Abstract
The Africa-derived ornamental geranium, Pelargonium × hortorum, is now widely planted in North America, leading to novel interactions with native herbivores such as the soybean looper, Chrysodeixis includens. Soybean looper eggs and larvae survive poorly on P. × hortorum. High mortality can be attributed specifically to glandular trichomes. Individual eggs treated with exudate from a single tall glandular trichome were significantly less likely to hatch than untreated eggs. Few early-instar larvae survived on either intact or excised leaves, but survival increased when trichome exudate was removed by rinsing leaves with ethanol or when the tall and short glandular trichomes were both plucked from the leaf surface. Surprisingly, final instar loopers often severed the veins of geranium leaves before feeding beyond the cuts, a behavior normally exhibited on plants with canal-borne exudates such as latex. To identify the cue that triggers vein cutting, loopers were tested with solutions of two known defensive compounds found in Pelargonium (l-quisqualic acid, 22:0 anacardic acid); both were inactive in eliciting vein cutting. However, exudate collected from the tall glandular trichomes triggered vein cutting, thus documenting for the first time the chemical stimulant for vein cutting in a plant species that lacks canal-borne exudates. Loopers severed leaf veins more frequently on plants previously fed upon indicating that the chemical trigger for vein cutting increases with damage. Vein cuts sever the major supply arteries in the leaf, thus potentially blocking inducible geranium responses to herbivory.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The intentional or inadvertent transport of organisms by humans has resulted in novel interactions between distant species and between long separated lineages. Plants in particular with their diverse agricultural and ornamental uses have been broadly distributed. In the continental USA, for example, over 2,000 species of alien plants are now established (Mooney et al. 2005). Native herbivores often colonize these alien plants. In California, for example, more than a third of the native butterfly species oviposit or feed on nonnative plants (Graves and Shapiro 2003). During initial encounters, native herbivores may be poorly adapted to the alien plants (Cox 2004). These new interactions between native and alien species have the potential to reveal hidden plant defenses and insect counter adaptations that are not always apparent with native plants and herbivores. In native systems, prolonged periods of coevolution often render important defenses ineffective against specialist herbivores (Spencer 1988; Heckel 2014; Jander 2014).
One such novel interaction occurs between the soybean looper (Chrysodeixis includens, formerly Pseudoplusia includens), an important agricultural pest, and geranium, Pelargonium × hortorum (Geraniaceae). C. includens (Noctuidae: Plusiinae) occurs exclusively in the New World from Canada to Argentina and Chile where it feeds on crops such as soybean and cotton, as well as on diverse wild plants including native Geraniaceae (Lafontaine and Poole 1991; Wagner et al. 2011). The genus Pelargonium, commonly known as geraniums, are found primarily in Southern Africa with a few species in Madagascar and Australia (Miller 2002). European explorers discovered Pelargonium within the past few 100 years and created cultivated varieties often by hybridizing multiple species (Miller 2002). In the hybrid P. × hortorum, most of the genetic contribution has been attributed to South African natives, Pelargonium inquinans and P. zonale (Grazzini et al. 1995b). With its showy flowers, P. × hortorum is now widely planted as an ornamental in North America (http://www.floridata.com/ref/p/pela_xho.cfm), where it undoubtedly interacts with thousands of new herbivore species, including soybean loopers. Their novel interaction provides an opportunity for investigating geranium defenses and looper counter adaptations in a system where the organisms have had little time to co-evolve.
Pelargonium × hortorum leaves are well protected. Both glandular and nonglandular trichomes occur in abundance on the leaf surfaces (Walters et al. 1989b). The plant produces a cocktail of defensive compounds (Grazzini et al. 1995b; Houghton and Lis-Balchin 2002; Farag et al. 2012; Liu et al. 2013), which include the neurotoxic amino acid, l-quisqualic acid, and the alkyl phenolic acids known as anacardic acids. l-Quisqualic acid was recently isolated from the flower petals of P. × hortorum (Ranger et al. 2011). The compound functions as a potent agonist of neural receptors for l-glutamic acid, an amino acid that acts at the neuromuscular junction of insects. Japanese beetles feeding on P. × hortorum flowers often become paralyzed after eating just one or two petals (Held and Potter 2003; Ranger et al. 2011). The beetles also become paralyzed from eating P. × hortorum leaves, although less frequently (Held and Potter 2003). Anacardic acids are produced by the tall glandular trichomes in P. × hortorum leaves; accumulation of trichome exudate occurs externally on the terminal gland cell, where it is readily encountered by herbivores (Gerhold et al. 1984). Anacardic acid occurs in both saturated and unsaturated forms. Mite resistance in P. × hortorum has been attributed specifically to unsaturated anacardic acids, which are sufficiently fluid to physically trap small arthropods (Stark 1975; Walters et al. 1990; Schultz et al. 2006). Topical application of exudate from the tall glandular trichomes to mites and small insects documented that the exudate is not only sticky, but also toxic (Stark 1975) presumably due to anacardic acids.
Despite these formidable defenses, soybean loopers can feed on P. × hortorum (hereafter geranium). We found final instar soybean loopers consuming leaves of mature geranium plants potted in a greenhouse in central Arkansas, USA (Dussourd unpub. obs). Curiously, the loopers severed the central leaf veins, often in a ring near the petiole (Fig. 1). A few loopers also produced a continuous line of bites that connected the vein cuts to form a trench. Similar vein-cutting and trenching behaviors have been previously observed in diverse caterpillars, beetles, and katydids feeding on plants with canal-borne exudates, such as latex (Dussourd 2009 and references cited). The cuts sever the canals, thus isolating a portion of the leaf from the pressurized canal system. Vein cutting and trenching reduce exudation distal to the cuts where the insects invariably feed. Unlike milkweeds and other plants with canal-cutting herbivores, geranium lacks secretory canals (Metcalfe and Chalk 1983). Furthermore, no visible exudate appears when the leaves are severed. Vein cutting and trenching are thus unexpected, although insects employing these behaviors have been previously reported on a few plant groups without secretory canals (Dussourd 1993, 2009). Heliconius caterpillars, for example, cut elaborate trenches that encircle a portion of Passiflora leaves where the larva feeds (Alexander 1961); the function of Heliconius trenching is not known. Given the extensive literature on geranium defenses (Simmonds 2002; Williams and Harborne 2002; Ranger et al. 2011; Liu et al. 2013), the geranium–looper interaction provides a model system for identifying why insects sever veins of plants lacking secretory canals. In this paper, we test if geranium defenses protect against soybean loopers and identify the cue that triggers vein cutting.
