Abstract
The safe disposal of coal combustion residues (CCRs) will remain a major public issue as long as coal is used as a fuel for energy production. Both dry and wet disposal methods of CCRs create serious environmental problems. The dry disposal method creates air pollution initially, and the wet disposal method creates water pollution as a result of the presence of trace and heavy metals. These leached heavy metals from fly ash may become more hazardous when they form toxic compounds such as arsenic sulfite (As2S3) and lead nitrate (N2O6Pb). The available studies on trace and heavy metals present in CCRs cannot ensure environmentally safe utilization. In this work, a novel approach has been offered for the retrieval of trace and heavy metals from CCRs. If the proposed method becomes successful, then the recovered trace and heavy metals may become a resource and environmentally safe use of CCRs may be possible.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thermal power plants remain the main sources of power generation in many countries including India. India is one of the largest electricity consumers, ranked 6th in the world. The government of India set a target to add approximately 78,000 MW of electricity generation by 2012. The total need for electricity in India is anticipated to surpass 950,000 MW by 2030.1 Annual generation of coal combustion residues (CCRs) (fly ash and bottom ash) worldwide is estimated to be approximately 600 million tonnes, with fly ash constituting about 500 million tonnes and bottom ash 100 million tonnes.2 Indian coal contains low sulfur, but it has a high ash content. As such, the combustion of such coal in thermal power plants generates huge amounts of ash. Therefore, annual CCR generation in India alone was 112 million tonnes in 2005–2006.1 The CCRs consist of a very high percentage of silica (60–65%), alumina (25–30%), magnetite, and Fe2O3 (6–15%) that enable its use for the synthesis of zeolite, alum, and precipitated silica. The other important physicochemical characteristics of CCRs such as bulk density, particle size, porosity, water holding capacity, and surface area make it suitable for use as adsorbents.2 Coal composition, combustion conditions, efficiency and type of emission control devices, and disposal methods are the major factors that affect the physical and chemical characteristics of CCRs.3 The safe disposal of CCRs remains a major public issue. A clear policy of handling CCRs is required to ensure the environmentally friendly disposal/utilization.
As shown in Table I, many trace elements are found in CCRs.4–7
Among these elements, V, Cr, Mn, Sr, and Ba are usually present in substantially higher concentrations. The presence of these elements in CCRs limits its use in place of soil as land-filling material or as building and road materials in combination with cement. Ahmaruzzaman2 addressed thoroughly the different methods of fly ash utilization with more emphasis on preparing adsorbents using fly ash for treatment of other possible contaminant removal. A similar review made by other researchers8,9 discussed in detail various fly ash utilization methods. They presented limited information on recovery of rarely available metals such as gallium and germanium from coal fly ash. Recovery of alumina from fly ash has been well documented10–13 This article specifically focuses on analyzing the potential ways of separating useful trace metals/minerals from coal fly ash. This process necessitates framing an operational policy for safe disposal or reuse of the CCRs to ensure no secondary pollution. The occurrence of secondary pollution may be reduced or eliminated if a method is identified for complete recovery/separation of the elements from CCRs.
Collection and Disposal of CCRs
In general, fly ash is collected by mechanical or electrostatic precipitators from the flue gases of power plants, whereas bottom ash is collected from the bottom of the boilers. Fly ash is disposed of either by a dry or by a wet disposal method. In the dry disposal method, the fly ash is stored in silos, and in the wet disposal method, in most of the events, both bottom ash and fly ash are mixed to form a slurry and stored in an artificial lagoon called an ash pond or a holding pond. Then the settled ash in the holding pond is dried by open dumping. Initially, ash ponds were built to solve the problem of fly ash from becoming airborne under the action of strong winds. But in the process of controlling air emissions, this strategy has generated a considerable amount of waste water. Pond ash contains about 80% fly ash and 20% bottom ash.14 Both dry and wet methods, the common practice of disposal is open dumping. The ash-to-water ratio in slurry may vary from 1:5 to 1:10 (by weight) to facilitate smooth transportation of slurry to the holding pond. Holding pond effluent is discharged directly into the river15 or recycled and reused for making slurry. If the same water is recycled continuously for wet disposal of ash, the water is expected to be saturated with heavy and trace metals present in pond ash. After reaching the saturation limit, the water is not expected to accept any trace and heavy metals from pond ash and the total quantity of those metals remains in the pond ash that is disposed of by an open dumping method along with ash from dry disposal. When the effluent from the holding pond is allowed to flow into the natural water courses without treatment, the concentrations of these trace and heavy metals in the receiving water keep on increasing continuously and contaminate the connected surface water bodies.
