Abstract
In the search for compounds that contribute to the host or habitat discrimination, antennae of Ips typographus were screened for sensitivity to volatiles released by spruce trap-trees using gas chromatography linked to electroantennography. The antennally active compounds were determined using comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometric detection. Data show that I. typographus antennae respond to compounds emitted by the host. In total, 18 of antennally active compounds were detected: α-pinene, camphene, sabinene, β-pinene, myrcene, Δ-3-carene, p-cymene, limonene, β-phellandrene, 1,8-cineole, γ-terpinene, terpinolene, nonanal, camphor, trans-pinocamphone, cis-pinocamphone, terpinen-4-ol, and verbenone. Unequivocal identification of all active minor compounds is provided and confirmed using synthetic standards. Compounds in minor quantities like 1,8-cineole, β-phellandrene, camphor, cis-pinocamphone, and trans-pinocamphone were more active than major spruce monoterpenes. We hypothesize that the minor spruce compounds may play so far unrecognized role in conveying information about host suitability for I. typographus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
European spruce bark beetle, Ips typographus (Linnaeus, 1758), is the most destructive Ips species in Eurasia as well as probably the most serious pest of Norway spruce, Picea abies (L.) H. Karst (Grégoire and Evans 2004). At endemic population densities, this species colonizes dead or severely weakened spruce trees while at epidemic conditions, it successfully attacks the healthy trees. Among current methods of I. typographus control, mass trapping by means of felled (or artificially stressed) trap-trees left for bark beetle colonization and debarked before the brood finishes their development (Courtois et al. 1961; Bakke 1989) represents a traditional approach widely used in Czechia (Švestka et al. 1996) and in Europe (Grégoire and Evans 2004). After identification of the I. typographus aggregation pheromone (Bakke 1970, 1976), the trap-tree technique was gradually replaced by traps baited with synthetic pheromone (pheromone traps) (Bakke 1985). Since the introduction of pheromone traps into the forest practice for control of I. typographus, many studies reported that host-specific odors may enhance the pheromone attractiveness (Raty et al. 1995; Bombosch 1983; Bombosch and Johann 1985; Austara et al. 1986; Erbilgin et al. 2007; Saint-Germain et al. 2007). As a consequence, pheromone-baited spruce trap-trees poisoned with insecticide were introduced as a more efficient alternative to pheromone traps (Klimetzek 1978; Raty et al. 1995).
Although the importance of volatiles in host selection by beetles in flight was generally known earlier (Person 1931) and has been reviewed for Ips and other bark beetles (Byers 1989, 1995, 2004), it is still not known whether host volatiles mediate the attraction of I. typographus. It is also uncertain whether tree volatiles are responsible for the enhanced attraction of pheromone-baited trap-trees compared to pheromone traps alone. In order to identify host compounds that may play a role in host selection and enhance attraction to aggregation pheromone, we studied volatiles released from trap-trees using comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometric detection (GC×GC/TOFMS). To identify the compounds with ecological potential, we studied physiological responses of I. typographus antennae to host volatiles using gas chromatography with electroantennographic detection (GC-EAD).
Materials and methods
Trap-trees
Three spruce trees were cut during February 2012 in production spruce forest (Dobřichovice, Central Bohemia region of the Czech Republic) of about 80 years old. The cut trees were allowed to age for a period of 3–6 months under the natural conditions. From each tree, one section of approximately same diameter and 1 m of length was taken into laboratory and their volatiles were collected.
Insects
Infested logs were collected from natural spruce stands and transferred to the laboratory where they were stored in a cool room (1–5 °C). Before experiments, the logs were transferred to a breeding room (25 ± 1 °C, R.H. 40 %, and 12:12 L:D). Under these conditions, beetles finished their development and emerged. After emergence, the beetles were provided with water and bark strips and were stored in plastic containers under 1–5 °C until used in electrophysiological experiments. Only males were used.
Solvents
For all operations with samples and cartridges were used following, prior the analyses under argon atmosphere redistilled solvents: hexane (for residual analysis, ≥99.0 %), acetone (CHROMASOLV® Plus, for HPLC, ≥99.9 %), both supplied by Sigma-Aldrich s.r.o. (Prague, Czech Republic).
Synthetic standards
Majority of synthetic standards was obtained from chemical inventory of the Institute of Organic Chemistry and Biochemistry (β-phellandrene, terpinolene, estragole) or purchased from Sigma-Aldrich and Bedoukian Research, Inc. (Danbury, CT, USA). Synthetic (1α,2β,5α)-2,6,6-trimethylbicyclo[3.1.1] heptan-3-one (cis-pinocamphone) and (1α,2α,5α)-2,6,6-trimethylbicyclo[3.1.1]heptan-3-one (trans-pinocamphone) were obtained via Alchimica s.r.o. (Prague, Czech Republic). Standards were diluted in hexane in concentrations of 10–100 ng µl−1 and analyzed in GC×GC/TOFMS or GC-EAD under the same conditions as trap-tree extracts. The EAD activity was considered to be confirmed if it was observed at least three times in the same area as in the GC-EAD analyses of trap-tree extracts.