Methods
Study organisms
A laboratory colony of soybean loopers (C. includens) was established by collecting adult moths in late September and early October 2011–2013 from central Arkansas, USA. The colony was maintained primarily on potted plantain (Plantago lanceolata, Plantaginaceae) grown in the greenhouse from field-collected seeds. Cuttings of geranium, P. × hortorum cv. Nittany Lion Red, were obtained from the Ornamental Plant Germplasm Center (Ohio State University, Columbus, OH). This diploid cultivar produces l-quisqualic acid (Ranger et al. 2011) and is known to be resistant to mites (Schultz et al. 2000). The cuttings were grown in greenhouses in Pro-mix general-purpose growing media; mature plants in 25- and 30-cm-diameter pots were used in all experiments. Details on experimental methods, including on rearing loopers and growing geranium, can be found in Hurley (2014).
Soybean looper egg hatch
To determine whether soybean looper eggs can develop and hatch on P. × hortorum, 15 females that had previously laid viable eggs were released into a 60 cm × 60 cm × 60 cm cage containing a mature geranium that had not been damaged by loopers within the last 90 days. An additional 15 fertile females were allowed to oviposit in an identical cage containing a mature plantain that had never experienced caterpillar damage. After 48 h, the moths were removed. Eggs were allowed to hatch for 7 days at 27 °C; then, the first 100 hatched or unhatched eggs encountered on mature leaves of each plant were counted. Soybean looper eggs ordinarily hatch within 5 days at this temperature (Hurley pers. obs.). Geranium and plantain were compared in the number of hatched and unhatched eggs using a 2 × 2 Chi-square test of independence; JMP version 11 was used in all statistical calculations with α = 0.05 except where multiple comparisons required Bonferroni adjustment.
To test if exudate from glandular trichomes kills soybean looper eggs, 50 eggs on wax paper were treated with exudate while an additional 50 were left untreated. Using a dissecting microscope, each of the treated eggs was rubbed on the side with the tip of a single tall glandular trichome from the edge of a mature P. × hortorum leaf. The orange exudate coats the side of the egg, but causes no visible damage to the shell (Online Resource 1). Eggs were allowed 7 days to hatch at 27 °C. The number of hatched and unhatched eggs in the treated and control groups were compared using a Chi-square test.
Caterpillar survival on intact and excised geranium
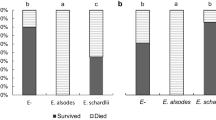
To determine whether soybean loopers can develop on leaves and flowers of intact-potted geranium plants, newly hatched caterpillars were randomly assigned to the following six groups with 10 plants/group and one caterpillar per plant: mature plantain leaves, mature leaves of geranium plants with flowers or flower buds, mature leaves of geraniums before flowers appeared, young leaves of geraniums with flowers or buds, young leaves of geraniums lacking flowers, and geranium flowers. This experiment tests if leaf age and plant reproductive status affect caterpillar survival. Caterpillars on leaves were enclosed within clip cages (3 cm diameter) for the first three instars and then within mesh sleeves until pupation. Larvae on flowers were contained within fine mesh sleeves from the first instar. Caterpillars were moved to new leaves or flowers on the same plant after they had consumed more than half of the surface available. Leaves were examined daily for vein cuts and to record caterpillar survival to pupation. Caterpillar survival for each of the five geranium treatments was compared with the plantain control using Fisher's exact tests with a Bonferroni adjusted α = 0.01.
Excising leaves from plants with canal-borne exudates effectively deactivates the canals and enables caterpillars to utilize plant species that otherwise would be unsuitable. Soybean loopers, for example, cut trenches less frequently and grow more rapidly on excised leaves of prickly lettuce, Lactuca serriola, which has latex canals (Dussourd and Denno 1994). To test if excising leaves of geranium likewise decreases vein cutting/trenching and improves survival, 750 newly hatched soybean loopers were divided into three groups: excised mature plantain leaves, excised mature geranium leaves, and excised geranium flowers. Each group of 250 was then subdivided into 10 containers with 25 caterpillars per container. Larvae received fresh leaves or flowers every 1–2 days. Data on survival to pupation did not meet the assumptions of parametric tests, so survival was compared across the three groups using a Kruskal–Wallis test and between paired treatments using Steel–Dwass tests.
In the above experiments with geranium leaves and flowers, soybean loopers only survived on the intact and excised mature leaves. Mature leaves were therefore used in all subsequent experiments.
Caterpillar survival on ethanol-rinsed leaves
To test if chemicals on the geranium leaf surface cause early-instar mortality, P. × hortorum leaf surfaces were rinsed with ethanol, which removed the orange exudate from the tall glandular trichomes without causing noticeable damage to the leaf surface (Online Resource 1). The orange exudate began to reappear on the tips of some trichomes within 4 h of leaf rinsing; almost all trichomes were colored orange 24 h after being rinsed. Orange exudate reappeared even on excised leaves. The orange pigment is not one of the anacardic acids secreted by the tall glandular trichomes (Gerhold et al. 1984); nevertheless, it provides a visual marker for exudate replenishment. Harman et al. (1996) found that P. × hortorum leaves required 14 days for full resistance to mites to reappear after rinsing leaves with water. Thus, in our experiment, the trichome exudate was probably only partially replenished after 24 h.
Newly hatched soybean loopers were tested on excised leaves in three treatments: leaves rinsed with ethanol and replaced every 12 h before the trichomes replenished, leaves rinsed with ethanol and replaced every 24 h, and unrinsed leaves replaced every 24 h. Each treatment had ten groups of 25 caterpillars. Each group was moved onto two new excised leaves in a plastic cassette (9 cm diameter by 4 cm) every 12 or 24 h; leaves showed visible signs of drying after 24 h. In all treatments, caterpillar survival was documented every 12 h for a 4-day period. Since the data did not meet assumptions of parametric tests, caterpillar survival at 24 h was compared with a Kruskal–Wallis one-way ANOVA and differences between treatments were compared using Steel–Dwass tests.