In the United States, approximately 600 power plants generated 130 million tonnes of CCRs annually, of which 56% is stored in surface water impoundments and landfills; the remaining is reused in the concrete, cement, and construction industries.16 In 2010, the total generation rates of CCRs from thermal power plants in EU-15 and EU-28 were 48 million tonnes and 105 tonnes, respectively. The EU-15 countries are nearing the target of 100% utilization of CCRs in various ways, such as construction, reclamation, and restoration.17 A large volume of CCR effluents is generated and disposed of into surface water bodies, and the environmental risks associated with these disposal practices are not well known.16 Moreover, because of the lack of CCR waste data, the effluents that are discharged from coal-fired power plants and permitted by the national and state regulatory bodies lack consistent monitoring and limit requirements that are relevant to the composition of CCR effluent.18 In most countries, at least 75% of the fly ash generated annually is dumped with no subsequent reutilization.19
Utilization of Fly Ash
The present CCR utilization policy in India requires minimum leaching when exposed to rain or water despite the minimal chance of leaching due to unfavorable leaching conditions. The quantity of CCRs used in construction industries is limited (maximum ~15% of the quantity of cement used for preparation of concrete). The physical state of CCRs in their used form (i.e., in a hardened state) leads to less permeability of water. In comparison, the CCRs in a holding pond are directly suspended in water for long periods under favorable conditions of leaching of trace and heavy metals. This serious environmental concern should be addressed with a proper policy framework.
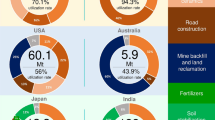
Many efforts have been made to use fly ash, such as preparation of building and road materials and preparation of lightweight aggregate for construction industries, mine backfill,2 and waste stabilization20 with required strength. Complete utilization of fly ash by these methods is not possible because of the presence of trace and heavy metals in fly ash. The Technology Information Forecasting and Assessment Council (TIFAC) under the Department of Science & Technology (DST), Government of India, New Delhi, started a fly ash utilization program in 1994 with its main objectives to make fly ash a useful by-product and to reduce environmental pollution and land required for fly ash disposal. The Ministry of Environment and Forest (MoEF)21 issued a notification (September, 14, 1999) to achieve an ambitious target of 100% fly ash utilization with the following regulatory measures: (I) No person shall manufacture clay bricks or tiles or blocks for use in construction activities without mixing at least 25% of CCRs with soil within a radius of 50 km from coal or lignite based thermal power plants; (II) every coal- or lignite-based thermal power plant shall make fly ash available for at least 10 years from the date of publication of this notification without any payment or any other considerations for manufacturing fly-ash-based products and any other construction activities; (III) the central and state government agencies of India (such as National Thermal Power Corporation, State Electricity Boards, etc.) shall facilitate land, electricity, and water for manufacturing activities and provide access to ash-lifting areas for promoting and setting up ash-based production units in the proximity of the area where ash is generated by thermal power plants; and (IC) Central Public Works Department (CPWD), State Public Works Department (SPWD), development authorities, housing boards, National Highway Authority of India (NHAI), and other construction agencies, including those in the private sector, shall prescribe the use of fly ash and fly-ash-based products in their respective schedule of specifications and construction applications including appropriate standards and code of practice within a period of 4 months from publication of this notification. The MoEF22 notification (November 3, 2009) mentioned that the thermal power plants in India are far from achieving the target of 100% utilization of the CCRs generated. Similarly, only 44% of total CCR is reused in the United States in construction industries because a very small quantity of CCRs can be added to cement to maintain the required strength.23
CCRs have been used for extraction of zeolites,2,24,25 mullite,26 glass-like materials,27 and composite materials such as plastic composites28 and metal composites.29 Several studies confirmed CCRs as potential adsorbents for removal of arsenic, boron, cadmium, chromium, fluoride, lead, mercury, nickel, pesticides, phosphate, zinc, color, and organic materials from waste water.2,24 Golden and Wilder30 proposed a conceptual commercial-scale design to recover various raw materials such as alumina, ferric oxide, gypsum, and alkali sulfate salts from fly ash based on a direct acid leaching process. Similarly, iron,31 hexavalent chromium,32 and alumina33 have been extracted from fly ash successfully. Fly ash has also been used in agriculture as a soil additive with some manure.2,24 Fly ash from coal-fired power stations has proved to have significant value-added potential as an ingredient in cementitious and other construction processes.24 In light of these many disposal options, CCRs are clearly not only a potential environmental burden, but they can also be viewed as an untapped resource of huge potential.24 CCRs do have a potential role as a value-added product in material preparation, waste management, recovery of materials. and agricultural applications. But there is a scarcity of information on the environmental impact of fly ash as an ingredient in the preparation of materials.2 There are many promising options of using fly ash, but with limited studies available on the critical comparison between fly-ash-generated products and processes, and those derived from more traditional routes to increase the acceptability of the fly-ash-generated products. The acceptability of the fly-ash-generated products can be enhanced by minimizing the leachability of the trace and heavy metals present in CCRs. There are no standards available to check the environmentally safe utilization of CCRs. Therefore, to increase the percentage use of fly ash, to enhance the acceptability of fly ash generated products, and to minimize the leaching potential of fly ash, it would be valuable to identify a method that can separate the trace and heavy metals from the CCRs.
