Abstract
Research on the novel technology of fluidized roasting reduction of samples of low-grade pyrolusite using biogas residual as reductant has been conducted. According to the response surface design and the analysis of results, orthogonal experiments have been conducted on the major factors, and the effects on the manganese reduction efficiency have been studied. The maximum manganese reduction efficiency could be optimized to nearly 100%, when the mass ratio of biogas residual to pyrolusite was 0.16:1, the dosage of sulfuric acid was 1.6 times that of the stoichiometric amount, the roasting temperature was 680°C, and the roasting time was 70 min. The results in terms of manganese reduction efficiency of the actual experiments were close to those anticipated by modeling the experiments, indicating that the optimum conditions had a high reliability. Other low-grade pyrolusites such as Guangxi pyrolusite (China), Hunan pyrolusite (China), and Guizhou pyrolusite (China) were tested and all these materials responded well, giving nearly 100% manganese reduction efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Manganese is a key strategic resource that plays important roles in many fields, such as ferrous metallurgy, nonferrous metal production, battery production, and fine chemicals.1,2 With the ever-increasing demand for manganese material and the shortage of high-grade ores, exploitation and utilization of low-grade manganese ores become important.3 Manganese is found in the form of MnO2 in pyrolusite. As manganese dioxide ores are stable in acidic or alkali oxidizing conditions, the extraction for manganese from pyrolusite must be carried out under reducting conditions to obtain acid-soluble manganese oxide.4 There are two kinds of reducting technologies for pyrolusite at present: high-temperature reduction and hydrometallurgical reduction. Roasting reduction can be carried out in a reverberatory furnace, a rotary kiln, or a fixed bed using CO5 or H2 6,7 gas as the reducting medium or organic materials as the reductants such as coal,8 cornstalk,9 or sawdust10 at high temperature. The approach has a number of disadvantages: high energy consumption, long roasting time, poor or insufficient heat and mass transfer, and easy sintering. The hydrometallurgical reduction proceeds mainly with the following reductants: reductive minerals, including pyrite,11 sphalerite,12 and galena13; organic reductive materials, including molasses,2,14 methyl alcohol,15,16 sawdust,17 cornstalk,18 and oxalic acid4; and inorganic reductants, including sodium sulfite,19 iron powder,20 ferrous sulfate,21 hydrogen peroxide,22–24 and sulfur dioxide.25–27
Biogas residual is one of the main wastes of the anaerobic digestion in a biogas production project, which consists of some undecomposed raw materials and new microorganisms. It could be divided into three parts28,29: organic material and humic acid,30 playing important roles in soil improvement; elements such as nitrogen, phosphorus, and potassium, meeting the growth demand of crops; and not thoroughly decomposed raw materials, fertilizers for farmland use to proceed with fermentation. Currently, biogas residual is mainly used in agriculture29 such as fertilization,31,32 nutrition soil and culture medium preparation, and in chemical engineering such as the adsorbent of Cr6+,33 Pb2+,34 and direct red 12B35 coloring agent in wastewater. As straw is one of the leading woody types of fiber biomass resource, application research on its anaerobic digestion is continuing to develop. Previous studies29 showed that anaerobic digestion had no effect on lignin in woody fiber composition, but it made the relative content of hemicellulose reduce and the relative content of cellulose increase. It is noted that cellulose can be hydrolyzed into the reductive glucose under the acidic condition, which has a positive significance for the resource utilization of biogas residual.
In this study, a novel technology based on fluidized roasting reduction of low-grade pyrolusite using biogas residual as reductant, was examined. This eco-friendly technology not only can solve the above noted environmental issues resulting from biogas residual30 but also can realize the sustainable development of ecological environment in the most effective way and expand its scope to achieve high value-added utilization.
Materials and Methods
Materials
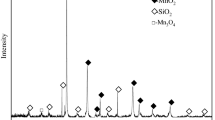
The sample of low-grade pyrolusite was obtained from Yunnan, China. The ore sample was crushed and ground into powder with particle sizes of less than 0.147 mm. The chemical multielemental analysis (as listed in Table I) showed that the pyrolusite used contained 21.43% Mn, 36.36% SiO2, and 8.11% Fe2O3. Powder x-ray diffraction (XRD) (Fig. 1) showed that manganese was mainly in the form of manganese dioxide in pyrolusite, and the main gangue minerals included quartz and feldspar.