Topical application of ethanol rinses to soybean loopers
To test if ethanol rinses of geranium leaves are toxic to soybean loopers, a concentrated leaf rinse was applied to the dorsal surface of second instar larvae reared on plantain, P. lanceolata. The rinse was obtained by dipping 50 mature P. × hortorum leaves into 250 ml ethanol, then concentrating the rinse to 0.01 and 0.001 leaf equivalent per 0.1 µl dose using a Savant Speedvac (model SC110A). Eighty caterpillars in the second instar that were raised on plantain were randomly assigned to receive 0.1 µl of either distilled water, ethanol, 0.01 leaf equivalent, or 0.001 leaf equivalent. Larvae were provided with fresh plantain leaves, and their survival was monitored for 48 h. Survival was compared across the four treatments using a Chi-square test. Treatment pairs were compared using Chi-square tests with a Bonferroni-corrected α = 0.0167. Image J software (v. 1.47) was used to determine the average surface area of ten mature geranium leaves; the areas of 0.01 and 0.001 leaf were calculated to equal squares with sides of 4.1 mm and 1.3 mm respectively.
Trichome removal
To determine whether geranium trichomes cause early-instar mortality, fine forceps were used to individually pluck trichomes from a circular 2-cm-diameter area on the lower surface of excised geranium leaves. Second instar soybean loopers were restricted to that area with 2-cm-diameter leaf clips (one caterpillar/leaf); their survival was monitored over a 24-hour period.
In the first experiment, second instars reared on excised geranium leaves were randomly divided into four treatments with excised geranium leaves (20 caterpillars per treatment): (1) leaves rinsed with ethanol to remove trichome exudate, (2) tall glandular trichomes removed, (3) nonglandular trichomes removed, or (4) both tall glandular and nonglandular trichomes removed (as illustrated in Online Resource 2). After 24 h, the number of surviving caterpillars was compared between each treatment and the ethanol rinse using three Chi-square tests with a Bonferroni-corrected α = 0.017.
The second experiment had six treatments: (1) excised plantain leaves, (2) intact geranium leaves on mature potted plants, (3) excised geranium leaves rinsed with ethanol, (4) excised geranium leaves with short glandular trichomes removed, (5) short and tall glandular trichomes removed, and (6) all trichomes removed (short and tall glandular trichomes plus nonglandular trichomes). Second instars reared on excised geranium leaves were randomly assigned to treatment (20 larvae/treatment and one larva/leaf). After 24 h, the number of surviving caterpillars was compared across all treatments using a Chi-square test. Survival between paired treatments was compared with six Fisher's exact tests with a Bonferroni-corrected α = 0.00625. Fisher's exact tests were employed since the sample size for two of the comparisons was insufficient for Chi-square analysis.
Vein-cutting stimulant
To identify the cue that triggers vein cutting by soybean loopers, two defensive compounds found in P. × hortorum were tested using a bioassay developed to identify trenching stimulants for another plusiine noctuid, the cabbage looper, Trichoplusia ni (Dussourd 2003). The two compounds, l-quisqualic acid and 22:0 anacardic acid, were both acquired from Sigma-Aldrich. Soybean loopers were reared on plantain, P. lanceolata, and thus had no previous experience with geranium chemicals or with vein cutting. None of the hundreds of soybean loopers that we have reared on plantain have ever left signs of vein cutting. As a final instar larva initiated feeding on an excised plantain leaf, three drops each approximately 0.5 μl were applied directly to its mouthparts using a Wiretrol capillary (total 1.5 µl). Each subsequent drop was applied only when the larva resumed feeding. The looper’s reaction to the drops and whether it initiated vein cutting was recorded. Both of the geranium compounds were tested at three concentrations with cucumber sap (Cucumis sativus) as a positive control and the dissolving solvent as a negative control. Cucumber phloem sap was obtained from straight eight cucumber fruits by making a shallow cut perpendicular to the long axis of the cucumber and drawing up exuding phloem sap with a capillary. In previous studies, cucumber phloem exudate collected from leaves or fruits triggered trenching behavior when applied to the mouthparts of final instar cabbage loopers, T. ni (Dussourd 1997 and unpub. data). Soybean loopers in the field routinely cut trenches across cucumber leaves before feeding distal to the cuts (Dussourd and Denno 1991). Fifteen caterpillars were tested with each solution; each caterpillar was randomly assigned to treatment and was only tested with a single solution. l-Quisqualic acid was dissolved in water, whereas anacardic acid was dissolved in 60 % acetone/40 % DMSO. Both were tested at 0.5, 5, and 50 nmol/1.5 µl together with the appropriate solvent control (water, 60 % acetone/40 % DMSO).
Finally, to determine whether the exudate of tall glandular trichomes triggers vein cutting, 40 final instar soybean loopers reared on plantain were randomly assigned to receive either trichome exudate or water. Approximately 0.5 µl of trichome exudate was obtained through capillary action by touching the tip of a drawn-out glass capillary to the top of 200 tall glandular trichomes. Each larva received three drops (each approximately 0.5 μl) of either freshly collected exudate or water applied to its mouthparts with the drawn capillary as it began to feed. Loopers in the exudate treatment received all three drops from the same geranium leaf; each larva was tested with a separate leaf, and each leaf was taken from a separate plant. The number of loopers cutting veins in the exudate treatment and water control was compared with a Chi-square test.
Frequency of vein cutting on damaged and undamaged plants
Many plants respond to herbivore damage by increasing levels of defensive compounds (Schaller 2008; Heidel-Fischer et al. 2014). To determine whether previous feeding by soybean loopers increases vein cutting and thus presumably levels of the vein-cutting stimulant, we recorded the number of soybean loopers cutting veins on plants with and without prior feeding. A total of 24 P. × hortorum plants that had not been fed upon within the last 90 days were randomly assigned to the damage or no damage treatments. For the 12 plants in the damage treatment, third instar soybean loopers reared on potted P. × hortorum were individually sleeved on the third, fourth, sixth, and seventh leaf from the apical meristem (four larvae/plant) and allowed to feed for 24 h before being removed. As expected, none of the caterpillars cut veins due to their small size. Each plant then received a single final instar soybean looper reared on potted geranium; it was sleeved on the fifth undamaged leaf between the four leaves that had just been damaged. An additional 12 undamaged control geraniums also received a final instar looper on the fifth leaf from the apical meristem, but no prior feeding damage on any leaves. The total number of final instar larvae that did and did not vein cut over a 24-h test period was compared between damaged and undamaged plants using a Chi-square test.