Headspace sampling procedure
Stem sections transferred to the laboratory were enclosed in Paclan PET heat-resistant baking foil sleeve (CeDo, Kąty Wrocławskie, Poland). A purified airstream (charcoal and 4 Å molecular sieves cartridges; 1 l min−1) was blown via PTFE tubing over foil-enclosed log section. Volatiles entrained by the airstream were trapped by 150 mg of SuperQ® adsorbent (Chrompack Inc., Florham Park, NJ, USA) placed in the tip of glass Pasteur-like-tube cartridge sealed by glass wool for 5 h. The identical adsorbent cartridge was used prior the airstream entrance into the sample for the final air pre-cleaning. One sample of each stem was obtained. After collection, the trapped volatiles were extracted with 500 μl of hexane. The extracts were concentrated to approximately 100 μl under a gentle stream of nitrogen and stored in a freezer until analysis. Between collection repetitions, the trap cartridges were washed with acetone and hexane, dried at room temperature, and conditioned for 1 h at 120 °C.
Comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometric detection (GC×GC/TOFMS) analyses
The GC×GC/TOFMS analyses were carried out on a LECO Pegasus 4D instrument (LECO Corp., St. Joseph, MI, USA) equipped with a quad-jet cryomodulator. A DB-5 column (J&W Scientific, Folsom, CA, USA; 30 m × 250 μm i.d. × 0.25 μm film) and a BPX-50 column (SGE Inc., Austin, TX, USA; 2 m × 100 μm i.d. × 0.1 μm of film) were used for the first and the second dimension analysis, respectively. Helium was used as a carrier gas at a constant flow of 1 ml min−1. Sample injection was done with the HP 7683 autosampler, and 1 μl of sample was injected in the split less mode. The temperatures of the GC×GC/TOFMS instrument were set at 220 °C at the injector, 260 °C at the transfer line, and 250 °C at the ion source. The temperature program on the primary GC oven was as follows: 40 °C for 2 min, the temperature was raised at 5 °C min−1 up to 190 °C and at 20 °C min−1 up to 320 °C with a hold for 2 min. The program in the secondary oven was 10 °C higher than in the primary one and was operated in the same ramping mode. The modulation period, the hot-pulse duration, and the cool time between the stages were set at 5, 0.8, and 1.7 s, respectively. The mass spectrometer was operated in the electron impact mode (EI, 70 eV). The detector voltage was 1,750 V. The data-acquisition rate was 100 Hz (scans per second) for the mass range of 29–400 amu. The purge time was 60 s at a flow of 60 ml min−1. The solvent delay time was 500 s. The data were processed and consecutively visualized on 2D and 3D chromatograms using LECO ChromaTOF® software. A series of n-alkanes (C8–C22; Sigma-Aldrich) was co-injected with authentic samples to determine their retention indices (I R; LRI-calculation method provided by LECO ChromaTOF® software). The volatiles were identified by a comparison of their mass spectra fragmentation patterns, the first and the second dimension retention times, and retention indices with previously published data [reference spectra NIST 2008 library, the Wiley/NBS Registry of mass spectral data (McLafferty and Stauffer 1989)] and published retention indices with synthetic standards. In the absence of standards, identifications were based on mass spectra and retention indices comparisons with previously published data (Adams 2007, www.pherobase.com, www.flavornet.org and specific references listed in the reference section).
Gas chromatography with electroantennographic detection (GC-EAD) experiments
Headspace samples were injected split less into a 5890A Hewlet-Packard gas chromatograph equipped with an Rxi-5Sil MS (Restek, Bellefonte, PA, USA; 30 m × 0.25 μm i.d. × 0.25 μm film) column. The column was split at the end by a Graphpack 3D/2 four-arm splitter (Gerstel GmbH & Co.KG, Mülheim an der Ruhr, Germany), allowing the division of the eluate to the flame ionization detection (FID) and antennal detector (EAD). Helium was used as a carrier gas at a constant flow of 1 ml min−1. The GC was operated at an initial temperature of 40 °C for 2 min then ramped up at a rate of 10 °C min−1 to 270 °C with a 10-min hold. The temperature of the GC inlet and detector was set to 250 and 270 °C, respectively. To allow a comparison of major antennal activities (EAD response) with individual compounds provided by GC×GC/TOFMS analysis, a series of saturated C8–C22 n-alkanes was co-injected with some of the analyzed samples. The linear retention indices (I R-EAD) of EAD active peaks were calculated (Van Den Dool and Kratz 1963), and the corresponding areas of GC×GC/TOFMS chromatograms were inspected in detail. All of the compounds present within these corresponding areas were identified, and their I R were calculated. The antennal activity of synthetic compounds was subsequently tested in GC-EAD experiments. The EAD activity was considered to be established if it was observed at least three times in exactly the same GC-EAD area. In total, about 200 GC-EAD recordings with different trap-tree extracts were attempted to yield 30 distinctive GC-EAD recordings.
Results
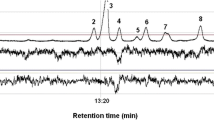
The GC×GC/TOFMS and GC-EAD analyses of spruce trap-tree volatiles revealed the presence of many terpenoid compounds with antennal activity (Table 1; Fig. 1). The most abundant compounds were monoterpene hydrocarbons making up 93 and 92.7 % in trap A and trap B, respectively. The rest was made up by oxidized monoterpenes and nonanal. Figure 1a shows a typical GC×GC/TOFMS 2D chromatogram. Each spot on the contour plot represents one compound, the concentration of which is color coded from zero (blue) to maximum (red). Numbers in the figure correspond to the serial numbers of the substances listed in Table 1, where each compound is characterized by observed retention times in the first and the second dimension, by measured and published linear retention indexes (I R), and previously reported or recorded antennal activity.
