Abstract
Global warming not only alters phenology but also nutritional quality and defense compounds in plants, which consequently hinders their defense against herbivorous insects. In this study, the performance of Spodoptera litura was analyzed to observe the effects of high temperatures on chemical-based defense in plants in the context of insect–plant interaction. Results show that high temperature reduced the nutritional value and content of defense compounds in the foliage of yellow cress (Rorippa dubia). These alterations negatively affected the performance of second instar S. litura larvae feeding on plants grown at high temperature. Low quality of the food source is likely the key cause of slow development of larvae. Adaptation of herbivorous insects known as compensatory feeding is projected resulting in more crop losses under global warming. Our data reveal temperature-induced reduction in the content of defensive compounds (in constitutive resistance) along with lower response capability against herbivore attacks (in induced resistance), which indicate a decrease in plant fitness. High temperatures caused by global warming negatively affect crop production and are expected to increase the burden on plant protection practices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The average global surface temperature increased by 0.74 °C from 1906 to 2005 (IPCC 2007), and most models predict a rise of at least 1.5–2.0 °C in global surface temperature by the end of this century (IPCC 2013). Insects and plants represent 50% of all identified species on earth (Price 1997). Global warming adversely affects plants–insect interaction, which is represented by its negative effects on crop protection (Evan et al. 2012). High temperatures modified the plant's physiological processes, growth, development, reproduction/mortality, and phenology, but in term of plant fitness, elevated temperature also alters the content of nutritional and defense compounds (Hatfield and Prueger 2015), which in turn affect the performance of phytophagous insects (Bale et al. 2002; Clissold 2013). An increase in the frequency of herbivore attacks was recorded recently (Pureswaran et al. 2018), and pest pressure is expected to increase in agriculture and forestry wrought by climate change (Battisti 2008; Bebber et al. 2014). However, whether the fitness of plants, with regard to defense against herbivore insects, is affected by high temperatures remains unclear.
The nutrient content in plants was found to be altered in response to warming condition because of enhanced photosynthesis (Luo 2007), which converts CO2 into carbohydrates in the presence of light for plant growth and development (Pan et al. 2012). Photosynthesis has been described as the most heat-sensitive process (Ashraf and Harris 2013; Berry and Bjorkman 1980), and temperature-induced stimulation is likely species specific; for example, soybean photosynthesis produced the highest foliar sugar concentrations in the temperature range of 28–32 °C (Vu et al. 2001). The increase in the concentration of soluble sugars, but a decrease in the concentrations of starch, total sugars, amino acids, and soluble protein typically are observed at elevated temperature (Lafta and Lorenzen 1995; Xia et al. 2015). In case of nitrogen absorption and conversion, high temperatures apparently increase the rates of nitrogen uptake and transport of nitrogen within trees both upward (xylem flow) and downward (phloem flow) (Rennenberg et al. 2006) until a threshold temperature is reached (Bassirirad 2000; Dong et al. 2001). For instance, in spruce, the uptake of ammonium was maximum at 20 °C (Gessler et al. 1998), whereas in soybean, the maximum levels of foliage nitrogen are typically observed at 24 °C (Rufty et al. 1981; Hungaria and Franco 1993). Some studies have recorded a reduction in total carbohydrate and nitrogen concentrations in plants grown at high temperatures, which indirectly negatively affects insect growth and fitness (Kingsolver and Woods 1998). As an adaptation to low-quality nutrition, compensatory feeding in herbivorous insect is likely to become a common phenomenon; leaf consumption typically increases with an increase in temperature (Yang and Joern 1994; Coggan et al. 2011).
The plant’s chemical-based resistance against insect herbivores based on secondary metabolites is known as defensive compounds which do not affect the normal vegetative growth and development (Hermilo and Alina 2017). These plant-derived secondary compounds target the digestive or metabolic enzymes of insects by disrupting (Sadasivam and Thayumanavan 2003) or inhibiting the normal chemical pathways of insect metabolism (Smith 2005). This interference result in reduction of performance, growth, and survival of insects (Li et al. 2000; Jadhav et al. 2012). To reduce the cost for defense in the absence of herbivorous threat, plant accumulated a constant concentration of defensive metabolites which provide stable protection from uninvited herbivore referred to as “constitutive resistance” (Underwood et al. 2005). Upon herbivorous threat, plants respond by activating the “induced resistance” which is due to locally and systemically increased production of defensive compounds to help host plant suppress the attackers (Brody and Karban 1992; War et al. 2012). Little is known about the impacts of elevated temperature on concentrations of plant’s secondary metabolites (Bidart-Bouzat and Imeh-Nathaniel 2008) in both plant’s constitutive and induced resistance. This topic is main target of our studies.
The plant’s constitutive resistances with basal protection are predicted to decrease in high temperatures based on the hypothesis that increased temperatures increase photosynthesis rates and reduce allocations toward plant defense (Herms and Mattson 1992). Indeed, levels of phenolic constituents (e.g., flavonoids and tannins) generally do decline with warming, but levels of terpenoids typically increase (Zvereva and Kozlov 2006). No consistent trend has been identified through few studies that have assessed the effects of high temperatures on plant secondary metabolites, and the outcome appears to be dependent on the plant species and chemical type. For example, the phenolic contents in Dark-leaved willow (Salix myrsinifolia) (Veteli et al. 2002) and white birch (Betula pendula R.) (Kuokkanen et al. 2001) recorded a significant decrease as a result of high temperature, whereas the glucosinolates content was found increasing in Broccoli (Brassica oleracea) (Pereira et al. 2002) that grew at elevated temperature. Although variation in phytochemical response to temperature makes it difficult to predict how future climate changes scenarios, but in terms of plant’s fitness, the question of whether or not how elevated temperature affects constitutive resistance against herbivorous insects still needs more practical evidences. This question is also an object of our study base on the example of Brassicaceae family (Rorippa dubia) and generalist insect of Spodoptera litura F.
An important mechanism of plant's defense response against herbivore insects is induced resistance, which will be affected or not by global warming process? This question is still need to be answer. From the example of domestication process, Moreira et al. (2018) provides the evidence of reduction of chemical-based resistance including both plant’s constitutive and induced resistance under influence of cabbage domestication. Although the global warming factors (temperature and CO2) are widely accepted as factors affecting all plant’s physiology, but adequate studies are not emphasized on the effect on plant’s induced resistance (Jamieson et al. 2012). To our knowledge, the elevated CO2 condition changed the herbivore-induced resistance of oilseed rape (Brassica napus) leaves with regard to glucosinolate concentrations (Himanen et al. 2008). Additional evidence is needed to answer the question of whether induced responses in plants are affected by high temperatures.