There are studies available on the methods for recovering materials from CCRs such as (I) the direct acid leaching process to obtain alumina, ferric oxide, gypsum, alkali sulfate salts, and spent fly ash (but the rate of return was only 20% of the investment);30 (II) the use of an extended arc reactor furnace to recover iron up to 90% from fly ash;31 (III) the use of a packed bed of fly ash pellets mixed with kaolin as the binder to recover hexavalent chromium;32 (IV) the use of chlorination and chemical transport to recover vanadium, nickel, and magnesium from fly ash of bitumen-in-water emulsion;34 and (V) a good number of processes to synthesize zeolites from fly ash.2,25,35 But there is a lack of an integrated approach by the coordination of technologists and manufacturers for the production of superior quality of fly-ash-based products to meet consumer acceptability and increased marketability.
Trace and Heavy Metal Contamination in Holding Pond Effluent and Proposed Solution of the Problem
Quina et al.36 reviewed removal of heavy metals such as zinc, led, copper, and cadmium from a municipal solid waste incinerator (MSWI) by adopting thermal treatment methods through evaporation at temperature lower than melting point, enabling recycling of the metals. Many promising studies have been reported in the literature about the separation of heavy metals by thermal treatment.1,37–40 The characteristics of the ashes resulting from sewage sludge combustion differ significantly from those of coal fly ash due to the much higher temperature involved in coal combustion (1500°C to 1700°C versus 800°C to 900°C), and coal is generally poor in nutrients such as phosphorous, which are concentrated in sewage sludge ash.41
Nazari et al.42 have used sulfuric acid as leaching agent for the simultaneous separation of vanadium and nickel from heavy fuel oil combusted fly ash. Similarly, Al-ghouti et al.43 used NH4OH solution as solvent for nickel recovery and Na2CO3 solution for vanadium recovery. Nayak and Panda12 also used sulfuric acid to recover aluminum from coal fly ash. It is proposed to find a suitable solvent(s) that can dissolve all the trace and heavy metals to saturation limits and treat the saturated solvent(s) by flash evaporation to recover the dissolved trace and heavy metals in the form of crystals and to reuse the solvent(s) (Fig. 1). Successful implementation of an integrated approach for extracting individual trace and heavy metals may provide a cost-effective recovery of the trace and heavy metals from the concentrated crystals rather than extracting directly from raw CCRs. The solid waste crystals rich in elements can be a resource for the iron and steel industries (for extraction of iron), aluminum industries (for extraction of alumina), copper industries (for extraction of copper), and other industries involved in producing pure elements for specific uses. In this way, the secondary pollution from the disposal of CCRs may be reduced as it will be relatively free from trace and heavy metals or these CCRs can safely be used in construction industries, mine filling, and agriculture (the major consumers of CCRs). Separation of the trace and heavy metals from CCRs using this technique can lower the environmental threats of the CCRs, and the management of the relatively small volume of the crystals rich in trace and heavy metals may become easier. The utilization of CCRs will be well accepted without a stringent law and enforcement when it becomes a resource without environmental threats. It is true that this technique will add substantial operating cost along with generation of solid waste crystals rich in elements.
References
V.C. Pandey, J.S. Singh, R.P. Singh, N. Singh, and M. Yunus, Resour. Conserv. Recycl. 55, 819 (2011).
H. Ahmaruzzaman, Prog. Energy Combust. 36, 327 (2010).
M. Karuppiah and G. Gupta, J. Hazard. Mater. 56, 53 (1997).
A. Das, M.K. Jain, and G. Singh, Int. J. Earth. Sci. Eng. 5, 305 (2013).
A. Sarkar, R. Rano, G. Udaybhanu, and A.K. Basu, Fuel Process. Technol. 87, 259 (2006).
A. Ugurlu, Environ. Geol. 46, 890 (2004).
A. Kumar and S.R. Samadder, Environ. Monit. Assess. 187, 370 (2015).
Z.T. Yao, X.S. Ji, P.K. Sarker, J.H. Tang, L.Q. Ge, M.S. Xia, and Y.Q. Xi, Earth-Sci. Rev. 141, 105 (2015).
R.S. Blissett and N.A. Rowson, Fuel 97, 1 (2012).
Z.T. Yao, M.S. Xia, P.K. Sarker, and T. Chen, Fuel 120, 74 (2014).
R.H. Matjie, J.R. Bunt, and J.H.P. van Heerden, Miner. Eng. 18, 299 (2005).