Results
Soybean looper egg hatch
None of the 100 eggs laid on geranium hatched, whereas 89 of 100 eggs hatched on plantain (χ 2 = 160.4, P < 0.0001). The low survival on geranium can be attributed at least in part to trichomes because only 20 % (10 of 50) of the eggs hatched after receiving exudate from a single tall glandular trichome compared to 70 % (35 of 50) of the untreated control eggs (χ 2 = 25.3, P < 0.0001).
Caterpillar survival on intact and excised geranium
For the caterpillars tested on intact plants, all ten larvae survived to pupation on plantain controls, fewer pupated on mature geranium leaves, and none survived on young geranium leaves or flowers (Fig. 2). Using a Bonferroni adjusted α = 0.01, each treatment differed significantly from the plantain control (Fisher's exact tests, P < 0.001) except for the mature geranium leaves on plants without flowers (Fisher's exact test, P = 0.09). All deaths occurred in the first instar within the first 4 days with the exception of two larvae on mature leaves that died after molting to the third instar. Larvae on petals sometimes hung by their prolegs and failed to respond to touch suggesting paralysis (Online Resource 1). Larvae that survived on geranium leaves completed six instars, whereas larvae on plantain pupated after the fifth instar. Only the eight final instars that survived on mature geranium leaves left conspicuous vein cuts; one of the larvae also damaged the area in-between the vein cuts to produce a partial trench.
For loopers tested on excised leaves and flowers, survival differed significantly across treatments (Kruskal–Wallis, P < 0.0001) with the highest survival on plantain and the lowest survival on geranium flowers (Fig. 3). All deaths occurred in the first instar, most occurring within the first 24 h. Vein cuts were not observed on any leaves or flowers.
Percentage of soybean loopers (means ± 1 SE) surviving from egg emergence until pupation on excised plantain leaves, excised geranium leaves, and excised geranium flowers. Bars with different letters differ significantly by Steel–Dwass posthoc tests. N = 10 samples per treatment, 25 caterpillars per sample
Caterpillar survival on ethanol-rinsed leaves
Washing the surface of geranium leaves with ethanol greatly improved the survival of first instar loopers (Fig. 4). For example, at 24 h, larval survival differed significantly between treatments (Kruskal–Wallis, P < 0.0001) with significantly higher survival on leaves rinsed every 12 or every 24 h compared to unrinsed leaves (Steel–Dwass tests, P = 0.0005). However, there was no difference between the 12 and 24 h rinses (Steel–Dwass, P = 0.47). On unwashed leaves, all larvae were dead within 60 h.
Topical application of ethanol rinses to soybean loopers
Caterpillars receiving a dorsal application of 0.1 µl water, ethanol, or leaf rinse (0.001 or 0.01 leaf equivalents) differed significantly in survival (χ 2 = 20.3, P = 0.0002; Fig. 5). Larvae receiving the higher dose of surface rinse (0.01 leaf equivalent) suffered significantly higher mortality than larvae in each of the other three treatments (χ 2 = 10.4, 14.5, and 14.5, P < 0.005).
Trichome removal
In the first experiment, second instars suffered high mortality even when the tall glandular trichomes, nonglandular trichomes, or both were removed, unlike the larvae on rinsed leaves, which almost all survived. Larval survival in each of the three trichome removal treatments differed significantly from the rinsed leaf control (χ 2 = 9.92, 9.92, and 11.87, P < 0.005; Fig. 6). Likewise, in the second experiment, removal of the short glandular trichomes did not improve survival; significantly fewer larvae survived on leaves with short glandular trichomes removed compared to rinsed geranium leaves (Fisher's exact test, P = 0.0008; Fig. 7). Survival did not differ on leaves with the short glandular trichomes removed compared with the intact geranium control (Fisher's exact test, P = 0.27). However, when both tall and short glandular trichomes were removed, survival was higher than on intact geranium (Fisher's exact test, P < 0.0001) and did not differ from the rinsed geranium leaves (P = 0.4872). Likewise, removal of all trichomes from the feeding area increased looper survivorship compared to intact geranium leaves (P < 0.0001; Fig. 7), but not compared to the rinsed geranium leaves (P = 0.64). As expected, rinsing the excised geranium leaves significantly increased caterpillar survival compared to intact geranium leaves (P < 0.0001); survival was similar on rinsed leaves and the plantain control (P = 0.49, Fig. 7).
Survival of second instar soybean loopers over 24 h on excised plantain leaves, unaltered geranium leaves on potted plants, excised geranium leaves rinsed with ethanol, and excised geranium leaves with short glandular trichomes removed, short and tall glandular trichomes removed, or all trichomes removed (short and tall glandular trichomes plus nonglandular trichomes). N = 20 caterpillars per treatment
On plantain, the loopers moved readily across the leaf, unlike on geranium where the loopers were largely stationary when glandular trichomes were present. The trichomes are sufficiently numerous that even a first instar caterpillar would have difficulty avoiding them. For example, we counted 420 tall glandular trichomes per 2-cm-diameter circle (mean of two counts) or ~1.3 trichomes/mm2; the trichomes occurred in highest density along the veins. Glandular trichomes were easily broken off with forceps, whereas removing the nonglandular trichomes often damaged the leaf surface. Larvae were often found feeding at locations where the leaf surface was disrupted.
Vein-cutting stimulant
None of the larvae tested with l-quisqualic acid, 22:0 anacardic acid, or solvent controls exhibited vein cutting or trenching. In contrast, a total of 28 out of 30 caterpillars tested in the two trials with fresh cucumber sap controls severed leaf veins and one additional larva cut a trench (a continuous line of bites connecting at least two of the vein cuts). l-Quisqualic acid was only deterrent at 50 nmol, causing regurgitation and temporary feeding cessation in all 15 caterpillars tested. Unlike loopers feeding on geranium flowers, the larvae remained responsive to touch during periods of inactivity. All larvae in all trials survived to pupation; no developmental abnormalities were observed.
