.R. Barnett and V.A. Bonham, Biol. Rev. Camb. Philos. Soc. 79, 461 (2004).
R.M. Kellogg and F.F. Wangaard, Wood Fiber Sci. 1, 180 (2007).
D.E. Kretschmann, in Wood Handbook: Wood as an Engineering Material, ed. R.J. Ross (Madison, WI: USDA Forest Service, Forest Products Laboratory, General Technical Report FPL- GTR-190, 2010), p. 5-1.
A.C. Wiedenhoeft, in Wood Handbook: Wood as an Engineering Material, ed. R.J. Ross (Madison, WI: USDA Forest Service, Forest Products Laboratory, General Technical Report FPL- GTR-190, 2010), pp. 3-1.
J.R. Barnett and G. Jeronimidis, Wood Quality and its Biological Basis, 1st ed. (Boca Raton: CRC Press, 2003).
A.J. Panshin and C.D. Zeeuw, Textbook of Wood Technology: Structure, Identification, Properties, and Uses of the Commercial Woods of the United States and Canada, 4th ed., Vol. 1 (New York: McGraw-Hill Book Co., 1980).
C. Plomion, G. Leprovost, and A. Stokes, Plant Physiol. 127, 1513 (2001).
D.W. Woodcock and A.D. Shier, Trees 16, 437 (2002).
D.W. Woodcock and A.D. Shier, Ann. Bot. 91, 529 (2003).
B. Lachenbruch, J.R. Moore, and R. Evans, in Size-and Age-Related Changes in Tree Structure and Function ed. F.C. Meinzer, B. Lachenbruch, and T.E. Dawson (Springer, 2011), p. 121.
B.L. Gartner, J. For. 103, 75 (2005).
P.W. West, Growing Plantation Forests, 2nd ed. (Berlin: Springer, 2006), pp. 1–22.
B.J. Zobel and J.P. van Buijtenen, Wood Variation: Its Causes and Control, 1st ed. (Berlin: Springer, 2012).
A.N. Burdett, Can. J. For. Res. 20, 415 (1990).
California Agricultural Statistics Review, 2014–2015. California Department of Food and Agriculture, California Agricultural Statistics Service, Fruit & Nut Crops. 2015, https://www.cdfa.ca.gov/statistics/PDFs/2015Report.pdf
S. Jung, S. Ficklin, T. Lee, C.H. Cheng, A. Blenda, P. Zheng, J. Yu, A. Bombarely, I. Cho, S. Ru, K. Evans, C. Peace, A.G. Abbott, L.A. Mueller, M.A. Olmstead, and D. Main, Nucl. Acids Res. 42, D1237 (2014).
C.A. Ledbetter, J. Genet. Breed. 50, 57 (1996).
M. Suzuki, S. Noshiro, A. Takahashi, K. Yoda, and L. Joshi, in The Himalayan Plants Volume 2, ed. H. Ohba and S.B. Malla (Tokio: The University Museum, The University of Tokyo, 1991).
E.A. Wheeler, “InsideWood” (Published on the Internet. 2004-onwards), http://insidewood.lib.ncsu.edu/search. Accessed 1 April 2016.
E.A. Wheeler, IAWA J. 32, 199 (2011).
H. Tuominen, F. Sitbon, C. Jacobsson, G. Sandberg, O. Olsson, and B. Sundberg, Plant Physiol. 109, 1179 (1995).
B. Horvath, I. Peszlen, P. Peralta, B. Kasal, and L. Li, IAWA J. 31, 29 (2010).
Q. Li, D. Min, J.P.Y. Wang, I. Peszlen, L. Horvath, B. Horvath, Y. Nishimura, H. Jameel, H.M. Chang, and V.L. Chiang, Tree Physiol. 31, 226 (2011).
S.E. Ruzin, Plant Microtechnique and Microscopy, 1st ed. (New York: Oxford University Press, 1999), p. 132.
P.E. Berry and A.C. Wiedenhoeft, Syst. Bot. 29, 125 (2004).
E.A. Wheeler, P. Baas, and P.E. Gasson, IAWA Bull. 10, 219 (1989).
A.C. Wiedenhoeft (Ph.D. Thesis, University of Wisconsin-Madison, Madison, 2008).
M.M. Chattaway, Trop. Woods 46, 16 (1936).
G.B. Williamson and M.C. Wiemann, Am. J. Bot. 97, 519 (2010).
J.E. Jakes, C.R. Frihart, J.F. Beecher, R.J. Moon, and D.S. Stone, J. Mater. Res. 23, 1113 (2008).
J.E. Jakes, C.G. Hunt, D.J. Yelle, L.F. Lorenz, K. Hirth, S.-C. Gleber, S. Vogt, W. Grigsby, and C.R. Frihart, ACS Appl. Mater. Interfaces 7, 6584 (2015).
R.R. Sokal and F.J. Rohlf, Biometry: The Principles and Practice of Statistics in Biological Research, 3rd ed. (New York: W.H. Freeman and Company, 1995).
R Core Team: R: A Language and Environment for Statistical Computing (Vienna, Austria: R Foundation for Statistical Computing, 2013). http://www.R-project.org/.
M.C. Wiemann and D.W. Green, Research Paper FPL-RP-643, Forest Products Laboratory, Forest Service (Madison: U.S. Department of Agriculture, 2007).
F. Pomar, F. Merino, and A. Ros Barceló, Protoplasma 220, 0017 (2002).
M. Himmel, M. Tucker, J. Baker, C. Rivard, K. Oh, K. Grohmann, and C.D. Scott, Seventh Symposium on Biotechnology for Fuels and Chemicals, ed. C.D. Scott (Hoboken: Wiley, 1986), p. 39.
J. Chave, D. Coomes, S. Jansen, S.L. Lewis, N.G. Swenson, and A.E. Zanne, Ecol. Lett. 12, 351 (2009).
Acknowledgements
The authors wish to thank Mr. Richard Soares for his micro-technical acumen in producing the histochemistry sections and the initial preparation of the nanoindentation blocks.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wiedenhoeft, A.C., Arévalo, R., Ledbetter, C. et al. Structure–Property Characterization of the Crinkle-Leaf Peach Wood Phenotype: A Future Model System for Wood Properties Research?. JOM 68, 2405–2412 (2016). https://doi.org/10.1007/s11837-016-2057-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-016-2057-0