GC×GC/TOFMS (a) and GC-EAD (b) chromatograms of spruce trap-tree volatiles: each dot on the plot a represents one compound, concentration of which is color coded from zero (blue) to maximum (red). Numbers on the picture represent identified volatile constituents as they are listed in Table 1. GC-EAD analysis b shows the compounds eliciting EAD responses
As could be seen from Fig. 1a and from Table 1, the most abundant compounds in spruce trap-tree volatiles were monoterpene hydrocarbons Δ-3-carene, α-pinene, β-pinene and (in total 58 and 57 % in trap A and B, respectively) followed by myrcene, limonene, camphene, sabinene, β-phellandrene, fenchone, terpinolene, p-cymene (forming up to 33.2 and 29.9 %, respectively), and oxygenated terpenoid compounds present in traces (≤1 %) forming 1.5 and 5.4 % in trap A and trap B, respectively, of the total amount of spruce tree-trap volatiles. A typical example of GC-EAD analysis of spruce trap-tree volatiles using I. typographus antenna as a biological detector is depicted on Fig. 1b. This figure shows that I. typographus antennae respond to many compounds from spruce trap-tree emissions and that many important stimuli are among the less abundant compounds. Sesquiterpenes, which accounted for <10 % of all collected volatiles, did not elicit antennal responses at concentrations present in analyzed samples. Among identified sesquiterpenes were α-cubebene, α-longipinene, α-gurjunene, α-copaene, β-longipinene, longifolene, (E)-β-caryophyllene, germacrene D, δ-cadinene, and α-cadinene. The identification of compounds was done based on correlation of GC×GC/TOFMS spectra and linear retention indices (I R) with authentic standards whenever possible. The determination of antennally active compounds was based on the precise correlation of (I R) obtained in both GC×GC/TOFMS and GC-EAD analyses. Tentative identification based on mass spectra and (I R) was in all cases confirmed by subsequent GC-EAD experiments using synthetic standards to determine whether the activities matched those found in GC-EAD analysis of trap-tree extracts. The process of compound identification is demonstrated in Fig. 2, where complementary sections of both GC×GC/TOFMS (Fig. 2a) and GC-EAD (Fig. 2b) analyses are displayed. The sections are focused on the area where camphor, trans-pinocamphone, cis-pinocamphone, terpinen-4-ol, and verbenone eluted. The compounds are numbered as listed in Table 1 (17: camphor, 18: trans-pinocamphone, 19: cis-pinocamphone, 20: terpinen-4-ol, 22: verbenone). Figure 2a shows the GC×GC/TOFMS chromatogram, while Fig. 2a depicts the GC-EAD analysis. Figure 2b shows that camphor, trans-pinocamphone, cis-pinocamphone, terpinen-4-ol, and verbenone elicit significant antennal responses. Subsequent GC-EAD analysis (Fig. 2c) of synthetic trans-pinocamphone and cis-pinocamphone confirms the antennal activities and supports the identification. Figure 2 also shows the advantage of GC×GC/TOFMS in the identification process of insect semiochemicals. While retention times of terpinen-4-ol and cis-pinocamphone are almost identical in one-dimensional GC-FID analysis and these two compounds often co-elute, forming one broad FID/EAD peak, in two-dimensional GC×GC/TOFMS analysis, terpinen-4-ol and cis-pinocamphone form clearly isolated spots in the chromatogram due to differences in retention times in the second dimension. Figure 3 compares MS spectra of natural and synthetic trans-pinocamphone and cis-pinocamphone. As can be seen from Fig. 2 and, the spectra and retentions times in both dimensions are essentially identical supporting unequivocal identification.
Detail of the corresponding parts of GC×GC/TOFMS (a) and GC-EAD (b) analysis of spruce trap-tree volatiles, (c) GC-EAD of synthetic trans- and cis-isopinocamphone (compound numbering as listed in Table 1)
Based on GC×GC/TOFMS and GC-EAD experiments, we observed that I. typographus antennae consistently respond to 18 compounds present in spruce trap-tree volatiles—specifically to α-pinene, camphene, sabinene, β-pinene, myrcene, Δ-3-carene, p-cymene, limonene, β-phellandrene, 1,8-cineole (eucalyptol), γ-terpinene, terpinolene, nonanal, camphor, trans-pinocamphone, cis-pinocamphone, terpinen-4-ol, and verbenone. To assess the effectiveness of trans-pinocamphone and cis-pinocamphone in comparison with the major monoterpenes α-pinene and β-pinene, EAD/FID ratio (EAD peak area/FID peak area) was calculated (the higher EAD/FID ratio, the lower efficiency; Table 2). The table shows mean values of EAD/FID ratios for trans-pinocamphone, cis-pinocamphone, α-pinene, and β-pinene calculated from three independent GC-EAD experiments. The EAD/FID ratio clearly shows that the effectiveness of α-pinene and β-pinene was 10–100× lower then cis- and trans-pinocamphone.