Of the 15 loopers receiving fresh trichome exudate, 11 cut plantain leaf veins, whereas none of the 15 tested with water controls exhibited vein cutting (χ 2 = 17.4, P < 0.0001). One larva severed veins after receiving just a single drop of exudate, whereas the others required a second (nine larvae) or third drop (three larvae) to initiate vein cutting. The trichome exudate was deterrent. All 15 loopers stopped feeding and regurgitated a drop of fluid onto their mouthparts; six of 15 wiped their mouthparts on the leaf.
Frequency of vein-cutting behavior on damaged and undamaged plants
Loopers on damaged geranium plants severed leaf veins more frequently than larvae on undamaged plants (χ 2 = 4.2, P = 0.041; Fig. 8). Two of the eight individuals that cut veins on damaged plants also cut partial trenches between the vein cuts. All of the caterpillars that cut veins or trenches did so within the first 12 h of the 24 h test.
Discussion
The sophisticated defenses employed by plants are matched by equally elaborate counteradaptations in insect herbivores (Dussourd 1993; Karban and Agrawal 2002; Heckel 2014). Both vein cutting and trenching are widely employed. Over 90 species in 13 families of caterpillars, beetles, and katydids disable secretory canals with vein cuts or trenches before feeding beyond the cuts (Dussourd 2009 and refs. cited). Severing the canals reduces distal exudation by isolating the insect’s feeding site from the elongate pressurized canals extending throughout the plant and by partially draining the canals beyond the cuts (Dussourd and Denno 1991; Oppel et al. 2009). Vein cutting and trenching also occur on plants lacking secretory canals. These behaviors in some cases appear to function simply to damage the main structural supports of the leaf to facilitate construction of a shelter, as suggested for animals as diverse as caterpillars and bats (Kunz et al. 1994; Greeney et al. 2012). Neither function explains vein cutting by soybean loopers on geranium since secretory canals are absent and leaf orientation is usually not substantially altered by the looper’s cuts. Furthermore, as documented in this paper, vein cutting is triggered specifically by the tall glandular trichomes, which are toxic to both eggs and larvae.
Due to the ubiquitous presence of these glandular trichomes, P. × hortorum is a poor host for soybean loopers. Adult females generally laid eggs on the underside of leaves where the eggs readily contacted tall glandular trichomes (Hurley unpub. obs.). None of 100 eggs laid on mature leaves survived. Eggs wiped with exudate from a single trichome suffered 80 % mortality versus 30 % for control eggs without exudate. The tall glandular trichomes occur on every plant part except the roots and flower petals, thus providing a formidable barrier against egg establishment. Early-instar larvae were also negatively impacted by the trichomes. Removal of glandular trichomes increased larval survival, but only if both tall and short glandular trichomes were eliminated. If either remained, larvae suffered high mortality. The tall glandular trichomes have a drop of orange exudate on their tip, whereas the short glandular trichomes appear translucent as illustrated in Online Resource 1 and 2. Despite differences in color and presumably chemical composition, both trichome types provided effective defense against loopers.
The short glandular trichomes have not been previously implicated in geranium resistance (Walters et al. 1989b), nor have these trichomes been analyzed for their chemical contents. In contrast, tall glandular trichomes are known to be lethal to small arthropods. Spider mite eggs and adults, aphids, and whiteflies died after receiving a topical application of exudate from the tall glandular trichomes (Stark 1975). Rinsing the geranium leaves first with chloroform or water significantly increased mite and aphid survival (Stark 1975; Walters et al. 1989a; Harman et al. 1996). Mite mortality was attributed specifically to derivatives of anacardic acid found in the exudate of the tall glandular trichomes (Gerhold et al. 1984); resistant cultivars have more tall glandular trichomes and produce more unsaturated anacardic acid than do susceptible cultivars (Grazzini et al. 1995a, b; Walters et al. 1989b). The anacardic acids are believed to be not just toxic; they also contribute to the adhesive nature of the trichome exudate. Both mites (Stark 1975) and aphids (Walters et al. 1990) often died stuck to trichomes.
In contrast, no loopers were observed trapped on trichomes. However, the trichomes were still lethal. Plucking glandular trichomes from the leaf or rinsing the leaf surface with ethanol increased larval survival. Reapplying the ethanol rinse to the dorsal surface of second instar larvae at a dose equal to 0.01 leaf equivalents killed most caterpillars (Fig. 5). One hundredth of a leaf represents a square with 4.1 mm sides, approximately the length of a second instar larva; thus, the immediate area around a young caterpillar contains enough chemical defense to be lethal. Since removing glandular trichomes improves survival, the toxin in the ethanol rinse is presumably from the trichomes. Unlike glandular trichomes, removing nonglandular trichomes from geranium leaves had little impact on young loopers.
Interestingly, virtually all soybean looper mortality occurred in the first two instars. Most larvae on intact geranium leaves that survived to the third instar successfully pupated. In many other insect–plant associations, plant defenses have their greatest impact on early-instar larvae, particularly the first instar (Zalucki et al. 2002). Larger larvae often have greater capabilities for enzymatically breaking down toxins (Yu 1983; Green et al. 2001; Zeng et al. 2013), as well as less surface area per unit volume in contact with trichomes.
Only the final instars exhibited vein cutting, a behavior that soybean loopers employ on other plants to disable host defenses. Soybean loopers in the field on cucumber or young mulberry saplings usually cut a trench that severs leaf veins and forms a continuous line of bites that connect the vein cuts (Dussourd and Denno 1991; Dussourd 2009). On mulberry, the loopers sometimes only cut the main veins; vein cutting suffices to disable the latex canals in this plant since the canals do not interconnect to form networks. Insects generally employ vein cutting on plants with canals in an arborescent arrangement, but produce trenches on plants with canals that form a net-like architecture (Dussourd and Denno 1991).
On P. × hortorum, soybean loopers severed individual leaf veins and sometimes connected two or more cuts with a trench. Exudate from the tall glandular trichomes sufficed to trigger vein cutting. When freshly collected exudate was applied to looper mouthparts, 73 % of larvae cut leaf veins of plantain, a species that ordinarily never triggers trenching. The more drops that were applied, the more loopers trenched.
