Abstract
The kinetics of extracting vanadium (V) from microwave-roasted (MR) vanadium slag (V-slag) with concentrated H2SO4 were investigated. The microwave irradiation experiments were performed in a modified microwave muffle furnace at temperatures ranging from 150°C to 750°C. The x-ray diffraction analysis indicated that the spinel phase of the V-slag is destroyed after 10 min of roasting. The phase composition of the V-slag was changed by the roasting process, and a new Fe2O3 phase appeared in the samples roasted at higher temperatures. Compared to the raw slag, the surface area, pore volume, and pore size of the MR slags were much lower. It was easier to leach V from the MR samples than the raw sample with the H2SO4 solution, and the leaching process was accelerated in the MR samples. When the V-slag was roasted at 150°C and 350°C (MR@150 and MR@350, respectively), the apparent activation energy was decreased from 77.65 kJ/mol to 68.42 kJ/mol and 66.68 kJ/mol, respectively. The process of leaching V from the raw and MR slags was controlled by both the surface chemical reactions and internal diffusion. The reaction orders of the raw, MR@150, and MR@350 V-slags, with respect to the H2SO4 concentration, were 1.23, 0.75, and 0.70, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
V is one of the most important rare metals because of its wide range of uses in ferrous metallurgy and the aerospace and chemical industries as a result of its excellent physical and chemical properties.1 Although it is widely distributed in nature, V never occurs in its pure state; it is found in combination with other elements.2 In China, the main sources of V are V-slag and stone coal. V-slag is a by-product of V-Ti magnetite and accounts for more than 40% of the V produced.3
In general, V(II), V(III), V(IV), and V(V) are the main states of V in nature. However, V(III) is the dominant state in V-slag because it can occupy the Fe, Mn, and Cr crystal-lattice sites to form an isomorphous spinel phase.4 When the V in the V-slag is embedded in the spinel phase, it is difficult to extract the V by direct acid leaching. The extraction of V from slag involves the oxidation of V(III) to either V(IV) or V(V), and then it is leached by acid or water.5 Typical methods for extracting V from V-slag include sodium-salt roasting/water leaching, calcified-salt roasting/acid leaching, and additive-free roasting/acid leaching techniques.6,7 Although the whole V recovery rate has been beyond 70% for a single flow from converter V-slag to V2O5 product with the traditional sodium-salt roasting/water leaching process, the roasting process still has a low energy efficiency and it causes a large amount of additive waste.8,9 Furthermore, a large volume of harmful gases, such as Cl2, HCl, and SO2, are discharged during the roasting process, which causes serious environmental pollution.10,11 Thus, a new method for extracting V, which is more efficient and environmentally friendly, must be developed.
Microwave irradiation is a unique and distinct technology in the field of materials processing.12 The advantages of microwave irradiation include clean energy, short processing time, non-thermal effects, and the selective and volumetric heating of materials.13 The application of microwave irradiation to the extraction of metals, such as Ni, Zn, and Au, has been reported in the literature.14–16 In the present study, a novel microwave-roasting and acid-leaching process for treating V-slag was investigated. The V-slag was subjected to microwave irradiation and then leached to recover the V. The results of the microwave-roasted (MR) V-slag were compared to that of raw V-slag. In addition, the effects of microwave roasting on the kinetics of extracting V from V-slag were systematically investigated.
Experimental Methods
Materials
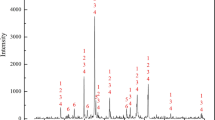
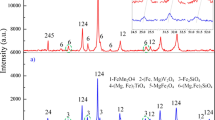
The V-slag sample was collected from the Panzhihua area of the Sichuan province of China. All the samples were crushed and screened to >0.075 mm for the roasting and leaching tests. The chemical composition of the V-slag was analyzed by XRF, and the results are shown in Table I. The x-ray diffraction (XRD) pattern of the raw V-slag is shown in Fig. 1, demonstrating that the main phases in the raw V-slag are spinel, fayalite, and titanomagnetite phases.
Instrumentation and Experimental Procedures
The MR slags were prepared in a modified HM-X08-16 type microwave muffle furnace manufactured by Kunming University of Science and Technology, Yun Nan province, China. The frequency of the microwave reactor was 2450 MHz. The reactor contained four 1.5-kW microwave generators that could be cooled via the circulation of water. To measure the temperature, a thermocouple was inserted into the sample through the top of the muffle furnace; the control cabinet was used to control the temperature.