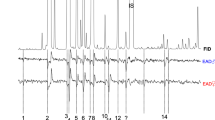
All antennal activities were confirmed with synthetic standards in subsequent GC-EAD experiments using same conditions as in the case of authentic spruce trap-tree samples (Fig. 4). During these experiments, we observed that individual antennal preparations responded with significant variability. In some preparations, certain compounds elicited small or no responses, while in others, quite significant responses were observed to the same compound(s). Some compounds elicited responses more often than the others, perhaps reflecting the sensillar abundance of the antenna. Compounds camphene, myrcene, Δ-3-carene, p-cymene, β-phellandrene, nonanal and especially 1,8-cineole, camphor, trans- and cis-pinocamphone, verbenone, and terpinen-4-ol elicited antennal responses almost in all antennal preparations, while responses to α-pinene, sabinene, β-pinene, p-cymene, limonene, and γ-terpinene were less frequent and less pronounced. Such variability in antennal sensitivity probably reflects different abundances in distributions of sensillae on I. typographus antennae. Less frequent sensillae are more difficult to encounter by the recording electrode. On Fig. 4, examples of GC-EAD responses to standards (concentrations 5–10 ng µl−1) are depicted. Trace 4a represents GC-EAD experiments with α-pinene, myrcene, Δ-3-carene, p-cymene, and limonene; trace 4b depicts GC-EAD responses to camphene, β-pinene, β-phellandrene, terpinolene, terpinen-4-ol, and verbenone; and trace 4c shows responses to camphene, sabinene, Δ-3-carene, 1,8-cineole, γ-terpinen, and camphor.
Examples of GC-EAD experiments with pure standards used to confirm antennal activities (concentrations 5–10 ng µl−1). Trace a depicts GC-EAD responses of α-pinene, myrcene, Δ-3-carene, cymene, and limonene; trace b depicts GC-EAD responses to camphene, β-pinene, β-phellandrene, terpinolene, terpinen-4-ol, and verbenone; trace c depicts responses to camphene, sabinene, Δ-3-carene, 1,8-cineole, γ-terpinene, and camphor
Discussion
Bark beetles that attack living trees including I. typographus invariably possess an aggregation pheromone, but are supposed to be weakly, if at all, attracted by host volatiles alone (primary attraction) (Pureswaran and Borden 2003). On the other hand, secondary bark beetle species often do not produce aggregation pheromones, but are strongly attracted to host monoterpenes, ethanol, acetaldehyde, or a combination (Byers et al. 1985; Kohnle 1985; Klimetzek et al. 1986; Moeck et al. 1981; Schroeder and Lindelöw 1989; Lindelöw and Risberg 1992; Sjödin et al. 1989). As demonstrated in the introductory part of this paper, data available for possible primary attraction in I. typographus are ambiguous. In this species, males locate a host tree and determine its suitability for colonization and reproduction. At endemic level, males prefer weakened less resistant trees, or trees that are in the initial stages of death and decay (Grégoire and Evans 2004). Such trees are scattered in the forest, and it might be helpful to use long-range olfactory signals for navigation. However, while some authors reported attractiveness of host odor or major host monoterpenes and their synergism with aggregation pheromone, others did not observe any of these effects (reviewed by Byers 1989, 2004) and speculated about random host selection (Moeck et al. 1981; Wood 1982; Byers 1996; Gries et al. 1989; Saint-Germain et al. 2007) based on observation that during dispersal flight beetles fly more than 40 km (Forsse and Solbreck 1985) and encounter several hosts and thus may check host suitability by close-range inspection (Moeck et al. 1981; Wood 1982).
Despite this ambiguity, our experiments show that I. typographus males perceive a broad spectrum of spruce trap-tree volatiles including both major and minor components. I. typographus antennae responded consistently to at least 18 compounds from collected spruce trap-tree volatiles including three major spruce monoterpene hydrocarbons (Δ-3-carene, α-pinene, and β-pinene) and less abundant compounds like camphene, myrcene, Δ-3-carene, p-cymene, limonene, β-phellandrene, 1,8-cineole, camphor, trans-pinocamphone, terpinen-4-ol, cis-pinocamphone, and verbenone. Antennal activities of individual compounds found in spruce tree-trap volatiles differed. Some compounds present in small quantities, like 1,8-cineole, camphor, trans-pinocamphone, terpinen-4-ol, and cis-pinocamphone, and verbenone, elicited more pronounced antennal responses then much more abundant spruce monoterpenes α-pinene and β-pinene. Antennally very active compounds like camphor, trans-pinocamphone, terpinen-4-ol, and cis-pinocamphone were not previously reported in fresh spruce odor (Borg-Karlson et al. 1993; Persson et al. 1993, 1996; Sjödin et al. 2000; Martin et al. 2002, 2003; Zhao et al. 2011). It should be noted, however, that the precise comparison of volatile composition obtained in different laboratories is hardly possible, since the volatile composition is influenced not only by the tree species, its age, previous and present physiology, and many other environmental factors that shaped phenotypes of individual trees, but depends also on the used methodology of sample preparation. Different techniques used for volatile collection produce different chromatographic patterns even for the same sample. Gas chromatograms of spruce volatile samples are complex blends containing many minor peaks that may remained previously unidentified because of limitation in experimental methodology or because peaks of minor volatile compounds might be overlaid by peaks of major ones or because researchers focused on more abundant compounds. The most active minor compounds observed in our study were oxygenated monoterpenes 1,8-cineole, camphor, trans-pinocamphone, cis-pinocamphone, terpinen-4-ol, and verbenone. In general, oxygenated monoterpenes are formed from nonoxygenated precursors by oxidation processes after exposure to the atmosphere or by the activity of microorganisms either present in the wood or transmitted by xylophagous insects (Leufvén et al. 1984, 1988; Leufvén and Nehls 1986; Hunt et al. 1989; Lindmark-Henriksson et al. 2003, 2004). Oxygenated monoterpenes camphor, trans-pinocamphone, and isopinocamphone were found among emanations released from I. typographus galleries created during spruce colonization, and in the surrounding bark (Leufvén and Birgersson 1987; Birgersson and Bergstrom 1989). Oxygenated monoterpenes can also be synthesized in the beetle gut via oxygenation of host monoterpenes as a part of bark beetle aggregation pheromones (Seybold et al. 2000). Trans-pinocamphone and isopinocamphone were found in headspace volatiles of I. typographus (Francke et al. 1995).