Biogas residual is the waste of the anaerobic digestion of woody fiber biomass, such as straw. It was prepared to the required particle size of less than 0.1 mm by pulverizing, ceramic milling, and screening. The main composition is shown in Table II, containing fiber for immediate use, crude fiber, mineral substance, and ash. The fiber composition is about 55.12%, which is considered relatively high.
Experimental Equipment
Figure 2 illustrates the experimental arrangement used for the tests. It consisted of the fluidized bed reactor, resistance furnace, and a flow rotameter. All the chemicals were of analytical grade and used without further purification.
Experimental Procedure
For each test, 10 g of pyrolusite and the required amount of biogas residual was mixed thoroughly according to the mass ratio of biogas residual to pyrolusite. The required amount of sulfuric acid with a concentration of 40 wt.% was then added. The mixture was blended uniformly and made into pellets with a diameter less than 0.147 mm. After the pellets were added into the quartz fluidized bed reactor, the roasting reduction was started at a required temperature for a given period of time. After this time, the process was stopped and then the roasted product was transferred into a small leaching tank containing 200 mL of water. The solution was stirred continuously until all the manganese sulfate was leached from the roasted product. After the sample was filtrated and dried, the manganese in the filtrate was analyzed to calculate the manganese leaching efficiency. It is noted that this corresponds to the manganese reduction efficiency since manganese dioxide is not dissolved by sulfuric acid. The manganese reduction efficiency can be calculated as follows:
where η is the manganese reduction efficiency (wt.%), V is the volume of the filtrate (mL), β is the manganese content in filtrate (g/mL), m is the mass of raw pyrolusite (g), and α is the manganese content in raw pyrolusite (wt.%).
The reduction of manganese dioxide in pyrolusite using biogas residual as reductant is mainly achieved by the reductive glucose into which the cellulose of biogas residual is hydrolyzed under acidic condition. The main related chemical reactions are shown as follows:
Results and Discussion
Single-Variable Experiments
Effect of Mass Ratio of Biogas Residual to Pyrolusite on Manganese Reduction Efficiency
In order to evaluate the effect of the mass ratio of biogas residual to pyrolusite, a series of experiments was carried out by varying the mass ratio from 0.05:1 to 0.4:1, while fixing the dosage of sulfuric acid at 1.5 times that of the stoichiometric amount, the roasting temperature at 600°C, and the roasting time at 80 min. From Fig. 3, it can be seen that the manganese reduction efficiency increased with the increase of the mass ratio when the mass ratio was below 0.2:1. The reason for this is probably due to the fact that the concentration of reductive glucose increases with the increase of the mass ratio, contributing to the increase of the manganese reduction efficiency. When the mass ratio was 0.2:1, the manganese reduction efficiency reached a maximum value of about 97.31%. Further increase in the mass ratio resulted in sharp decrease in the manganese reduction efficiency. Therefore, the optimum mass ratio of biogas residual to pyrolusite was considered as 0.2:1.
Effect of Dosage of Sulfuric Acid on Manganese Reduction Efficiency
The effect of the sulfuric acid dosage (a factor times that of the stoichiometric amount) on the manganese reduction efficiency from 0.5 to 2.5 is presented in Fig. 4, while fixing the mass ratio of biogas residual to pyrolusite at 0.2:1, the roasting temperature at 600°C, and the roasting time at 80 min. From Fig. 4, it is seen that the manganese reduction efficiency increased with the increase of the dosage of sulfuric acid. This is mainly because the increase in the dosage of sulfuric acid contributes to intensifying and catalyzing the hydrolysis of cellulose in biogas residual into reductive glucose, improving the reductive capacity of the reaction system. When the dosage of sulfuric acid was above 1.5, no improvement of the manganese reduction efficiency could be observed. Therefore, the optimum dosage of sulfuric acid was considered as 1.5 times that of the stoichiometric amount.