For each experiment, 50 g of V-slag was added to a 100-mL porcelain crucible, and insulating materials were then packed around the crucible. After the samples were roasted at the chosen temperature (±50°C) for 10 min, the device was shut down and cooled by the circulating water. The leaching experiments were performed in a three-necked flask (500 mL) with a water bath. The samples were leached with H2SO4 using a liquid-to-solid ratio of 70:1 (350 mL of H2SO4 to 5 g of slag). The mixed solution was stirred at a speed of 350 rpm and heated at the chosen reaction temperature (±1°C). At fixed intervals, less than 5 mL of the reaction solution was removed from the flask. After separating the solid and liquid via vacuum filtration, the liquid was diluted to analyze its chemical composition.
Results and Discussion
Characterization of the MR V-slags
The XRD patterns of the raw and MR V-slags are shown in Fig. 1. The diffraction peaks of the MR samples appear to shift, especially the peak positions of the (Mn, Fe)(V, Cr)2O4 phase (Reference code: 00-035-0550). Furthermore, a new Fe2O3 phase is present in the XRD patterns of the slags roasted at 350°C, 550°C, and 750°C (MR@350, MR@550, and MR@750, respectively); the peaks corresponding to this new phase appear at 2θ = 33.37°, 35.85°, 49.65°, and 64.20°. In addition, because there are differences in the microwave-absorbing properties of the various phases, the microwave roasting causes phase transformations. In the microwave field, the heating rate of Si is very slow, but the heating rate of V and Fe is fast. Due to the difference of thermal expansion coefficient, different minerals generate a stress in the lattice, thereby causing cracks in the mineral particles.17
The calculated surface areas, pore volumes, and pore sizes of the raw and MR V-slags are shown in Table II. Compared to the raw slag, the surface area, pore volume, and pore size of the MR@150 slag has changed little. However, the slags roasted at 350°C or higher exhibit sharp decreases in the surface area, pore volume, and pore size.
According to the thermodynamics analysis of the oxidation processes of spinel, the products for oxidation reactions are V (V) and Cr(VI).18 In the MR V-slags, the following reactions may occur with the O2 in the air during the roasting and leaching processes:
The V-extraction rates for the raw and MR slags are shown in Fig. 2. The microwave roasting has a positive effect on the V-extraction rate; approximately 32% of the V was extracted from the MR@150 slag after 5 min of leaching, whereas only approximately 18% was leached from the raw slag after the same dissolution time. As the roasting temperature increases from 150°C to 350°C, the V-extraction rate increases significantly. Under the same experimental conditions, the extraction rate for the MR@350 slag is 30% higher than that of the raw sample after 60 min of leaching. Thus, the enhancing effect of microwave roasting on the V-extraction rate of V-slag is significant. However, because of the altered lattice structure of the slag, such as the formation of the new Fe2O3 phase and decreasing surface area, roasting temperatures above 350°C do not significantly enhance the reactivity of the V-slag.
Effect of the Leaching Temperature
The effects of the leaching temperature on the dissolution of V in the raw, MR@150, and MR@350 slags are presented in Fig. 3. The results indicate that the leaching of V from all the slags increases with increasing leaching temperature (within 50–90°C). In addition, the V-leaching behavior of the raw slag exhibits a greater monotonic increase over time compared to that of the MR slags. When the leaching temperature is 90°C, the V-extraction rate for the MR@350 slag reaches approximately 88% after 60 min. However, under the same leaching conditions, only about 60% of the V was extracted from the raw slag. These results indicate that a greater proportion of the active materials in the MR slags than that in the raw slag can be dissolved into the H2SO4 solution.
Effects of H2SO4 Concentration
The effects of the H2SO4 concentration on the V-extraction rate of the raw and MR slags was investigated using H2SO4 concentrations ranging from 100 g/L to 250 g/L, with all other conditions held constant. Figure 4 shows that the V-extraction efficiencies of the MR samples increase sharply before 5 min, especially for the MR@350 slag. The V-extraction rate of the MR@350 slag reaches approximately 94% after 60 min in the 250 g/L solution of H2SO4.