Spodoptera litura Fabricius, known as the common armyworm, tobacco cutworm, or cluster caterpillar, is a polyphagous defoliating insect pest worldwide, particularly in tropical countries. It is resistant to multiple commonly used insecticides (Huang and Han 2007). Gradually, S. litura has become a crucial insect pest (Dhaliwal et al. 2010). However, future climate change with increasing temperature is projected to provide favorable conditions for S. litura (Zhang et al. 2017); consequently, crop losses are expected to increase (Smriti et al. 2018).
In this study, based on experimental proofs from model of generalist insect (S. litura) and yellow cress (Rorippa dubia), an annual herb in Brassicaceae family, we examined the effects of high temperatures on plant chemical profiles including nutrient content and defense compounds, which consequently affected the performance of generalist herbivory insects. With regard to chemical-based resistance, not only the constitutive resistance but also the induction response of plants, which was changed under the influence of high temperatures, was studied.
Materials and methods
Greenhouse conditions
Two temperature conditions, namely ambient temperature (24/21 °C day/night) and high temperature (29/26 °C day/night), were set up in four greenhouse rooms (two rooms for ambient and two other for elevated temperatures) with a photoperiod of 16:8 h (light/daily) maintained in all treatments. The ambient temperatures used in the study were based on the average November temperatures in Taichung between 2013 and 2016 (data from the Central Weather Bureau, Taiwan), when the production of brassica vegetables is at its peak in Taiwan. The high-temperature setting was based on the predicted maximum temperature in the next century, which is predicted to be at least 5 °C higher than current temperatures (Flato et al. 2013).
Plant cultivation conditions
Yellow cress (Rorippa dubia) was collected in Taichung, Taiwan, and then maintained under greenhouse conditions at National Chung Hsing University in Taichung, Taiwan. Seeds of R. dubia were soaked in water at 65 °C for 5 min before sowing them in standard potting soil (MOS‐010; Known‐You Seed Company) in 104-well plates. Plants were watered daily and maintained at temperatures of 24 °C and 21 °C (day and night, respectively). After germination, plants with 1 or 2 true leaves were transplanted into individual plastic pots (10.5 cm [height] × 12 cm [diameter]) filled with commercial soil (N: 1%; P: 0.2%, and K: 0.1%) (Known-You Seed Company, Taichung, Taiwan), and soil moisture was maintained by watering daily. Plants were maintained at ambient temperatures (24 °C and 21 °C day and night, respectively) and high temperatures (29 °C and 26 °C day and night, respectively), and no fertilizer and pesticide were applied. To reduce the effects of within-chamber variation, we randomly rearranged pots once a week. After each plant exhibited a fully expanded sixth leaf (5 weeks, after seedling), we initiated herbivore-induced manipulations on R. dubia foliage. After 48 h of induction, experimental foliage were collected to analyze chemical compounds as well as insect feeding assays.
Insect rearing
Spodoptera litura eggs were collected manually from a taro field in Taichung County, Taiwan, and then maintained on an artificial diet in a growth chamber at a constant temperature of 25 °C ± 2 °C and 70% ± 3% relative humidity (RH) for 14:10 h (light/dark) at the Insect–Plant Interaction Laboratory, Entomology Department, National Chung Hsing University, Taichung, Taiwan.
An artificial diet for rearing larvae was made with some modifications from composition described in (Gupta et al. 2005), including flower bean (9.85%), wheat germ (3.61%), yeast powder (3.91%), agar (2.4%), other vitamins, and distilled water. Subsequently, adult males and females were paired and fed with saturated sugar in a glass cylinder (22 [height] × 14.5 cm [diameter]) lined with tissue paper for egg collection. The larvae used in the experiments were obtained from eggs laid by F3 females.
Manipulation of induced resistance of foliage through herbivore attack
Fourth instar larvae of S. litura were individually starved from the night prior to the beginning of the experiment. The larvae were then placed, one per leaf, on the experimental foliage, and the leaf was covered with a 12 × 15 cm2 mesh net. The larvae were allowed to feed for approximately 2–3 h and were removed (after approximately 20% of the leaf area had been consumed) from experimental leaves. All herbivorous-damaged leaves and intact leaves (control) were collected for experiments after 48 h of induction.
Experimental design
A two-factor experiment was set up in four temperature-controlled greenhouse rooms to estimate the effects of temperature and herbivore-induced responses in terms of changes in the profile of defense compounds that might consequently affect the development of generalist insects.
The experiment was set up with a total of 80 plants, with 40 plants each maintained at ambient and high temperatures. In each temperature condition, two sets of treatments were designated as intact treatment (for constitutive resistance, n = 20) and herbivore-induced foliage treatment (for induced resistance, n = 20). Herbivore induction experiments allowed direct herbivorous attack at 48 h before sample (foliage) collection.
For each plant, four middle-aged foliages were designated for the experiment including the second, third, fourth, and fifth leaves (counted from the bottom). The youngest leaf (fifth leaf) was used for the bioassay to estimate insect performance (relative growth rate [RGR]), and three other leaves (the second, third, and fourth leaf) were used for the chemical analysis of nutritional and defensive compounds.
All experimental leaves (intact and 48 h-induced foliage) were collected at the same time to estimate the temperature-induced effects on altering the concentration of plant’s nutritional and defensive compounds which play an important role in chemical-based resistance including both constitutive and induced resistance. Furthermore, the indirect effect of temperature on growth rate (RGR) of generalist insect fed on temperature-induced plant was estimated by larval bioassay.
Insect performance
A total of 80 larvae (20 larvae/treatment) were fed individually on the experimental foliage of R. dubia grown at different conditions of temperature and herbivorous induction described above. Fresh leaves were cut using sterilized scissors, and stems were placed in 2-mL tubes containing distilled water before being placed in plastic cups (10 cm [diameter] × 5.7 cm [height]). Newly molted second instar larvae of S. litura were individually weighed using an M2P Sartorius microbalance (Sartorius AG, Goettingen, Germany) before gently placing them on the experimental foliage (1 larva/leaf/cup).
Feeding assay has been conducted in chamber condition (25 °C ± 1 °C; 14:10 h [light:dark]; 75% RH) for 2.5 days until the larvae reached the late second instar stage; then, all individuals were collected and weighed. The fresh weights of the larvae before and after the experiment were used to calculate the RGR by using the following equation: RGR (mg/mg/day) = (final weight − initial weight)/(initial weight × days).