The identity of the vein-cutting stimulant is not yet known. Trichome exudate contains multiple constituents, so there may be several stimulants or a combination of compounds that elicit the behavior. The two Pelargonium compounds tested in the bioassay were both inactive, although the loopers were often deterred and sometimes regurgitated. It was surprising that l-quisqualic acid, which acts as an agonist of l-glutamic acid receptors, was inactive; diverse neurotoxins trigger trenching by cabbage loopers (Dussourd 2003). l-quisqualic acid occurs at especially high concentration in petals of P. × hortorum cv. Nittany Red, the same cultivar that we used (~7 µg per petal, Ranger et al. 2011). Whether the compound occurs specifically in glandular trichomes is not known. Japanese beetle feeding on geranium petals or on agar plugs infused with l-quisqualic acid become paralyzed; consumption of as little as 5 ng induced 100 % paralysis (Ranger et al. 2011). Similarly, final instar soybean loopers feeding on geranium petals often hung by their hind prolegs unresponsive to touch (Online Resource 1). In the vein-cutting assays, loopers each received 9.5 µg of l-quisqualic acid at the highest concentration tested. No vein cutting or paralysis was observed even at this extraordinary dose; the larvae perhaps avoided ingesting the compound by regurgitating after receiving each drop and by wiping their mouths repeatedly on the leaf. The second compound tested in the vein-cutting assay, 22:0 anacardic acid, is one of 19 anacardic acids present in P. × hortorum glandular trichomes (Hesk et al. 1992; Grazzini et al. 1995a). Gerhold et al. (1984) documented that mite resistance correlated with the presence of unsaturated anacardic acids, whereas we tested a commercially available saturated anacardic acid, which occurs in greater abundance in geranium cultivars susceptible to spider mites (Hesk et al. 1992). We cannot exclude the possibility that other anacardic acids might elicit vein cutting. We also cannot exclude one or more of the abundant terpenoids in geranium as possible stimulants of vein cutting. The one terpenoid that we tested, geraniol, proved to be inactive in eliciting vein cutting (Hurley 2014), but it has since been shown to be absent from P. × hortorum (Farag et al. 2012; Liu et al. 2013) although abundant in other Pelargonium species (Lis-Balchin 2002).
Glandular trichomes have a broad distribution in plants being found on the aerial surfaces of ~20–30 % of all vascular plant species (Duke et al. 2000; Tissier 2012). In many cases, a defensive role has been documented (Levin 1973; Kelsey et al. 1984; Wagner 1991; Dalin et al. 2008). Given that the glandular trichomes of geranium elicit vein cutting by soybean loopers, it would be surprising if other plants with trichomes did not also have vein-cutting herbivores. Indeed, in Arkansas, USA, we have found soybean loopers severing leaf veins and cabbage loopers cutting trenches in leaves of the East Asian tree, Paulownia tomentosa (Scrophulariaceae) (Dussourd unpub. obs.). The Scrophulariaceae are not reported to have secretory canals (Metcalfe and Chalk 1983), and no visible exudate appears when leaves are cut; however, leaves of P. tomentosa are covered by a dense investiture of glandular hairs (Kobayashi et al. 2008). Are the vein cuts and trenches produced by insects on plants with secretory canals also triggered by trichomes and not by canal exudate? Although theoretically possible, two studies document that canal exudates elicit the behaviors: Vein cutting by monarchs is triggered by milkweed latex (Helmus and Dussourd 2005) and trenching by cabbage loopers is stimulated by lettuce latex and cucurbit phloem exudate (Dussourd 1997).
Whether vein cuts in geranium leaves benefit soybean loopers is not known. Vein cutting could simply be a nonadaptive response to an alien plant, a behavior that does not provide any advantages with respect to trichome defenses. However, we found that loopers cut veins on intact plants, but not on excised leaves. Furthermore, the loopers exhibited vein cutting more frequently on previously damaged plants suggesting that the chemical trigger increases with damage. By severing the major supply arteries in a leaf, soybean loopers could potentially block inducible geranium responses to herbivory. Recent studies with tomato and other Solanaceae suggest that plants are capable of increasing production of diverse defensive compounds within glandular trichomes in response to damage (Laue et al. 2000; Hare and Walling 2006; Van Schie et al. 2007; Peiffer et al. 2009; Tian et al. 2012). Since geranium trichome exudate triggers vein cutting, our observation that loopers on previously damaged geraniums were more likely to cut veins of mature leaves likewise suggests that trichome chemistry changed. Mature geranium leaves do not increase trichome density; new tall glandular trichomes only appear on young, undeveloped leaves of P. × hortorum (Walters et al. 1989b).
In summary, we provide the first example of a plusiine noctuid employing vein cutting and trenching on a host plant lacking pressurized defensive canal systems. The behavior is triggered by the exudate not of secretory canals, but of glandular trichomes. The trichomes are lethal to looper eggs and early-instar larvae. The few individuals that survive to the final instar rarely employ vein cutting if transferred to new plants, but routinely utilize vein cutting on damaged hosts. How the vein cuts affect the chemistry of the glandular trichomes remains to be determined.