Analysis of the Leaching Kinetics
Kinetic Model
The leaching of V from the V-slag in a H2SO4 solution is a solid-liquid heterogeneous reaction. The shrinking-core model is a typical kinetic model for heterogeneous reactions. In this model, the rate-controlling step of the leaching process is the surface chemical reactions, internal diffusion, or external diffusion. In this study, it was found that the leaching of V was independent of the stirring speed when it is above 300 rpm. Thus, to ignore the effects of external diffusion, a stirring speed of 350 rpm was used.
If the leaching process is controlled by the surface chemical reactions, the leaching kinetics can be expressed with Eq. 6:19
where:
If the leaching process is controlled by the internal diffusion, the leaching kinetics can be expressed with Eq. 8:19
where:
If the leaching process is a controlled by the surface chemical reactions and internal diffusion, the leaching kinetics can be expressed with Eq. 10:20
where:
In Eqs. 6–11, x is the V-extraction rate (%), k 1 is the rate constant of the surface chemical reactions, k 2 is the rate constant of the internal diffusion, k 3 is the rate constant of the multi-phase reaction, and F(k 1, k 2) is the function describing the relationship between k 1 and k 2. In addition, t is time (min), ρ is the density of the solid reactant (kg/m3), M is the molecular weight of the solid reactant (kg/kmol), C 0 is the initial concentration of the H2SO4 solution (mol/L), n is the reaction order, k is the reaction-rate constant, D is coefficient of diffusion (m2/min), b is the mole ratio of H2SO4 consumed when 1 mol of the solid reactant is leached, and r is the initial radius of the solid reactant.
To confirm the rate-controlling step and kinetics equation of the leaching of V from the raw and MR V-slags in H2SO4 solutions, Eqs. 6, 8, and 10 were applied to the experimental data. Compared to Eqs. 6 and 8, the regression coefficient of Eq. 10 is better. This indicates that the leaching step is controlled by both the surface chemical reactions and internal diffusion. Therefore, Eq. 10 was used to describe the kinetics of leaching V from the raw and MR slags. Figure 5 shows a plot of (ln(1 − x)/3) + [(1 − x)−1/3 − 1] as a function of t for different reaction temperatures.
Calculating the Activation Energy and Reaction Order
From the results in Fig. 5, the rate constants for different reaction temperatures (k 3T) were calculated and an Arrhenius plot was constructed (Fig. 6). The activation energies for the leaching of V from the raw, MR@150, and MR@350 V-slags were calculated to be 77.65 kJ/mol, 68.42 kJ/mol, and 66.68 kJ/mol, respectively. Thus, it is confirmed that the activation energy decreases after the microwave-roasting process. These results also indicate that the microwave-roasting process enhances the reactivity of the V-slag, and thus, increases the extraction rate.
The reaction orders with respect to the H2SO4 concentration were determined with the aforementioned kinetic model. As the volume of the H2SO4 solution is large enough, the concentration of the acid is assumed to remain constant. From the results of the leaching data and calculations used to fit the plots, the values of the rate constants at different H2SO4 concentrations (k 3A) and the correlation coefficients were calculated, as shown in Table III. The relationship between ln[H2SO4] and ln[k 3A] is shown in Fig. 7. The reaction orders for the leaching of V from the raw, MR@150, and MR@350 V-slags, with respect to the H2SO4 concentration, were found to 1.23, 0.75, and 0.70, respectively. The decrease in the reaction order after the microwave-roasting process indicates that microwave roasting might decrease the dependency of the V-extraction rate on the H2SO4 concentration.
To describe the kinetics clearly, a full equation that contains the effects of the main leaching factors is indispensable. In this study, the control factors were the temperature and concentration of the H2SO4 solution. The effects of these factors on the rate constant can be expressed with Eq. 12:21
where k 0 is a constant determined by both k 3T and k 3A (min−1), [H2SO4] is the concentration of the H2SO4 solution (g/L), E a is the activation energy of the leaching process (kJ/mol), R is an ideal gas constant (8.314 kJ/(kmol K)), and T is temperature (K).
Thus, Eqs. 13, 14, and 15 can be used to describe the kinetics of extracting V from the raw, MR@150, and MR@350 V-slags in a H2SO4 solution, respectively.