Chemical analysis
For measuring the trypsin inhibitor (TI), polyphenol oxidase (PPO), peroxidase (POD), and protein content, the fresh fourth leaf was collected and preserved in − 20 °C (for less than 1 week) until analysis. For measuring content of nitrogen, carbohydrate, and total phenolic compound, the second and third foliage were collected, treated with liquid nitrogen, dried in a freeze dryer (Alpha 1-4, Christ, Germany) for 48 h, and subsequently ground into powder. Details of analysis were described as follows:
Nitrogen
Total amount of nitrogen from the foliage of R. dubia was determined using the standard micro-Kjeldahl assay described by Lang (1958) with some modification. Fifty milligram of sample (leaf powder) or standard (Glycine) was digested in glass tube containing 4 mL of digestion mixture, which was prepared 48 h before use by combining 7 g of lithium sulfate (Sigma), 0.21025 g of selenium powder (Alfa), 175 mL of hydrogen peroxide (Sigma), and 210 mL of sulfuric acid (Fluka, Honeywell).
Acid digestion (Parkinson and Allen 1975) was performed for 3 h (30 min at 200 °C and then increasing the temperature up to 380 °C in 150 min). After the digested material cooled down, 75 mL of distilled water was added for dilution. To the diluted solution (500 µL), 4.5 mL of polyvinyl alcohol (PVA, 0.05 g [Sigma] in 500 mL distilled water) was added. Next, 200 µL of Nessler’s reagent (Hach, USA) was added. The sample and standard solutions were vortexed and allowed to stand for 10 min before measuring absorbance at 460 nm (ΔOD460).
Total amount of nitrogen was calculated on the basis of a standard curve (y = 0.0336x − 0.0072 with R2 = 0.9981) generated using glycine (Riedel-de Haën) concentrations.
Total carbohydrate
Total carbohydrate (soluble carbohydrate and insoluble) was extracted twice from dried and powdered foliage (1.5 mg/Eppendorf) with 2 mL of 80% ethanol at 80 °C in 10 min followed by centrifugation at 5000 rpm for 10 min. Extraction resulted in soluble and insoluble fractions representing soluble and insoluble carbohydrates, respectively.
Soluble carbohydrate concentration was determined using the 3,5-dinitrosalicylic acid (DNS) assay described by Miller (1959) and Wood and Bhat (1988) with some modification. The DNS reagent was prepared according to a previously reported method (Coughlan and Moloney 1988) by mixing solutions A and B. Solution A was prepared by dissolving 1 g of 3,5-DNS acid by gentle heating in 20 mL of 2 N NaOH. Solution B was prepared by slowly adding 30 g of sodium potassium tartrate tetrahydrate (Sigma, CAS 6381-59-5) to 50 mL of distilled water. The final volume of the DNS reagent was fixed up to 0.1 L with distilled water. A sample of soluble carbohydrate (500 µL) was added to 100 µL of 2 N HCL and then boiled in water bath for 30 min. After cooling down to room temperature, 400 µL of 1.2 N NaOH and 500 µL of the DNS reagent were added to the solution and placed in a boiling water bath for 5 min. The solution was diluted using 3.5 mL of distilled water. Absorbance was read at 511 nm (ΔOD511) by using a Genesys 10 UV–Visible Spectrophotometer (Thermo Spectronic, USA).
The standard of d-glucose (Katayama, Japan) in 80% ethanol was manipulated using the same procedure as that used for the sample. The standard curve (y = 1.2259x + 0.2769 with R2 = 0.992) was generated on the basis of glucose concentrations, and the soluble carbohydrate concentration was calculated from the calibration curve.
Insoluble carbohydrate was determined from the insoluble fraction through ethanol extraction by using the method of Rose et al. (1991) with some modification. The samples were suspended in 0.5 mL of 0.2 N NaOH and boiled for 12 min to gelatinize starch. After cooling, pH was adjusted to 4.5 by using 100 µL of acetic acid. A volume (100 µL) of sodium acetate buffer (0.1 M, pH 4.5) containing 15 IU of amyloglucosidase from Aspergillus niger (Sigma; CAS 9032-08-0) was added, and the mixture was incubated for 10 min at 55 °C for enzymatic hydrolysis of the insoluble material. The soluble fraction was collected through centrifugation at 5000 rpm for 10 min; then, glucose present in the solution was measured following the protocol of soluble carbohydrate described previously. The standard curve (y = 0.3223x + 0.1049 with R2 = 0.9945) was generated using a solution of d-glucose (Katayama chemical Co., Ltd) in sodium acetate (Sigma) by using the same procedure as that used for the sample.
Protein content
Protein was extracted and its activity was measured according to the method described by Stout et al. (1997) with minor modifications. Fresh leaf samples (0.5 g) were ground in liquid nitrogen and homogenized in 5 mL of grinding buffer containing 7% (w/v) polyvinylpolypyrrolidone (Sigma) in potassium phosphate buffer (0.1 M, pH 8); 10% solution of triton X-100 was added to the homogenate. The supernatant was collected through centrifugation at 10,000 rpm at 4 °C for 15 min.
The quantification of protein was performed as described by Bradford (1976) with modifications. To each 10 μL of the leaf extract, 40 μL of potassium phosphate buffer (pH 7.0) was added in 2 mL Eppendorf tubes. Each tube contained 5 μL of 0.1 N HCL, 40 μL of distilled water, and then they were mixed with 1750 μL of 20% Coomassie Blue Dye (Bio-Rad 500-0006). Bovine serum albumin (BSA) in potassium phosphate buffer (pH 7.0) was used as standard protein solution and manipulated using the same method as that used for the foliar extract. The dying sample (200 μL) was then delivered to each well on 96-well plates (Greiner MICROLON), and absorbance was measured at 570 nm (ΔOD570) on VersaMax™ Microplate Reader.
Protein content was calculated from the calibration curve (y = 0.0129x + 0.3142 with R2 = 0.9953) with y = ΔOD570 and x = sample protein content.
Trypsin inhibition activity
Trypsin inhibition (TI) activity was determined using a method described by Rodriguez-Saona et al. (2005) with some modifications. Leaf tissues were ground in liquid nitrogen and homogenized in a Tris–HCl extraction buffer (pH 7.8; 3 µL/mg fresh mass). The homogenized mass was centrifuged at 12,000 rpm at 4 °C for 20 min to collect the supernatant. Three sets (sample, blank, and standard) were prepared for the TI assay, with absorbance measured at 280 nm (A280) by using a spectrophotometer (Genesys 10S UV–Vis) as described previously by Lee and Lin (1995).
The percentage of inhibition was calculated using the following equation: [(A280 of standard + A280 of blank − A280 of sample)/A280 of standard] × 100%. TI activity was converted from the percentage of inhibition and expressed by unit/gram of fresh foliage. One unit was defined as the amount of TI that inhibited 1 mg of trypsin within a 20-min period.