Many of spruce tree-trap terpenoids that elicited antennal responses in I. typographus are not exclusively specific for spruce, but are present in the fragrances of many other species of conifers as well as deciduous trees and plants (Knudsen et al. 1993; Sjödin et al. 2000; Faldt et al. 2001; Vrkočová et al. 2000; Dudareva et al. 2004). This indicates that very likely none of the identified compounds alone serves as a signature compound for host plant selection in I. typographus. Attraction to and recognition of appropriate host likely involves a suite of compounds released in particular ratios (Bruce et al. 2005) and perhaps in particular enantiomeric composition (Borg-Karlson et al. 1993). Our study show that except abundant spruce monoterpenes, also minor compounds are perceived by I. typographus antennae and thus likely participate in host discrimination. Given that some minor compounds are much more active then the major ones, their behavioral role in I. typographus host discrimination should be further investigated. The involvement of minor spruce compounds in I. typographus host recognition was previously considered only by two researchers. Tømmerås (1985) and Tømmerås and Mustaparta (1987) reported that bark emanations strongly stimulate certain olfactory receptor cells. Bark extraction, extract fractionation, and subsequent GC-ESG recording showed that important stimuli for these cells occur among minor compounds, which, however, remained unidentified due to their trace amounts (Tømmerås 1985; Tømmerås and Mustaparta 1987). Based on these observations, Tømmerås and Mustaparta (1987) speculated that since the major constituents are present in all trees, minor constituents may be those that convey important information about species specificity or host suitability. Recently, minor plant volatiles attract scientific attention, and number of reports implicates their role in attractant or deterrent roles in many insect–plant relationships (reviewed by McCormick et al. 2014).
Our data are in a good agreement with concurrent GC-EAD analysis of spruce volatiles (Schiebe 2012). In addition to compounds identified in our study, Schiebe observed responses to thujan-4-ol, styrene, and pinocarvone, which we have not observed. The discrepancy between the two studies might be due to differences in spruce varieties and methodology of volatile collection and GC–MS analysis. In addition, electrophysiological recordings from bark beetles antennae may provide distinct differences in measured activities. The different olfactory receptor cells located in different sensillar types on ventral side of I. typographus flattered terminal antennal segment have uneven distribution (Hallberg 1981; Andersson et al. 2009). Thus, the variation in the size and location of recording electrode may contribute to the observed variations in antennal activities. In spite of these minor variabilities, both studies provide clear evidence that in addition to major spruce monoterpenes, also minor compounds are perceived by I. typographus antennae and thus likely play an role in host discrimination. Electrophysiological studies, however, cannot provide information, whether antennally active compounds mediate attraction or repulsion, neither that all antennally active compounds must be necessarily involved in eliciting behavioral response from I. typographus. Nevertheless, identifying the range of host-associated volatiles that insects can detect represents an important step toward recognition of ecologically important compounds and understanding the role of olfaction in modulating insect behavior.
In majority of previous research focused on the behavioral reactions of I. typographus to spruce volatiles, only major spruce monoterpenes were tested and results have been inconclusive (Billings et al. 1976; Miller and Borden 1990, 2000; Erbilgin and Raffa 2000; Erbilgin et al. 2003, 2007; Saint-Germain et al. 2007; Coyne and Lott 1976; Raffa and Smalley 1995; Wallin and Raffa 2000; Byers 2012). From minor compound, verbenone which is thought to be formed by autooxidation and/or by microorganisms from α-pinene in the galleries of bark beetles possess significant repellent influence on several bark beetle genera including I. typographus (Byers and Wood 1980; Bakke 1981; Byers et al. 1989; Miller et al. 1995; Rudinsky et al. 1974; Lingren and Miller 2002; Schlyter et al. 1989) and regulates bark beetle aggregation and colonization (Byers 1989). Recently, behavioral roles of 1,8-cineole and camphor for I. typographus were investigated (Andersson et al. 2010). Thus, 1,8-cineole [repellent, toxic, and antifeedant compound for storage insect pests and mosquitoes, (Klocke et al. 1987; Sfara et al. 2009; Obeng-Ofori et al. 1997)], has been shown to inhibit attraction of I. typographus to its aggregation pheromone (Andersson et al. 2010). Camphor, on the other hand, enhances the pheromone attractiveness (Schlyter and Jakuš, personal communication). Camphor, isopinocamphone, terpinen-4-ol, and verbenone form the attractive kairomone blends for spruce bark beetles parasites Coeloides bostrichorum and Rhopalicus tutela (Sulivan and Berisford 2004).