Effect of Roasting Temperature on Manganese Reduction Efficiency
The fluidized roasting reduction experiments at different roasting temperature from 400°C to 800°C were carried out while fixing the mass ratio of biogas residual to pyrolusite at 0.2:1, the dosage of sulfuric acid at 1.5 times that of the stoichiometric amount, and the roasting time at 80 min (Fig. 5). The results indicated that the manganese reduction efficiency increased as the roasting temperature was initially increased. When the roasting temperature was 600°C, the manganese reduction efficiency increased only slightly with further increase of the roasting temperature. Hence, the optimum roasting temperature was considered as 600°C.
Effect of Roasting Time on Manganese Reduction Efficiency
The manganese reduction efficiency also depended on the roasting time (Fig. 6). The experiment was carried out with fixing the mass ratio of biogas residual to pyrolusite at 0.2:1, the dosage of sulfuric acid at 1.5 times that of the stoichiometric amount, and the roasting temperature at 600°C. When the roasting time ranged from 20 min to 80 min, longer roasting times benefited manganese reduction efficiency. When the roasting time was 80 min, further increase in the roasting time improved the manganese reduction efficiency only marginally. Considering the equipment and the roasting cost, the optimum roasting time was considered to be 80 min.
Response Surface Analysis and Experimental Optimization
Variables and Levels Selection
As noted above, the primary variables for manganese reduction efficiency included the mass ratio of biogas residual to pyrolusite, the dosage of sulfuric acid, roasting temperature, and roasting time. In order to obtain a relatively high manganese reduction efficiency, the conditions of the single factors should be: the mass ratio of biogas residual to pyrolusite, 0.2:1; the dosage of sulfuric acid, 1.5 times that of the stoichiometric amount; the roasting temperature, 600°C; and the roasting time, 80 min; respectively, according to the single-factor experiments. Based on the results above, it was considered sufficient to proceed to the Box-Behnken design of experiments considering four factors or variables and three levels (Table III).
Experimental Results and Analysis
The results of orthogonal or “unbiased” experiments are shown in Table IV. The sum of squares and summary statistics of various fitting models between the manganese reduction efficiency and every factor are listed in Tables V and VI, respectively.
The results indicated that the fitting model of quadratic terms was relatively great and suggested. The F value of the quadratic fitting model was 609.37, and the p value was smaller than 0.0001, indicating that this model had statistical significance. The related coefficient value of R 2 was 0.9973, indicating that this model fit well with the experimental results and using this model for fitting actual experiments was feasible.
The confidence degree analysis of the quadratic model is shown in Table VII. The results indicated that the fitting results of quadratic model were significant. The impact sequence of single factors to the manganese reduction efficiency is shown as follows: the dosage of sulfuric acid > the mass ratio of biogas residual to pyrolusite > roasting temperature > roasting time. The impact sequence of interaction factors to the manganese reduction efficiency is presented as follows: (roasting temperature × roasting time) > (the dosage of sulfuric acid × roasting time) > (the dosage of sulfuric acid × roasting temperature) > (the mass ratio of biogas residual to pyrolusite × the dosage of sulfuric acid) > (the mass ratio of biogas residual to pyrolusite × roasting temperature) > (the mass ratio of biogas residual to pyrolusite × roasting time).
The quadratic model between the manganese reduction efficiency and every factor could be expressed as follows:
Normal plot of residuals is shown in Fig. 7. The points were almost distributed in a line, indicating that the model fit relatively well.
Contours between the mass ratio of biogas residual to pyrolusite and the dosage of sulfuric acid for the manganese reduction efficiency are shown in Fig. 8, under conditions of a roasting temperature at 600°C and the roasting time at 80 min. The results indicated that the dosage of sulfuric acid should be gradually reduced with the increase of the mass ratio when the manganese reduction efficiency was 86.5483% and the conditions of the roasting temperature and the roasting time were at an intermediate level.
The response surface between the roasting temperature and the roasting time for the manganese reduction efficiency is shown in Fig. 9, while fixing the mass ratio of biogas residual to pyrolusite at 0.2:1 and the dosage of sulfuric acid at 1.5 times that of the stoichiometric amount. The manganese reduction efficiency increased with the extension of the roasting time when the conditions of the mass ratio and the dosage of sulfuric acid were intermediate level. The manganese reduction efficiency increased with the increase of the roasting temperature. When the roasting temperature was 600°C, the manganese reduction efficiency was maximized, which agreed with the results of the single-factor experiments.