Polyphenol oxidase activity
Polyphenol oxidase (PPO) was extracted using the method of protein extraction. PPO activity was determined as described previously (Ryan et al. 1982). Accordingly, 15 μL of the leaf extract was added to 500 μL of 10 mM caffeic acid (Sigma) in potassium phosphate buffer (0.1 M, pH 8). Absorbance of the mixture was measured at 470 nm (ΔOD470) for 60 s. PPO activities were reported as ΔOD470/mg fresh weight/min.
Peroxidase activity
Peroxidase (POD) was extracted using freshly ground foliage in liquid nitrogen and homogenized in grinding buffer (describe in PPO extraction). The activity of POD from the leaf extracted was measured through reaction with the substrate of POD. The substrate was prepared by combining 62 μL of guaiacol (Sigma) with 2 μL of hydrogen peroxide (RdH) in 9.36 mL of potassium phosphate buffer (0.1 M, pH 8). Fifteen microliter of the leaf extract was mixed with 500 mL of the substrate, and absorbance was measured at 470 nm (ΔOD470) after 30 s. POD activity was reported as ΔOD470/mg fresh weight/min.
Total phenolic content
Total phenolic content was extracted and quantified using the Folin–Ciocalteu method, as described previously (Makkar et al. 1997; Siddhuraju and Becker 2003), with some modifications. Briefly, 2 mg of powdered freeze-dried foliage was extracted twice with 80% methanol in 1.7-mL aliquots for 30 min by using an ultrasonic cleaner (DC200H, Taiwan). For analyzing total phenolic compounds, a 100-µL sample extract was prepared for reaction with 750 µL of 10% Folin–Ciocalteu phenol reagent (Sigma). After 5 min, 750 µL of sodium carbonate solution (Merck) was added, and tubes were placed in dark for 90 min at room temperature; absorbance was recorded at 725 nm.
The standard curve (y = 0.0058x − 0.0075 with R2 = 0.9914) was constructed using gallic acid concentrations (Sigma), and the amount of total phenolic compounds was calculated as gallic acid equivalents from the calibration curve.
Statistical analysis
Data on insect RGR and chemical compounds (nitrogen; carbohydrate; protein; activity of TI, PPO, and POD; and total phenolic compounds) in the plant are presented as means ± standard error of the mean (SE). The effects of elevated temperature and larval attack on larval RGR and foliage chemistry were examined using a two-way analysis of variance (ANOVA) with “temperature” and “induction” and their interaction as factors, by using SPSS for Windows (IBM SPSS ver. 2.0). Tukey’s HSD (honestly significant difference) test at α = 0.05 was used to assess differences between treatments.
Student’s t test (paired-sample t test; α = 0.05; IBM SPSS ver. 2.0) was performed to analyze differences between constitutive resistance (intact foliage) and induced resistance (induced foliage) in terms of plant chemical profile and insect performance parameters.
Graphs with bar represent the means ± SE for insect performance (RGR) and plant chemical profiles (content of nitrogen, carbohydrate, and protein; activity of TI, PPO, and POD; and total phenolic compounds) were generated using SigmaPlot (ver. 12; Systat Software Inc. 2011).
Results
Insect performance
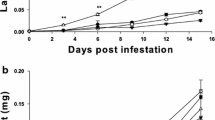
On average, higher temperature decreased larval RGR by 28.56% (F(1,76) = 33.065, p < 0.001). Plant’s induced response to herbivorous insects caused a reduction in the performance of S. litura larvae (RGR) by 18.13% (F(1,76) = 12.335, p < 0.001). No interactions were observed between temperature and induction factor on larval growth rate (F(1,76) = 1.416, p = 0.238) (Fig. 1).
Relative growth rate (RGR) of second instars larvae of S. litura fed on intact and induced foliage of R. dubia grown at ambient and high temperatures. Each bar represents the mean RGR (mean ± standard error of mean [SE]) determined independently from larvae fed on either intact or herbivore-induced leaves from 20 plants. Asterisk indicates p (*p < 0.05; **p < 0.01 and ***p < 0.001) in paired-sample t test
For temperature-induced effect, the reduction of larval RGRs by 31.17% (T[19] = 6.482, p < 0.001) and 25.96% (T[19] = 2.572, p = 0.0187) was found on insect that fed on foliage grown at high temperatures in comparison to those of larvae that fed on foliage grown at ambient temperature in context of constitutive (intact) and herbivorous-induced condition, respectively.
For herbivorous-induced responses, the reduction of larval RGRs by 21.12% (T[19] = 2.73, p = 0.0133) and 15.15% (T[19] = 2.238, p = 0.0374) were recorded on insect fed in 48 h post-induced foliage of R. dubia grown at ambient and high temperatures, respectively.
Nutrition content
Nitrogen content
On average, higher temperature decreased the nitrogen content of foliage by 16.54% (F(1,76) = 29.17, p < 0.001). Herbivore induction negatively affected nitrogen content levels, but the effect was marginally significant at p = 0.063 after 48 h of damage. No interactions were observed between temperature and induction factor on foliage’s nitrogen (F(1,76) = 0.148, p = 0.701) (Fig. 2a).
Nitrogen content (a) protein content (b) on intact and induced foliage of R. dubia growth at ambient and high temperatures. Each bar represents the mean of a primary compound (mean ± SE) determined dependently on either intact or induced leaves from 20 plants (n = 20). Asterisk indicates p (*p < 0.05; **p < 0.01 and ***p < 0.001) in paired-sample t test between intact and induced foliage
With intact plants, the nitrogen content of foliage grown at high temperatures was 17.15% lower (T[19] = 4.326, p < 0.001) than that of foliage grown at ambient temperatures. In herbivorous-induced plants, the content of nitrogen foliage was 15.93% lower (T[19] = 4.554, p < 0.001) in plants grown at high temperatures than that of the foliage of plants grown at ambient temperatures (Fig. 2a).
Protein content
Higher temperature decreased the protein content of foliage by 21.12% (F(1,92) = 22.498, p < 0.001). Herbivore induction caused a reduction of foliage’s protein content by 19.66% (F(1,92) = 19.121, p < 0.001) after 48 h of damage. No interactions were observed between temperature and induction factor on foliage’s protein (F(1,92) = 0.06, p = 0.937). Hence these two factors affected protein content independently (Fig. 2b).
For temperature-induced effect, the reduction of protein content by 19.39% (T[23] = 3.928, p < 0.001) and 22.84% (T[23] = 3.355, p = 0.0027) found on foliage grown at high temperatures in comparison to those at ambient temperature in the context of constitutive (intact) and herbivorous-induced condition, respectively.
In response to herbivorous attack, the decrease of protein content by 17.9% (T[23] = 3.292, p = 0.0032) and 21.42% (T[23] = 3.393, p = 0.0025) was found in 48 h post-induced foliage of plants grown at ambient and high temperatures, respectively.