Conclusion
The spruce trap-tree scent contains many substances that are not specific to spruce but are widespread among plants. The olfactory system of I. typographus perceives wide spectrum of these volatiles including those present in small quantities. Based on these observations, we hypothesize that I. typographus discriminates suitable host based on complex mixture of spruce volatiles where minor compounds play an important role.
References
Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry, 4th edn. Allured Publishing Corporation, Carol Stream
Andersson MN, Larsson MC, Schlyter F (2009) Specificity and redundancy in the olfactory system of the bark beetle Ips typographus: single-cell responses to ecologically relevant odors. J Insect Physiol 55:556–567
Andersson MN, Larsson MC, Blaženec M, Jakuš R, Zhang Q-H, Schlyter F (2010) Peripheral modulation of pheromone response by inhibitory host compound in a beetle. J Exp Biol 213:3332–3339
Angioni A, Barra A, Coroneo V, Dessi S, Cabras P (2006) Chemical composition, seasonal variability, and antifungal activity of Lavandula stoechas L. ssp. stoechas essential oils from stem/leaves and flowers. J Agric Food Chem 54:4364–4370
Austara O, Bakke A, Midtgaard F (1986) Response in Ips typographus to logging waste and synthetic pheromones. J Appl Entomol 101:194–198
Bakke A (1970) Evidence of population aggregating pheromone in Ips typographus (Coleoptera: Scolytidae). Contrib Boyce Thompson Inst Plant Res 24:309
Bakke A (1976) Spruce bark beetle Ips typographus pheromone production and field response to synthetic pheromones. Naturwissenschaften 63:92
Bakke A (1981) Inhibition of the response in Ips typographus to the aggregation pheromone; field evaluation of verbenone and ipsenol. Z Angew Entomol 92:172–177
Bakke A (1985) Deploying pheromone baited traps for monitoring Ips typographus populations. Z Angew Entomol 99:33–39
Bakke A (1989) The recent Ips typographus outbreaks in Norway: experiences from a control program. Holarct Ecol 12:515–519
Bilia AR, Flamini G, Taglioli V, Morelli I, Vincieri FF (2002) GC–MS analysis of essential oil of some commercial fennel teas. Food Chem 76:307–310
Billings RF, Gara RI, Hrutfiord BF (1976) Influence of ponderosa pine resin volatiles on the response of Dendroctonus ponderosae to synthetic trans-verbenol. Environ Entomol 5:171–179
Birgersson G, Bergstrom G (1989) Volatiles released from individual spruce bark beetle entrance holes quantitative variations during the first week of attack. J Chem Ecol 15:2465–2483
Bombosch S (1983) Considerations on the foundation of using pheromone traps for controlling the bark beetle Ips typographus. J Appl Entomol 96:242–247
Bombosch S, Johann M (1985) Notes on the host selection of Ips typographus. In: Johann M (ed) The role of the host in population dynamics of forest insects, Proceedings of Join Meeting of UIFRO S2-07-05 and S2-07-06, Victoria, BC, CA, Pacific Forest Research Centre, Canadian Forestry Service, pp 46–51
Borg-Karlson A-K, Lindström M, Norin T, Persson M, Valterová I (1993) Enantiomeric composition of monoterpene hydrocarbons in different tissues of Norway spruce, Picea abies (L.) Karst. A multidimensional gas-chromatography study. Acta Chem Scand 47:138–144
Bruce TJA, Wadhams LJ, Woodcock CM (2005) Insect host location: a volatile situation. Trends Plant Sci 10:269–274
Byers JA (1989) Chemical ecology of bark beetles. Experientia 45:271–283
Byers JA (1995) Host tree chemistry affecting colonization in bark beetles. In: Cardé RT, Bell WJ (eds) Chemical ecology of insects 2. Chapman and Hall, New York, pp 154–213
Byers JA (1996) An encounter rate model of bark beetle populations searching at random for susceptible host trees. Ecol Model 91:57–66
Byers JA (2004) Chemical ecology of bark beetles in a complex olfactory landscape. In: Lieutier F, Day KR, Battisti A, Grégoire J-C, Evans HF (eds) Bark and wood boring insects in living trees in Europe: a synthesis. Springer, Dordrecht, pp 89–134
Byers JA (2012) Bark beetles, Pityogenes bidentatus, orienting to aggregation pheromone avoid conifer monoterpene odors when flying but not when walking. Psyche J Entomol. doi:10.1155/2012/940962
Byers JA, Wood DL (1980) Interspecific inhibition of the response of the bark beetles Dendroctonus brevicomis and Ips paraconfusus, to their pheromones in the field. J Chem Ecol 6:149–164
Byers JA, Lanne BS, Löfqvist J, Schlyter F, Bergström G (1985) Olfactory recognition of host-tree susceptibility by pine shoot beetles. Naturwissenschaften 72:324–326
Byers JA, Lanne BS, Löfqvist J (1989) Host-tree unsuitability recognized by pine shoot beetles in flight. Experientia 45:489–492