Experimental Optimization
Based on the experimental results and the fitting model analysis, the optimization for the level of each variable or factor could be conducted on the basis of the maximum manganese reduction efficiency (Table VIII). When the mass ratio of biogas residual to pyrolusite was 0.16:1, the dosage of sulfuric acid was 1.6 times that of the stoichiometric amount, the roasting temperature was 680°C, and the roasting time was 70 min, the maximum manganese reduction efficiency, at nearly 100%, could be obtained. The optimized contours and response surface between the roasting temperature and the roasting time for the manganese reduction efficiency of the No. 1 optimization scheme are shown in Figs. 10 and 11, respectively, under the conditions that the mass ratio of biogas residual to pyrolusite was 0.16:1, and the dosage of sulfuric acid was 1.6 times that of the stoichiometric amount. Three verification experiments were conducted according to the No. 1 optimization scheme and the results of the manganese reduction efficiency were 99.94%, 99.98%, and 100%, respectively, which were close to the results of the fitting model, indicating that the optimum solution had a relatively high reliability.
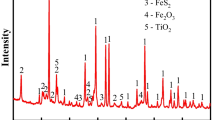
The XRD image of the fluidized roasting reductive leaching residual of pyrolusite under the No. 1 optimization scheme is shown in Fig. 12. The main minerals included quartz and a small amount of hematite. The diffraction peak of manganese dioxide has virtually disappeared. The fact that the maximum manganese reduction efficiency of pyrolusite under this condition could be obtained has been further confirmed.
Reduction Behavior of Other Low-Grade Pyrolusite
Other low-grade pyrolusite such as Guangxi pyrolusite (China), Hunan pyrolusite (China), and Guizhou pyrolusite (China) were also tested. For these tests, a 10-g ore sample was used, the dosage of sulfuric acid 1.6 times that of stoichiometric amount, the roasting temperature 680°C, and the results are shown in Table IX. The amount of biogas residual for these materials was varied to optimize the manganese reduction efficiency. The amounts corresponding to maximum reduction efficiency showed that the amount of the required biogas residual was dependent on the manganese content of pyrolusite and the requirement decreased with the decrease in Mn content. The current study established the suitability of biogas residual as reductant for various low-grade pyrolusite.
Conclusions
-
1.
The novel technology of fluidized roasting reduction of low-grade pyrolusite using biogas residual as reductant for the manganese exploitation and utilization is considered “green” and eco-friendly; it handles a biogas residual material and produces a value added product in a sustainable development fashion.
-
2.
A number of variables was examined. The results indicated that the most effective single variable or factor for the manganese reduction efficiency was the dosage of sulfuric acid, and the most effective interaction factor for the manganese reduction efficiency was (roasting temperature × roasting time). The quadratic model between the manganese reduction efficiency and each variable could be expressed as follows (where the terms A to D are given in the article):
-
3.
According to the optimization scheme of the experiments, when the mass ratio of biogas residual to pyrolusite was 0.16:1, the dosage of sulfuric acid was 1.6 times that of the stoichiometric amount, the roasting temperature was 680°C, and the roasting time was 70 min, a maximum manganese reduction efficiency of close to 100% was obtained. The results of the manganese reduction efficiency of the actual experiments were close to those of the fitting model by the verification experiments, indicating that the optimum solution had a relatively high reliability.
-
4.
This technology is generally applicable to the extraction of various low-grade pyrolusite, and it is promising to be utilized widely in manganese industry due to its high efficiency, good availability, and low cost.
References
F. Pagnanelli, M. Garavini, F. Vegliò, and L. Toro, Hydrometallurgy 71, 319 (2004).
H. Su, Y. Wen, F. Wang, Y. Sun, and Z. Tong, Hydrometallurgy 93, 136 (2008).
H. Su, H. Liu, F. Wang, X. Lü, and Y. Wen, Chin. J. Chem. Eng. 18, 730 (2010).
R.N. Sahoo, P.K. Naik, and S.C. Das, Hydrometallurgy 62, 157 (2001).
J.M.M. Paixdo, J.C. Amaral, L.E. Memória, and L.R. Freitas, Hydrometallurgy 39, 215 (1995).