Soluble carbohydrate (sugar)
On average, higher temperature increased the soluble carbohydrate of foliage by 12.95% (F(1,76) = 7.749, p = 0.0057). Herbivore induction caused a reduction of sugar content by 45.33% (F(1,76) = 192.472, p < 0.001) after 48 h of damage. No interactions were observed between temperature and induction factor on foliage’s soluble carbohydrate (F(1,76) = 0.18, p = 0.6715) (Fig. 3a).
Soluble carbohydrate (a) and non-soluble carbohydrate (b) on intact and induced foliage of R. dubia grown at ambient and high temperatures. Each bar represents the mean of a primary compound (mean ± SE) determined dependently on either intact or herbivore leaves from 20 plants (n = 20). Asterisk indicates p (*p < 0.05; **p < 0.01 and ***p < 0.001) in paired-sample t test between intact and induced foliage
For temperature-induced effect, the increase of soluble carbohydrate by 10.97% (T[19] = 2.682, p < 0.001) and 14.94% (T[19] = 2.329, p = 0.0226) was found on foliage grown at high temperatures in comparison to those at ambient temperature in the context of constitutive (intact) and 48 h-induced condition, respectively.
In response to herbivorous attack, the decrease of soluble carbohydrate by 45.99% (T[19] = 12.516, p < 0.001) and 44.67% (T[19] = 10.242, p < 0.001) was found in 48 h post-induced foliage of plants grown at ambient and high temperatures, respectively.
Non-soluble carbohydrate
On average, higher temperature decreased the non-soluble carbohydrate of foliage by 20.46% (F(1,76) = 15.249, p < 0.001). Herbivore induction does not affect the non-soluble carbohydrate content of R. dubia foliage (F(1,76) = 3.751, p = 0.056) after 48 h of damage. In addition, no interactive effect was observed between temperature and induction on the foliar non-soluble carbohydrate content (F(1,76) = 0.034, p = 0.854) (Fig. 3b).
In temperatures-exposed treatments, the reduction of non-soluble carbohydrate by 20.29% (T[19] = 3.13, p = 0.006) and 20.63% (T[19] = 2.551, p = 0.02) found in foliage of plant grown at high temperature in comparison to those at ambient temperature in the context of constitutive (intact) and herbivorous-induced condition, respectively.
Secondary metabolism
Trypsin inhibitor (TI)
On average, higher temperature decreased the foliage’s TI activity by 18.31% (F(1,76) = 42.698, p < 0.001). Herbivore induction caused an increase of TI activity by 12.23% (F(1,76) = 14.258, p < 0.001) after 48 h of damage. No interactions were observed between temperature and induction factor on foliage’s TI activity (F(1,76) = 1.762, p = 0.1929) (Fig. 4a).
Trypsin inhibitor (TI) (a) and total phenolic compounds (b) on intact and induced foliage of R. dubia grown at ambient and high temperatures. Each bar represents the mean of a primary compound (mean ± SE) determined dependently on either intact or induced leaves from 20 plants (n = 20). Asterisk indicates p (*p < 0.05; **p < 0.01 and ***p < 0.001) in paired-sample t test between intact and induced foliage
For temperature-induced effect, the decrease of TI activity by 15.92% (T[19] = 3.914, p < 0.001) and 20.71% (T[19] = 4.416, p < 0.001) was found on foliage grown at high temperatures in comparison to those at ambient temperature in the context of constitutive (intact) and 48 h-induced condition, respectively.
In response to herbivorous attack, the increase of TI activity by 15.52% (T[19] = 3.935, p < 0.001) and 8.93% (T[19] = 2.168, p = 0.0431) was found in 48 h post-induced foliage of plants grown at ambient and high temperatures, respectively.
Phenolic compounds
On average, higher temperature increased the phenolic compounds of R. dubia foliage by 28.7% (F(1,76) = 20.075, p = 0.001). Herbivore damage causes the plant’s response by increasing of phenolic by 23.58% (F(1,76) = 14.325, p < 0.001) after 48 h of induction. In addition, no interactive effect was observed between the temperature and induction on the foliar phenolic content (F(1,76) = 0.132, p = 0.291) (Fig. 4b).
For temperature-induced effect, the increase of phenolic compounds by 24.33% (T[19] = 2.379, p = 0.028) and 33.07% (T[19] = 4.163, p < 0.001) was found on foliage grown at high temperatures in comparison to those at ambient temperature in context of constitutive (intact) and 48 h-induced condition, respectively.
In response to herbivorous attack, the increase of phenolic compounds by 19.38% (T[19] = 2.245, p = 0.0369) and 27.77% (T[19] = 3.336, p = 0.00347) was found in 48 h post-induced foliage of plants grown at ambient and high temperatures, respectively.
Polyphenol oxidase (PPO)
On average, higher temperature decreased the PPO activity of R. dubia foliage by 34.05% (F(1,76) = 52.089, p < 0.001). Herbivore damage causes the plant’s response by increasing of PPO activity by 21.3% (F(1,76) = 12.186, p < 0.001) after 48 h of induction. In addition, no interactive effect was observed between temperature and induction on the foliar PPO activity (F(1,76) = 2.303, p = 0.133) (Fig. 5a).
Polyphenol oxidase (a) and peroxidase (b) activity in intact and induced foliage of R. dubia grown at ambient and high temperatures. Each bar represents the mean of a primary compound (mean ± SE) determined dependently on either intact or induced leaves from 20 plants (n = 20). Asterisk indicates p (*p < 0.05; **p < 0.01 and ***p < 0.001) in paired-sample t test between intact and induced foliage
For temperature-induced effect, the decrease of PPO activity by 30.88% (T[19] = 3.291, p = 0.00384) and 37.22% (T[19] = 6.697, p < 0.001) was found on foliage grown at high temperatures in comparison to those at ambient temperature in the context of constitutive (intact) and 48 h-induced condition, respectively.
In response to herbivorous attack, the increase of PPO activity by 27.13% (T[19] = 3.097, p = 0.0059) and 15.47% (T[19] = 2.111, p = 0.0483) was found in 48 h post-induced foliage of plants grown at ambient and high temperatures, respectively.
Peroxidase (POD)
On average, higher temperature decreased the POD activity of R. dubia foliage by 20.35% (F(1,76) = 26.559, p < 0.001). Herbivore damage causes the plant’s response by increasing the POD activity by 22.12% (F(1,76) = 20.534, p < 0.001) after 48 h of induction. In addition, no interactive effect was observed between the temperature and induction on the foliar POD activity (F(1,76) = 1.308, p = 0.255) (Fig. 5b).