Courtois JE, Chararas C, Debris MM (1961) Recherches preliminaires sur les glucidases presentes dans un coleoptere xylophage Ips typographus L. Bull Soc Chim Biol 43:698
Coyne JF, Lott LH (1976) Toxicity of substances in pine oleoresin to southern pine beetles. J Georgia Entomol Soc 11:301–305
Dudareva N, Pichersky E, Gershenzon J (2004) Biochemistry of plant volatiles. Plant Physiol 135:1893–1902
Erbilgin N, Raffa KF (2000) Opposing effects of host monoterpenes on responses by two sympatric species of bark beetles to their aggregation pheromones. J Chem Ecol 26:2527–2548
Erbilgin N, Powell JS, Raffa KF (2003) Effect of varying monoterpene concentrations on the response of Ips pini (Coleoptera: Scolytidae) to its aggregation pheromone: implications for pest management and ecology of bark beetles. Agric For Entomol 5:269–274
Erbilgin N, Krokene P, Kamme T, Christiansen E (2007) A host monoterpene influences Ips typographus (Coleoptera: Curculionidae, Scolytinae) responses to its aggregation pheromone. Agric For Entomol 9:135–140
Faldt J, Sjödin K, Persson M, Valterová I, Borg-Karlson A-K (2001) Correlations between selected monoterpene hydrocarbons in the xylem of six Pinus (Pinaceae) species. Chemoecology 11:97–106
Forsse E, Solbreck C (1985) Migration in the bark beetle Ips typographus duration timing and height of flight. Z Angew Entomol 100:47–57
Francke W, Bartels J, Meyer H, Schröder F, Kohnle U, Baader E, Vité JP (1995) Semiochemical from bark beetles: new results, remarks, and reflections. J Chem Ecol 21:1043–1063
Grégoire JC, Evans HF (2004) Damage and control of BAWBILT organisms: an overview. In: Lieutier F, Day KR, Battisti A, Grégoire J-C, Evans HF (eds) Bark and wood boring insects in living trees in Europe: a synthesis. Springer, Dordrecht, pp 19–37
Gries G, Nolte R, Sanders W (1989) Computer simulated host selection in Ips typographus. Entomol Exp Appl 53:211–217
Hallberg (1981) Antennal sensilla in I. typographus. Protoplasma 111:206–2014
Högnadóttir A, Rouseff RL (2003) Identification of aroma active compounds in orange essence oil using gas chromatography–olfactometry and gas chromatography–mass spectrometry. J Chromatogr A 998:201–211
Hunt DWA, Borden JH, Lindgren BS, Gries G (1989) The role of autoxidation of α-pinene in the production of pheromones of Dentroctonus ponderosae (Coleoptera: Scolytidae). Can J For Res 19:1275–1282
Juliani HR, Simon JE (2002) Antioxidant activity of basil. In: Janick J, Whipkey A (eds) Trends in new crops and new uses. ASHS Press, Alexandria, pp 575–579
Klimetzek D (1978) Ips typographus: Erhöhung der lockwirkung begifteter und unbegifteter Fangbäume durch synthetische Pheromone. Allg Forst Jagdztg 148:120–123
Klimetzek E, Kőhle J, Vité JP, Kohnle U (1986) Dosage response to ethanol mediates host selection by “secondary” bark beetles. Naturwissenschaften 73:270–272
Klocke JA, Darlington MV, Balandrin MF (1987) A mosquito feeding and oviposition repellent. J Chem Ecol 13:2131–2141
Knudsen JT, Tollsten L, Bergström G (1993) Floral scents: a checklist of volatile compounds isolated by head-space techniques. Phytochemistry 33:253–280
Kohnle U (1985) Investigations of chemical communication systems in secondary bark beetles. Z Angew Entomol 100:197–218
Leufvén A, Birgersson G (1987) Quantitative variation of different monoterpenes around galleries of Ips typographus (Coleoptera: Scolytidae) attacking Norway spruce. Can J Bot 65:1038–1044
Leufvén A, Nehls L (1986) Quantification of different yeasts associated with the bark beetle Ips typographus, during its attack on a spruce tree. Microb Ecol 12:237–243
Leufvén A, Bergström G, Falsen E (1984) Interconversion of verbenols and verbenone by identified yeasts isolated from the spruce bark beetle Ips typographus. J Chem Ecol 10:1349–1361
Leufvén A, Bergström G, Falsen E (1988) Oxygenated monoterpenes produced by yeasts, isolated from Ips typographus (Coleoptera: Scolytidae) and grown in phloem medium. J Chem Ecol 14:353–362
Lindelöw Å, Risberg B (1992) Attraction during flight of scolytids and other bark- and wood-dwelling beetles to volatiles from fresh and stored spruce wood. Can J For Res 22:224–228
Lindmark-Henriksson M, Isaksson D, Sjödin K, Högberg H-E, Vaněk T, Valterová I (2003) Transformation of pinene using a Picea abies suspension culture. J Nat Prod 66:337–343
Lindmark-Henriksson M, Isaksson D, Vaněk T, Valterová I, Högberg HE, Sjödin K (2004) Transformation of terpenes using a Picea abies suspension culture. J Biotechnol 107:173–184
Lingren BS, Miller DR (2002) Effect of verbenone on five species of bark beetles (Coleoptera: Scolytidae) in lodgepole pine forests. Environ Entomol 31:759–765