T.J.W. De Bruijn, T.H. Soerawidjaja, W.A. De Jongt, and P.J. Van Den Berg, Chem. Eng. Sci. 35, 1591 (1980).
A. Jerez and M.A. Alario, Thermochim. Acta 58, 333 (1982).
J. Chen, P.F. Tian, X.A. Song, N. Li, and J.X. Zhoum, J. Iron. Steel Res. Int. 17, 13 (2010).
Z. Cheng, G. Zhu, and Y. Zhao, Hydrometallurgy 96, 176 (2009).
J.J. Song, G.C. Zhu, P. Zhang, and Y.N. Zhao, Acta Metall. Sin. 23, 223 (2010).
A.G. Kholmogorov, A.M. Zhyzhaev, U.S. Kononov, G.A. Moiseeva, and G.L. Pashkov, Hydrometallurgy 56, 1 (2000).
L. Yaozhong, Miner. Eng. 17, 1053 (2004).
H. Long, L. Chai, and W. Qin, Trans. Nonferr. Met. Soc. China 20, 897 (2010).
T.A. Lasheen, M.N. El Hazek, and A.S. Helal, Hydrometallurgy 98, 314 (2009).
F.W.Y. Momade and Z.G. Momade, Hydrometallurgy 51, 103 (1999).
F.W.Y. Momade and Z.G. Momade, Hydrometallurgy 54, 25 (1999).
D. Hariprasad, B. Dash, M.K. Ghosh, and S. Anand, Miner. Eng. 20, 1293 (2007).
X. Tian, X. Wen, C. Yang, Y. Liang, Z. Pi, and Y. Wang, Hydrometallurgy 100, 157 (2010).
J. Zheng, H. Luo, X. Tian, L. Wang, C. Yang, and Z. Pi, J. China Univ. Geosci. 18, 163 (2007).
M.S. Bafghi, A. Zakeri, Z. Ghasemi, and M. Adeli, Hydrometallurgy 90, 207 (2008).
S.C. Das, P.K. Sahoo, and P.K. Rao, Hydrometallurgy 8, 35 (1982).
M.N. El Hazek, T.A. Lasheen, and A.S. Helal, Hydrometallurgy 84, 187 (2006).
S. Do, B. Batchelor, H. Lee, and S. Kong, Chemosphere 75, 8 (2009).
A.A. Nayl, I.M. Ismail, and H.F. Aly, Int. J. Miner. Process. 100, 116 (2011).
D. Grimanelis, P. Neou-Syngouna, and H. Vazarlis, Hydrometallurgy 31, 139 (1992).
P.K. Naik, L.B. Sukla, and S.C. Das, Hydrometallurgy 54, 217 (2000).
W. Sun, S. Ding, S. Zeng, S. Su, and W. Jiang, J. Hazard. Mater. 192, 124 (2011).
R. Jiao, Energy Conserv. Environ. Protect. 3, 70 (2011).
L. Liu, H. Chen, and Y. Han, Trans. CSAE 26, 277 (2010).
G. Zhang, S. Wu, H. Wang, S. Wei, K. Wang, Y. Long, and L. Deng, China Biogas 28, 21 (2009).
L. Zhu and J. Lu, J. Agro-Environ. Sci. 26, 176 (2007).
Q. Guo, D. Niu, H. Cheng, and Y. Zhao, China Resourc. Comprehens. Utilization 12, 11 (2005).
C. Namasivayam and R.T. Yamuna, Chemosphere 30, 561 (1995).
C. Namasivayam and R.T. Yamuna, Bioresour. Technol. 52, 125 (1995).
C. Namasivayam and R.T. Yamuna, Environ. Pollut. 89, 1 (1995).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (no. 21176026), the National High Technology Research and Development Program (863 program) of China (no. 2012AA062401), and the National Key Technology R&D Program of China (no. 2012BAB14B05).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, Z.L., Feng, Y.L., Li, H.R. et al. Optimization of Fluidized Roasting Reduction of Low-Grade Pyrolusite Using Biogas Residual as Reductant. JOM 64, 1296–1304 (2012). https://doi.org/10.1007/s11837-012-0453-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-012-0453-7