For temperature-induced effect, the decrease of POD activity by 18.08% (T[19] = 2.973, p = 0.0063) and 22.61% (T[19] = 4.524, p < 0.001) was found on foliage grown at high temperatures in comparison to those at ambient temperature in the context of constitutive (intact) and 48 h-induced condition, respectively.
In response to herbivorous attack, the increase of POD activity by 25.59% (T[19] = 4.152, p < 0.001) and 18.65% (T[19] = 2.327, p = 0.0269) was found in 48 h post-induced foliage of plants grown at ambient and high temperatures, respectively.
Discussion
At high temperatures, plants grow faster and increase in vegetative size (Yang et al. 2018); however, in our study, the R. dubia exhibited reduction in both nutritional value and chemical-based defense against herbivorous insects. In terms of fitness, at high temperature, plants exhibited lower constitutive resistance and weaker induced resistance against generalist insects than at ambient temperatures, which consequently reduce the defense capability of plants. From this evidence, we highlighted the role of temperature in providing favorable condition for growth processes; however, we also reported negative effects on plant fitness, particularly in chemical-based defense, which are likely to cause a considerable burden on agricultural production, particularly on plant protection activities.
The content of nutrients, such as foliage nitrogen, protein, and non-soluble carbohydrate (starch), was reduced in response to high temperature. Lower nutritional value of foliage consequently affects the performance of the generalist insect S. litura. Our data showed that the RGR of an early larval stage (second instar) was lower by 31.17% in larvae-fed intact foliage grown at high temperatures than in those fed foliage grown at ambient temperatures. According to our knowledge, in early larval stages, when detoxification enzymes are not fully activated (Reda et al. 2013), the nutritional content of foliage plays a vital role in insect growth. The decrease in larval RGR likely resulted from intake of low-quality food.
Defensive compounds, namely, TI, PPO, and POD, exhibited a reduction in activity (exception for total phenolic compounds) in intact foliage of R. dubia in response to high temperatures. The lower content/activity of TI, PPO, and POD in intact foliage was referred as lower plant’s basal protection and consequently, plants with lower constitutive defense are considered more vulnerable to attackers at high temperature of global warming context.
In terms of induced defense, our data showed a reduction in induced response in R. dubia grown at high temperature. Lower increase in foliar activities of TI, PPO, and POD at 48 h after induction at high temperatures is considered a weaker response of induced defense of a plant toward an insect. In accordance with comparative bioassay data of insects fed on intact and induced foliage, the induced response in plants suppressed larvae RGR by 21.12% at ambient temperatures, whereas at high temperatures, larvae RGR was reduced by only 15.15%. Thus, we provided evidence of weak response at high temperature; furthermore, similar to the results of a study on the effect of high CO2 on induced response (Bidart‐Bouzat et al. 2005), we provided an answer to the question of whether the chemical-based defense of plants is affected by global warming.
Common cutworm (S. litura), a generalist herbivorous insect, is gradually emerging as an economical insect pest in tropical regions. This polyphagous defoliate species is known to possess a detoxification enzyme (fully be activated in later instars), which enables them to overcome a plant’s chemical-based defense system (Lindroth 1991). Our data also showed lower performance (in terms of RGR) of second instar larvae fed on plants grown at high temperatures (because of low nutritional content), but the same trend was not observed in later phases of larvae development. Data from a similar study (Zhang et al. 2017) on fourth instar larvae reported a higher RGR of S. litura at high temperature. In another of our studies (unpublished), we reported an increase in RGR and food consumption of fourth instar larvae of S. litura at high temperature. Most leaf-chewing insects exhibit a compensatory increase in food consumption (Lee et al. 2002) if nutritional value is low.
In conclusion, plants with low nutritional contents and defensive compounds at high temperatures are projected more vulnerable to defoliating insects; this causes concerns regarding plant protection. Additional studies on the combined effects of high temperature and high CO2 on insect–plant interactions are needed to clarify the question of whether global warming is affecting the future of agricultural production.
References
Ashraf M, Harris PJC (2013) Photosynthesis under stressful environments: an overview. Photosynthetica 51:163–190. https://doi.org/10.1007/s11099-013-0021-6
Bale J, Masters GJ, Hodkins ID, Awmack C, Bezemer TM, Brown VK, Butterfield J, Buse A, Coulson JC, Farrar J, Good JEG, Harrington R, Hartley S, Jones TH, Lindroth RL, Press MC, Symrnioudis I, Watt AD, Whittaker JB (2002) Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Glob Change Biol 8:1–16. https://doi.org/10.1046/j.1365-2486.2002.00451.x
Bassirirad H (2000) Kinetics of nutrient uptake by roots: responses to global change. New Phytol 147:155–169. https://doi.org/10.1046/j.1469-8137.2000.00682.x
Battisti A (2008) Forests and climate change—lessons from insects. iForest Biogeosci For 1(1):1–5. https://doi.org/10.3832/ifor0210-0010001
Bebber DP, Holmes T, Gurr SJ (2014) The global spread of crop pests and pathogens. Glob Ecol Biogeogr 23:1398–1407. https://doi.org/10.1111/geb.12214
Berry J, Bjorkman O (1980) Photosynthetic response and adaptation to temperature in higher-plants. Annu Rev Plant Physiol 31:491–543. https://doi.org/10.1146/annurev.pp.31.060180.002423
Bidart-Bouzat MG, Imeh-Nathaniel A (2008) Global change effects on plant chemical defenses against insect herbivores. J Integr Plant Biol 50:1339–1354. https://doi.org/10.1111/j.1744-7909.2008.00751.x
Bidart-Bouzat MG, Mithen R, Berenbaum MR (2005) Elevated CO2 influences herbivory-induced defense responses of Arabidopsis thaliana. Oecologia 145:415–424. https://doi.org/10.1007/s00442-005-0158-5