References
Alexander AJ (1961) A study of the biology and behavior of the caterpillars, pupae and emerging butterflies of the subfamily Heliconiinae in Trinidad, West Indies. Part I. some aspects of larval behavior. Zoologica 46:1–25
Cox GW (2004) Alien species and evolution: the evolutionary ecology of exotic plants, animals, microbes, and interacting native species. Island Press, Washington
Dalin P, Ågren J, Björkman C, Huttunen P, Kärkkäinen K (2008) Leaf trichome formation and plant resistance to herbivory. In: Schaller A (ed) Induced plant resistance to herbivory. Springer, New York, pp 89–105
Duke SO, Canel C, Rimando AM, Tellez MR, Duke MV, Paul RN (2000) Current and potential exploitation of plant glandular trichome productivity. Adv Bot Res 31:121–151
Dussourd DE (1993) Foraging with finesse: caterpillar adaptations for circumventing plant defenses. In: Stamp NE, Casey TM (eds) Caterpillars: ecological and evolutionary constraints on foraging. Chapman and Hall, New York, pp 92–131
Dussourd DE (1997) Plant exudates trigger leaf-trenching by cabbage loopers, Trichoplusia ni (Noctuidae). Oecologia 112:362–369
Dussourd DE (2003) Chemical stimulants of leaf-trenching by cabbage loopers: natural products, neurotransmitters, insecticides, and drugs. J Chem Ecol 29:2023–2047
Dussourd DE (2009) Do canal-cutting behaviours facilitate host-range expansion by insect herbivores? Biol J Linn Soc 96:715–731
Dussourd DE, Denno RF (1991) Deactivation of plant defense: correspondence between insect behavior and secretary canal architecture. Ecology 72:1383–1396
Dussourd DE, Denno RF (1994) Host range of generalist caterpillars: trenching permits feeding on plants with secretory canals. Ecology 75:69–78
Farag M, Ahmed MH, Yousef H, El-Badawey SS, Abd El-Ghany MA, Abdel-Rahman AAH (2012) Repellent and insecticide activity of Pelargonium × hortorum against Spodoptera littoralis (Boisd.). Z Naturforsch C-J Biosci 67(7):398–404
Gerhold DL, Craig R, Mumma RO (1984) Analysis of trichome exudate from mite-resistant geraniums. J Chem Ecol 10:713–722
Graves SD, Shapiro SM (2003) Exotics as host plants of the California butterfly fauna. Biol Conserv 110:413–433
Grazzini R, Hesk D, Yerger E, Cox-Foster D, Medford J, Craig R, Mumma RO (1995a) Distribution of anacardic acids associated with small pest resistance among cultivars of Pelargonium × hortorum. J Am Soc Hortic Sci 120:343–346
Grazzini R, Hesk D, Yerger E, Cox-Foster D, Medford J, Craig R, Mumma RO (1995b) Species distribution of biochemical and morphological characters associated with small pest resistance in Pelargonium × hortorum. J Am Soc Hortic Sci 120:336–342
Green ES, Zangerl AR, Berenbaum MR (2001) Effects of phytic acid and xanthotoxin on growth and detoxification in caterpillars. J Chem Ecol 27:1763–1773
Greeney HF, Dyer LA, Smilanich AM (2012) Feeding by lepidopteran larvae is dangerous: a review of caterpillars’ chemical, physiological, morphological, and behavioral defenses against natural enemies. Invert Surviv J 9:7–34
Hare JD, Walling LL (2006) Constitutive and jasmonate-inducible traits of Datura wrightii. J Chem Ecol 32:29–47
Harman J, Paul P, Craig R, Cox-Foster D, Medford J, Mumma RO (1996) Development of a mite bioassay to evaluate plant resistance and its use in determining regeneration of spider mite resistance. Entomol Exp Appl 81:301–305
Heckel DG (2014) Insect detoxification and sequestration strategies. In: Voelckel C, Jander G (eds) Annual plant reviews, vol 47., Insect-plant interactionsWiley, Oxford, pp 77–114
Heidel-Fischer HM, Musser RO, Vogel H (2014) Plant transcriptomic responses to herbivory. Ann Plant Rev 47:155–196
Held DW, Potter DA (2003) Characterizing toxicity of Pelargonium spp. and two other reputedly toxic plant species to Japanese beetles (Coleoptera: Scarabaeidae). Environ Entomol 32:873–880
Helmus MR, Dussourd DE (2005) Glues or poisons: which triggers vein cutting by monarch caterpillars? Chemoecology 15:45–49
Hesk D, Craig R, Mumma RO (1992) Comparison of anacardic acid biosynthetic capability between insect-resistant and susceptible geraniums. J Chem Ecol 18:1349–1364
Houghton P, Lis-Balchin M (2002) Chemotaxonomy of Pelargonium based on alkaloids and essential oils. In: Lis-Balchin M (ed) Geranium and Pelargonium: the genera Geranium and Pelargonium. Taylor and Francis, New York, pp 166–173
Hurley KW (2014) Tricky trichomes: chemical defense in geranium and counteradaptations by soybean loopers. Masters dissertation, University of Central Arkansas
Jander G (2014) Revisiting plant-herbivore co-evolution in the molecular biology era. In: Voelckel C, Jander G (eds) Annual plant reviews, vol 47., Insect-plant interactionsWiley, Oxford, pp 361–384
Karban R, Agrawal AA (2002) Herbivore offense. Ann Rev Ecol Syst 33:641–664
Kelsey RG, Reynolds GW, Rodriguez E (1984) The chemistry of biologically active constituents secreted and stored in plant glandular trichomes. In: Rodriguez E, Healey PL, Mehta I (eds) Biology and chemistry of plant trichomes. Plenum Press, New York, pp 187–241
Kobayashi S, Asai T, Fujimoto Y, Kohshima S (2008) Anti-herbivore structures of Paulownia tomentosa: morphology, distribution, chemical constituents and changes during shoot and leaf development. Ann Bot 101:1035–1047
Kunz TN, Fujita MS, Brooke AP, McCracken GF (1994) Convergence in tent architecture and tent-making behavior among neotropical and paleotropical bats. J Mamm Evol 2:57–78
Lafontaine JD, Poole RW (1991) Noctuoidea: Noctuidae (part). In: Dominick RB et al. (eds) The Moths of America North of Mexico, Fascicle 25.1. Wedge Entomological Research Foundation, Washington
Laue G, Preston CA, Baldwin IT (2000) Fast track to the trichome: induction of N-acyl nornicotines precedes nicotine induction in Nicotiana repanda. Planta 210:510–514
Levin DA (1973) The role of trichomes in plant defense. Q Rev Biol 48:3–15
Lis-Balchin M (2002) Essential oils from different Pelargonium species and cultivars: their chemical composition (using GC, GC/MS) and appearance of trichomes (under EM). In: Lis-Balchin M (ed) Geranium and Pelargonium: the genera Geranium and Pelargonium. Taylor and Francis, New York, pp 147–165