N. Nayak and C.R. Panda, Fuel 89, 53 (2010).
A. Shemi, R.N. Mpana, S. Ndlovu, L.D. van Dyk, V. Sibanda, and L. Seepe, Miner. Eng. 34, 30 (2012).
A. Ghosh, J. Mater. Civ. Eng. 22, 343 (2010).
S. Sushil and V.S. Batra, Fuel 85, 2676 (2006).
L. Ruhl, A. Vengosh, G.S. Dwyer, H. Hsu-Kim, A.R. Schwartz, and S.D. Smith, Environ. Sci. Technol. 46, 12226 (2012).
European Coal Combustion Products Association (ECOBA), http://www.ecoba.com/. Accessed April 2016.
J. Hanlon, Memorandum (Washington, DC: U.S Environmental Protection Agency, 2010).
A. Malik and A. Thapliyal, Crit. Rev. Environ. Sci. Technol. 39, 333 (2009).
C.J. Morati, K.W. Wiken, O.E. Manz, and C.A. Wentz, in Proceedings of American Institute of Chemical Engineers National Meeting, paper 41e (1987).
Ministry of Environment and Forests (MoEF), Ministry of Environment and Forests Notification (September 14, 1999), Government of India, New Delhi (1999).
Ministry of Environment and Forests (MoEF), Ministry of Environment and Forests Notification (November 3, 2009), Government of India, New Delhi (2009).
American Coal Ash Association (ACAA), https://www.acaa-usa.org/Portals/9/Files/PDFs/2008_ACAA_CCP_Survey_Report_FINAL_100509.pdf. Accessed March 2016.
R.S. Iyer and J.A. Scott, Resour. Conserv. Recycl. 31, 217 (2001).
P.K. Mehta, in Proceedings of 3rd International Conference of American Concrete Research Institute, Detroit, 1 (1989).
T. Ohtake, K. Uchida, F. Ikazaki, M. Kawamura, T. Ohkubo, and K. Kamiya, J. Ceram. Soc. Jpn. 99, 239 (1991).
L. Barbieri, I. Isabella, T. Manfredini, I. Querait, J. Ma-Rincon, and M. Romero, Fuel 78, 271 (1999).
P.A. Jarvala and P.K. Jarvala, J. Mater. Sci. 31, 3853 (1999).
R.Q. Guo, P.K. Rohatgi, and D. Nath, J. Mater. Sci. 32, 3971 (1997).
D.M. Golden and R.P. Wilder, US Department Energy, Morgantown Energy Technology Center (Report) DOE METC 85-6018, vol. 2 (Morgantown, 1985), p. 733.
C.A. Pickles, A. Mclean, C.N. Alcock, and Z.N. Nikolic, Can. Metall. Q. 29, 193 (1990).
G.P. Dasmahaputra, T.K. Pal, and B. Bhattacharya, Chem. Eng. Technol. 21, 89 (1998).
D.J. O’Connor, in Proceedings of 13th Australian Chemical Engineering Conference (Chemeca 85), (Perth, 1985), p. 127.
M. Kuniaki, K. Nishikawa, T. Ozaki, K. Machida, G. Adachi, and T. Suda, J. Alloy. Compd. 264, 151 (1998).
R. Moriyamaa, S. Takedab, M. Onozakia, Y. Katayamaa, K. Shiotac, T. Fukudac, H. Sugihara, and Y. Tani, Fuel 84, 1455 (2005).
M.J. Quina, J.C. Bordado, and R.M. Quinta, Waste Manag. 28, 2097 (2008).
P.O. Auer, B. Eichler, C. Ludwing, S. Stucki, and J. Wochele, in 4th World Congress—Recovery, Recycling, Re-Integration, vol IV (1999), p. 328.
A. Jakob, S. Stucki, and P. Kuhn, Environ. Sci. Technol. 29, 2429 (1995).
A. Jakob, S. Stucki, and R.W. Struis, Environ. Sci. Technol. 30, 3275 (1996).
S. Stucki and A. Jakob, Waste Manag. 17, 231 (1997).
S. Donatelloa and C.R. Cheeseman, Waste Manag. 33, 2328 (2013).
E. Nazari, F. Rashchi, M. Saba, and S.M.J. Mirazimi, Waste Manag. 34, 2687 (2014).
M.A. Al-ghouti, Y.S. Al-degs, A. Ghrair, H. Khoury, and M. Ziedan, Chem. Eng. J. 173, 191 (2011).
Acknowledgements
The authors acknowledge the support provided by the Department of Environmental Science and Engineering, Indian School of Mines, Dhanbad, for carrying out the research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, A., Samadder, S.R. & Elumalai, S.P. Recovery of Trace and Heavy Metals from Coal Combustion Residues for Reuse and Safe Disposal: A Review. JOM 68, 2413–2417 (2016). https://doi.org/10.1007/s11837-016-1981-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-016-1981-3