Bradford MM (1976) A rapid and sensitive for the quantitation of microgram quantitites of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Brody AK, Karban R (1992) Lack of a tradeoff between constitutive and induced defenses among varieties of cotton. Oikos 65:301–306. https://doi.org/10.2307/3545022
Clissold FJ, Coggan N, Simpson SJ (2013) Insect herbivores can choose microclimates to achieve nutritional homeostasis. J Exp Biol 216:2089–2096. https://doi.org/10.1242/jeb.078782
Coggan N, Clissold FJ, Simpson JS (2011) Locusts use dynamic thermoregulatory behavior to optimize nutritional outcomes. Proc R Soc B 278:2745–2752. https://doi.org/10.1098/rspb.2010.2675
Coughlan MP, Moloney AP (1988) Isolation of 1,4-b-d-glucan 4-glucanohydrolases of Talaromyces emersonii. In: Wood WA, Kellogg ST (eds) Methods in enzymology, vol 160. Academic Press, London, p 365
Dhaliwal GS, Jindal V, Dhawan AK (2010) Insect pest problems and crop losses: changing trends. Indian J Ecol 37:1–7
Dong SF, Scagel CF, Cheng LL, Fuchigami LH, Rygiewicz PT (2001) Soil temperature and plant growth stage influence nitrogen uptake and amino acid concentration of apple during early spring growth. Tree Physiol 21:541–547. https://doi.org/10.1093/treephys/21.8.541
Evan HD, Paul DN, Jorge AZ, May RB (2012) Climate change: resetting plant–insect interactions. Plant Physiol 160:1677–1685. https://doi.org/10.1104/pp.112.204750
Flato G, Marotzke J, Abiodun B, Braconnot P, Chou SC, Collins WJ, Eyring V (2013) Evaluation of climate models. In: Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Clim Change 5:741–866
Gessler A, Schneider S, Von Sengbusch D, Weber P, Hanemann U, Huber C, Rothe A, Kreutzer K, Rennenberg H (1998) Field and laboratory experiments on net uptake of nitrate and ammonium by the roots of spruce (Picea abies) and beech (Fagus sylvatica) trees. New Phytol 138:275–285
Gupta GP, Rani S, Birah A, Raghuraman M (2005) Improved artificial diet for mass rearing of the tobacco caterpillar, Spodoptera litura (Lepidoptera: Noctuidae). Int J Trop Insect Sci 25:55–58. https://doi.org/10.1079/IJT200551
Hatfield JL, Prueger JH (2015) Temperature extremes: effect on plant growth and development. Weather Clim Extrem 10:4–10. https://doi.org/10.1016/j.wace.2015.08.001
Hermilo S-S, Alina M-C (2016) Chemical plant defense against herbivores (chapter 1). In: Shields VDC (ed) Herbivores. IntechOpen. https://doi.org/10.5772/67346
Herms DA, Mattson WJ (1992) The dilemma of plants: to grow or to defend. Quart Rev Biol 67:283–335
Himanen SJ, Nissinen A, Auriola S, Poppy GM, Stewart CN, Holopainen JK, Nerg AM (2008) Constitutive and herbivore-inducible glucosinolate concentrations in oilseed rape (Brassica napus) leaves are not affected by Bt Cry1Ac insertion but change under elevated atmospheric CO2 and O3. Planta 227:427–437. https://doi.org/10.1007/s00425-007-0629-5
Huang SJ, Han ZJ (2007) Mechanisms for multiple resistances in field populations of common cutworm, Spodoptera litura (Fabricius) in China. Pestic Biochem Physiol 87:14–22. https://doi.org/10.1016/j.pestbp.2006.05.002
Hungaria M, Franco AA (1993) Effects of high temperature on nodulation on nitrogen fixation by Phaseolus vulgaris L. Plant Soil 149:95–102. https://doi.org/10.1007/BF00010766
IPCC (2007) Climate change 2007: the physical science basis. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, et al. (eds) Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
IPCC (2013) Working Group I Contribution to the IPCC Fifth Assessment Report. In: Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, et al. (eds) Climate change 2013: the physical sciences basis summary for policymakers. Cambridge University Press, Cambridge
Jadhav DR, Mallikarjuna N, Rathore A, Pokle D (2012) Effect of some flavonoids on survival and development of Helicoverpa armigera (Hübner) and Spodoptera litura (Fab) (Lepidoptera: Noctuidae). Asian J Agric Environ Sci 4:298–307
Jamieson MA, Trowbridge AM, Raffa KF, Lindroth RL (2012) Consequences of climate warming and altered precipitation patterns for plant–insect and multitrophic interactions. Plant Physiol 160(4):1719–1727. https://doi.org/10.1104/pp.112.206524
Kingsolver JG, Woods HA (1998) Interactions of temperature and dietary protein concentration in growth and feeding of Manduca sexta caterpillars. Physiol Entomol 23:354–359. https://doi.org/10.1046/j.1365-3032.1998.234105.x
Kuokkanen K, Julkunen-Tiitto R, Keinanen M, Niemela P, Tahvanainen J (2001) The effect of elevated CO2 and temperature on the secondary chemistry of Betula pendula seedlings. Trees (Berlin) 15:378–384. https://doi.org/10.1007/s004680100108
Lafta AM, Lorenzen JH (1995) Effect of high temperature on plant growth and carbohydrate metabolism in potato. Plant physiol 109(2):637–643. https://doi.org/10.1104/pp.109.2.637
Lang CA (1958) Simple micro determination of Kjeldahl nitrogen in biological materials. Anal Chem 30:1692–1694. https://doi.org/10.1021/ac60142a038
Lee TM, Lin YH (1995) Trypsin inhibitor and trypsin-like protease activity in air or submergence-grown rice (Oryza sativa L.) coleoptiles. Plant Sci 106:43–54. https://doi.org/10.1016/0168-9452(95)04058-3
Lee KP, Behmer ST, Simpson SJ, Raubenheimer D (2002) A geometric analysis of nutrient regulation in the generalist caterpillar Spodoptera littoralis (Boisduval). J Insect Physiol 48:655–665. https://doi.org/10.1016/S0022-1910(02)00088-4
Li Q, Eigenbrode SD, Stringham GR, Thiagarajah MR (2000) Feeding and growth of Plutella xylostella and Spodoptera eridania on Brassica juncea with varying glucosinolate concentrations and myrosinase activities. J Chem Ecol 26:2401–2419. https://doi.org/10.1023/A:1005535129399
Lindroth RL (1991) Differential toxicity of plant allelochemicals in insects: roles of enzymatic detoxification systems. In: Bernays EA (ed) Insect–plant interactions. CRC Press, Boca Raton, pp 1–33
Luo YQ (2007) Terrestrial carbon-cycling feedback to climate warming. Annu Rev Ecol Evol Syst 38:683–712. https://doi.org/10.1146/annurev.ecolsys.38.091206.095808
Makkar HPS, Becker K, Abel H, Pawelzik E (1997) Nutrient contents, rumen protein degradability and antinutritional factors in some colour- and white-flowering cultivars of Vicia faba beans. J Sci Food Agric 75:511–520. https://doi.org/10.1002/(SICI)1097-0010(199712)75:4%3c511:AID-JSFA907%3e3.0.CO;2-M