Liu XC, Yang K, Wang SY, Wang XG, Liu ZL, Cheng J (2013) Composition and insecticidal activity of the essential oil of Pelargonium hortorum flowering aerial parts from China against two grain storage insects. J Med Plant Res 7:3263–3268
Metcalfe CR, Chalk L (1983) Anatomy of the dicotyledons, vol II. Clarendon Press, Oxford
Miller D (2002) The taxonomy of Pelargonium species and cultivars, their origins and growth in the wild. In: Lis-Balchin M (ed) Geranium and Pelargonium: the genera Geranium and Pelargonium. Taylor and Francis, New York, pp 49–79
Mooney HA (2005) Invasive alien species: the nature of the problem. In: Mooney HA, Mack RN, McNeely JA, Neville LE, Schei PJ, Waage JK (eds) Invasive alien species: a new synthesis. Island Press, Washington, pp 1–15
Oppel CB, Dussourd DE, Garimella U (2009) Visualizing a plant defense and insect counterploy: alkaloid distribution in Lobelia leaves trenched by a plusiine caterpillar. J Chem Ecol 35:625–634
Peiffer M, Tooker JF, Luthe DS, Felton GW (2009) Plants on early alert: glandular trichomes as sensors for insect herbivores. New Phytol 184:644–656
Ranger CM, Winter RE, Singh AP, Reding ME, Frantz JM, Locke JC, Krause CR (2011) Rare excitatory amino acid from flowers of zonal geranium responsible for paralyzing the Japanese beetle. Proc Natl Acad Sci USA 108:1217–1221
Schaller A (ed) (2008) Induced plant resistance to herbivory. Springer, New York
Schultz DJ, Medford JI, Cox-Foster D, Grazzini RA, Craig R, Mumma RO (2000) Anacardic acids in trichomes of Pelargonium: biosynthesis, molecular biology, and ecological effects. Adv Bot Res 31:175–192
Schultz DJ, Wickramasinghe NS, Klinge CM (2006) Anacardic acid biosynthesis and bioactivity. Rec Adv Phytochem 40:131–156
Simmonds MSJ (2002) Interactions between arthropod pests and pelargoniums. In: Lis-Balchin M (ed) Geranium and Pelargonium: the genera Geranium and Pelargonium. Taylor and Francis, New York, pp 291–298
Spencer KC (ed) (1988) Chemical mediation of coevolution. Academic Press, New York
Stark RS (1975) Morphological and biochemical factors relating to spider mite resistance in the geranium. Dissertation, Pennsylvania State University
Tian D, Tooker J, Peiffer M, Chung SH, Felton GW (2012) Role of trichomes in defense against herbivores: comparison of herbivore response to woolly and hairless trichome mutants in tomato (Solanum lycopersicum). Planta 236:1053–1066
Tissier A (2012) Glandular trichomes: what comes after expressed sequence tags? Plant J 70:51–68
Van Schie CCN, Haring MA, Schuurink RC (2007) Tomato linalool synthase is induced in trichomes by jasmonic acid. Plant Mol Biol 64:251–263
Wagner DL, Schweitzer DF, Sullivan JB, Reardon RC (2011) Owlet caterpillars of eastern North America. Princeton University. Press, Princeton
Wagner GJ (1991) Secreting glandular trichomes: more than just hairs. Plant Physiol 96:675–679
Walters DS, Craig R, Mumma RO (1989a) Glandular trichome exudate is the critical factor in geranium resistance to foxglove aphid. Entomol Exp Appl 53:105–109
Walters DS, Grossman H, Craig R, Mumma RO (1989b) Geranium defensive agents. IV. Chemical and morphological bases of resistance. J Chem Ecol 15:357–372
Walters DS, Craig R, Mumma RO (1990) Effects of mite resistance mechanism of geraniums on mortality and behavior of foxglove aphid (Acyrthosiphon solani). J Chem Ecol 16:877–886
Williams CA, Harborne JB (2002) Geranium and Pelargonium: phytochemistry of the genus Pelargonium. In: Lis-Balchin M (ed) Geranium and Pelargonium: the genera Geranium and Pelargonium. Taylor and Francis, New York, pp 99–115
Yu SJ (1983) Age variation in insecticide susceptibility and detoxification capability of fall armyworm (Lepidoptera: Noctuidae) larva. J Econ Entomol 76:219–222
Zalucki MP, Clarke AR, Malcolm SB (2002) Ecology and behavior of first instar larval Lepidoptera. Ann Rev Entomol 47:361–393
Zeng RS, Wen Z, Niu G, Berenbaum MR (2013) Aflatoxin B1: toxicity, bioactivation and detoxification in the polyphagous caterpillar, Trichoplusia ni. Insect Sci 20:318–328
Acknowledgments
Special thanks to Reid Adams, K.C. Larson, Jerry Manion, Rick Noyes, Christopher Ranger, and Patrick Ward for helpful advice, to Pablo Jourdan at the Ornamental Plant Germplasm Center (Ohio State University and USDA-ARS) for donating geranium cuttings, to K.C. Larson and two anonymous reviewers for thorough reviews and many helpful suggestions, and to the University of Central Arkansas (CNSM Student Research grants) and the Arkansas Center for Plant-Powered Production (P3) for funding. The P3 Center is funded through the RII: Arkansas ASSET Initiatives (AR EPSCoR) I (EPS-0701890) and II (EPS-1003970) by the National Science Foundation and the Arkansas Science and Technology Authority.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Michael Smith.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11829_2014_9348_MOESM1_ESM.tif
(A) Soybean looper egg daubed with orange exudate from a single tall glandular trichome from P. × hortorum. (B) Soybean loopers hanging immobilized after consuming geranium petals. (C) Focus stacked image of tall glandular trichomes on the abaxial surface of a geranium leaf. Orange exudate is clearly visible at the tip of the trichomes. (D) Same trichomes after the leaf was rinsed with ethanol; the orange trichome exudate has been removed. Scale bars equal 100 µm (TIFF 8489 kb)
11829_2014_9348_MOESM2_ESM.tif
(A) Focus stacked images of trichomes found on the edge of a geranium leaf: tall glandular trichomes with orange tips, short glandular trichomes with translucent tips, and nonglandular trichomes with pointed tips. (B) Leaf edge after nonglandular trichomes have been removed with fine forceps; the glandular trichomes remain. (C) Leaf edge after tall glandular trichomes have been removed; nonglandular and short glandular trichomes remain. Scale bar equals 100 µm (TIFF 19810 kb)
Rights and permissions
About this article
Cite this article
Hurley, K.W., Dussourd, D.E. Toxic geranium trichomes trigger vein cutting by soybean loopers, Chrysodeixis includens (Lepidoptera: Noctuidae). Arthropod-Plant Interactions 9, 33–43 (2015). https://doi.org/10.1007/s11829-014-9348-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-014-9348-6