Conclusion
In this study, it was found that the leaching of V from V-slag is affected by microwave roasting. The microwave roasting decreased the intensity of the peaks in the XRD patterns and altered the spinel structure of the V-slag. Compared to the raw slag, the surface area, pore volume, and pore size of MR slags were significantly decreased by the high-temperature microwave roasting. The leaching results for the raw and MR slags at different temperatures showed that the microwave roasting enhanced the reactivity of the V-slag. The analysis of the leaching kinetics indicated that the microwave roasting increases the leaching of V from the V-slag while decreasing its sensitivity to temperature. The apparent activation energies of the leaching of V from the raw, MR@150, and MR@350 V-slags were calculated to be 77.65 kJ/mol, 68.42 kJ/mol, and 66.68 kJ/mol, respectively. The kinetics of the raw and MR V-slags were controlled by both the surface chemical reactions and internal diffusion. The reaction orders of the leaching of V from the raw, MR@150, and MR@350 V-slags, with respect to the H2SO4 concentration, were 1.23, 0.75, and 0.70, respectively.
References
R.R. Moskalyk and A.M. Alfantazi, Miner. Eng. 16, 793 (2003).
Y.M. Zhang, S.X. Bao, T. Liu, T.J. Chen, and J. Huang, Hydrometallurgy 109, 116 (2011).
D.S. He, Q.M. Feng, G.F. Zhang, M. Oule, and Y.P. Lu, Miner. Eng. 20, 1184 (2007).
G.Q. Zhang, T.A. Zhang, G.Z. Lü, Y. Zhang, Y. Liu, and Z.L. Liu, Int. J. Miner. Metall. Mater. 22, 21 (2015).
M.Y. Wang, L.S. Xiao, Q.G. Li, X.W. Wang, and X.Y. Xiang, Rare Met. 28, 1 (2009).
W.Z. Mu, T.A. Zhang, Z.H. Dou, G.Z. Lü, and Y. Liu, Trans. Nonferrous Met. Soc. China 21, 2078 (2011).
X.S. Li, B. Xie, G.E. Wang, and X.J. Li, Trans. Nonferrous Met. Soc. China 21, 1860 (2011).
G.Q. Zhang, T.A. Zhang, G.Z. Lü, Y. Zhang, Y. Liu, and Z.L. Liu, Rare Met. (2014). doi:10.1007/s12598-014-0225-3.
M.Y. Wang, P.F. Xian, X.W. Wang, and B.W. Li, JOM 67, 369 (2015).
K. Mazurek, Hydrometallurgy 134, 26 (2013).
G.Q. Zhang, T.A. Zhang, G.Z. Lü, Y. Zhang, Y. Liu, and G. Xie, Rare Metal. Mat. Eng. 44, 1894 (2015).
A.H. Mohammad and K. Sam, Hydrometallurgy 73, 189 (2004).
Z.W. Peng and J.Y. Hwang, Int. Mater. Rev. 60, 30 (2015).
X.J. Zhai, Y. Fu, X. Zhang, L.Z. Ma, and F. Xie, Hydrometallurgy 99, 189 (2009).
A.H. Mohammad and K. Sam, Chem. Eng. Process. 46, 883 (2007).
R.K. Amankwah and G. Ofori-Sarpong, Miner. Eng. 24, 541 (2011).
G.Q. OuYang, X.Y. Zhang, X.D. Tian, Y. Li, and S. Xie, China J. Nonferrous Met. 18, 750 (2008).
H.Y. Li, H.X. Fang, K. Wang, W. Zhou, Z. Yang, X.M. Yan, W.S. Ge, Q.W. Li, and B. Xie, Hydrometallurgy 156, 124 (2015).
H.Y. Jiang, Physical and Chemical for Hydrometallurgical Process, 1st ed. (Beijing: Metallurgical Industry Press, 1984), pp. 71–106.
C.F. Dickinson and G.R. Heal, Thermochim. Acta 341, 89 (1999).
R. Dehghan, M. Noaparast, and M. Kolahdoozan, Hydrometallurgy 96, 275 (2009).
Acknowledgements
This work was financially supported by the Chinese National Programs for High Technology Research and Development (No. 2012AA062303), the National Natural Science Foundation of China (Nos. U1402271, U1202274, 51004033, 51204040, 51504059, and 50974035), the National Science and Technology Support Program (No. 2012BAE01B02), and the Doctoral Fund Project (No. 20120042110011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, G., Zhang, Ta., Lü, G. et al. Effects of Microwave Roasting on the Kinetics of Extracting Vanadium from Vanadium Slag. JOM 68, 577–584 (2016). https://doi.org/10.1007/s11837-015-1736-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-015-1736-6