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Moreira X, Abdala-Roberts L, Gols R (2018) Plant domestication decreases both constitutive and induced chemical defences by direct selection against defensive traits. Sci Rep 8:12678. https://doi.org/10.1038/s41598-018-31041-0
Pan J, Lin S, Woodbury NW (2012) Bacteriochlorophyll excited-state quenching pathways in bacterial reaction centers with the primary donor oxidized. J Phys Chem B 116:2014–2022. https://doi.org/10.1021/jp212441b
Parkinson JA, Allen SE (1975) A wet oxidation process suitable for the determination of nitrogen and mineral nutrients in biological material. Commun Soil Sci Plant Anal 6:1–11. https://doi.org/10.1080/00103627509366539
Pereira FMV, Rosa E, Fahey JW, Stephenson KK, Carvalho R, Aires A (2002) Influence of temperature and ontogeny on the levels of glucosinolates in broccoli (Brassica oleracea var. italica) sprouts and their effect on the induction of mammalian phase 2 enzymes. J Agric Food Chem 50:6239–6244. https://doi.org/10.1021/jf020309x
Price PW (1997) Insect ecology. Wiley, New York
Pureswaran DS, Roques A, Battisti A (2018) Forest insects and climate change. Curr For Rep 4:35–50. https://doi.org/10.1007/s40725-018-0075-6
Reda FA, Bakr RFA, Elaziz MFA, Awad MH, El-Halim HME (2013) The activity of some detoxification enzymes in Spodoptera littoralis (Boisd.) larvae (Lepidoptera–Noctuidae) treated with two different insect growth regulators. Egypt Acad J Biol Sci 5:19–27. https://doi.org/10.21608/eajbsc.2013.16092
Rennenberg H, Loreto F, Polle A, Brilli F, Fares S, Beniwal RS, Gessler A (2006) Physiological responses of forest trees to heat and drought. Plant Biol 8:556–571. https://doi.org/10.1055/s-2006-924084
Rodriguez-Saona C, Chalmers JA, Raj S, Thaler JS (2005) Induced plant responses to multiple damagers: differential effects on an herbivore and its parasitoid. Oecologia 143:566–577. https://doi.org/10.1007/s00442-005-0006-7
Rose R, Rose CL, Omi SK, Forry KR, Durall DM, Bigg WL (1991) Starch determination by perchloric acid vs. enzymes: evaluating the accuracy and precision of six colorimetric methods. J Agric Food Chem 39:2–11. https://doi.org/10.1021/jf00001a001
Rufty TW, Raper CD, Jackson WA (1981) Nitrogen assimilation, root growth and whole plant responses of soybean to root temperature and to carbon dioxide and light in the aerial environment. New Phytol 88:607–619. https://doi.org/10.1111/j.1469-8137.1981.tb01736.x
Ryan CA, Gregory P, Tingey W (1982) Phenolic oxidase activities in glandular trichomes of Solanum berthaultii. Phytochemistry 21:1885–1887. https://doi.org/10.1016/0031-9422(82)83008-2
Sadasivam S, Thayumanavan B (2003) Molecular host plant resistance to pests. Marcel Dekker, New York, p 479
Siddhuraju P, Becker K (2003) Studies on antioxidant activities of mucuna seed (Mucuna pruriens var. utilis) extracts and certain non-protein amino/imino acids through in vitro models. J Sci Food Agric 83:1517–1524. https://doi.org/10.1002/jsfa.1587
Smith CM (2005) Plant resistance to arthropods: molecular and conventional approaches. Springer, Dordrecht, p 423
Smriti S, Rubaljot K, Singh SS, Ramesh A (2018) Impact of elevated temperature and carbon dioxide on insect performance indices of Spodoptera litura Fabricius. J Entomol Res 42:315–324. https://doi.org/10.5958/0974-4576.2018.00053.1
Stout MJ, Workman KV, Bostock RM, Duffey S (1997) Specificity of induced resistance in the tomato, Lycopersicon esculentum. Oecologia 113:74–81. https://doi.org/10.1007/s004420050355
Underwood N, Anderson K, Inouye BD (2005) Induced vs. constitutive resistance and the spatial distribution of insect herbivores among plants. Ecology 86:594–602. https://doi.org/10.1890/03-0290
Veteli TO, Kuokkanen K, Julkunen-Tiitto R, Roininen H, Tahvanainen J (2002) Effects of elevated CO2 and temperature on plant growth and herbivore defensive chemistry. Glob Change Biol 8:1240–1252. https://doi.org/10.1046/j.1365-2486.2002.00553.x
Vu JCV, Gesh RW, Pennanen AH, Allen LH Jr, Boote KJ, Bowes G (2001) Soybean photosynthesis, Rubisco, and carbohydrate enzyme function at supraoptimal temperatures in elevated CO2. J Plant Physiol 158:295–307. https://doi.org/10.1078/0176-1617-00290
War AR, Paulraj MG, Ahamad T, Buhroo AA, Hussain B, Ignacimuthu S, Sharma HC (2012) Mechanisms of plant defense against insect herbivores. Plant Signal Behav 7:1306–1320. https://doi.org/10.4161/psb.21663
Wood TM, Bhat KM (1988) Methods for measuring cellulase activities. In: Wood WA, Kellogg ST (eds) Methods in enzymology, vol 160. Academic Press, Inc., London, pp 87–112
Xia J, Zhao YH, Wang WK, He YH (2015) Elevated temperature altered photosynthetic products in wheat seedlings and organic compounds and biological activity in rhizopshere soil under cadmium stress. Sci Rep. https://doi.org/10.1038/srep14426
Yang Y, Joern A (1994) Influence of diet quality, developmental stage, and temperature on food residence time in the grasshopper Melanoplus differentialis. Physiol Zool 67:598–616
Yang LY, Yang SL, Li JY (2018) Effects of different growth temperatures on growth, development, and plastid pigments metabolism of tobacco (Nicotiana tabacum L.) plants. Bot Stud 59(5):1–13. https://doi.org/10.1186/s40529-018-0221-2
Zhang YF, Wan GJ, Liu B, Zhang XG, Xing GN, Chen FJ (2017) Elevated CO2 and temperature alter development and food utilization of Spodoptera litura fed on resistant soybean. J Apply Entomol 142:250–262. https://doi.org/10.1111/jen.12463
Zvereva EL, Kozlov MV (2006) Consequences of simultaneous elevation of carbon dioxide and temperature for plant–herbivore interactions: a meta-analysis. Glob Change Biol 12:27–41. https://doi.org/10.1111/j.1365-2486.2005.01086.x
Acknowledgements
The study was supported by grant from Ministry of Science and Technology (MOST), Taiwan (Grant No. 105-2313-B-005-003-MY3). We also thank Dr. Melissa Andrews from Wallace Academic Editing for editing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Heikki Hokkanen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pham, T.A., Hwang, SY. High temperatures reduce nutrients and defense compounds against generalist Spodoptera litura F. in Rorippa dubia. Arthropod-Plant Interactions 14, 333–344 (2020). https://doi.org/10.1007/s11829-020-09750-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-020-09750-z