Martin DM, Tholl D, Gershenzon J, Bohlman J (2002) Methyl jasmonate induces traumatic resin ducts, terpenoid resin biosynthesis and terpenoid accumulation in developing xylem of Norway spruce stems. Plant Physiol 129:1003–1018
Martin DM, Gershenzon J, Bohlmann J (2003) Induction of volatile terpene biosynthesis and diurnal emission by methyl jasmonate in foliage of Norway spruce. Plant Physiol 132:1586–1599
Marzoug HNB, Romdhane M, Lebrihi A, Mathieu F, Couderc F, Abderraba M, Khouja ML, Bouajila J (2011) Eucalyptus oleosa essential oils: chemical composition and antimicrobial and antioxidant activities of the oils from different plant parts (stems, leaves, flowers and fruits). Molecules 16:1695–1709
McCormick AC, Gershenzon J, Unsicker SB (2014) Little peaks with big effects: establishing the role of minor plant volatiles in plant-insect interactions. Plant Cell Environ. doi:10.1111/pce.12357
McLafferty FW, Stauffer DB (1989) The Wiley/NBS registry of mass spectral data, vol 1–7. Wiley, New York
Miller DR, Borden JH (1990) β-Phellandrene: kairomone for pine engraver, Ips pini (Say) (Coleoptera, Scolytidae). J Chem Ecol 16:2519–2531
Miller DR, Borden JH (2000) Dose-dependent and species specific responses of pine bark beetles (Coleoptera: Scolytidae) to monoterpenes in association with pheromones. Can Entomol 132:183–195
Miller DR, Borden JH, Lindgren BS (1995) Verbenone: dose-dependent interruption of pheromone based attraction of three sympatric species of pine bark beetles (Coleoptera: Scolytidae). Environ Entomol 24:692–696
Moeck HA, Wood DL, Lindahl KQ (1981) Host selection behavior of bark beetles (Coleoptera: Scolytidae) attacking Pinus ponderosa, with special emphasis on the western pine beetle, Dendroctonus brevicomis. J Chem Ecol 7:49–83
Obeng-Ofori CH, Bekele RJ, Hassanali A (1997) Biological activity of 1,8-cineole, a major component of essential oil of ocimum kenyense (Ayobangira) against stored product beetles). J Appl Entomol 121:237–243
Person HL (1931) Theory in explanation of the selection of certain trees by the western pine beetle. J For 29:696–699
Persson M, Borg-Karlson A-K, Norin T (1993) Enantiomeric composition of six chiral monoterpene hydrocarbons in different tissues of Picea abies. Phytochemistry 33:303–307
Persson M, Sjödin K, Borg-Karlson A-K, Norin T, Ekberg I (1996) Relative amount and enantiomeric compositions of monoterpene hydrocarbons in xylem and needle of Picea abies. Phytochemistry 42:1289–1297
Pureswaran DS, Borden JH (2003) Test of semiochemical mediated host specificity in four species of tree killing bark beetles. Environ Entomol 32:963–969
Raffa KF, Smalley EB (1995) Interactions of pre-attack and induced monoterpene concentrations in host conifer defense against bark beetle-microbial complexes. Oecologia 102:285–295
Raty L, Drumont A, Dewindt N, Gregoire JC (1995) Mass trapping of the spruce bark beetle Ips typographus L.: traps or trap trees. For Ecol Manag 78:191–205
Rudinsky JA, Morgan ME, Libbey LM, Putnam TB (1974) Antiaggregative rivalry pheromone of the mountain pine beetle, and a new arrestant of the southern pine beetle. Environ Entomol 3:90–97
Saint-Germain M, Christopher MB, Drapeau P (2007) Primary attraction and random landing in host-selection by wood-feeding insects: a matter of scale? Agric For Entomol 9:227–235
Schiebe C (2012) Attraction and resistance in the Picea abies–Ips typographus system host choice in the Eurasian spruce bark beetle. Dissertation, Swedish University of Agricultural Sciences, Alnarp, Sweden
Schlyter F, Birgersson G, Leufvén A (1989) Inhibition of attraction to aggregation pheromone by verbenone and ipsenol density regulation mechanisms in bark beetle Ips typographus. J Chem Ecol 15:2263–2277
Schroeder LM, Lindelöw Ä (1989) Attraction of scolytids and associated beetles by different absolute amounts and proportions of α-pinene and ethanol. J Chem Ecol 15:807–817
Seybold SJ, Bohlmann J, Raffa KF (2000) Biosynthesis of coniferophagous bark beetle pheromones and conifer isoprenoids: evolutionary perspective and synthesis. Can Entomol 132:697–753
Sfara V, Zerba EN, Alzogaray RA (2009) Fumigant insecticidal activity and repellent effect of five essential oils and seven monoterpenes on first-instar nymphs of Rhodnius prolixus no access. J Med Entomol 46:511–515
Sjödin K, Schroeder LM, Eidmann HH (1989) Attack rates of scolytids and composition of volatile wood constituents in healthy and mechanically weakened pine trees. Scand J For Res 4:379–391
Sjödin K, Persson M, Fäldt J, Ekberg I, Borg-Karlson A-K (2000) Occurrence and correlations of monoterpene hydrocarbon enantiomers in Pinus sylvestris and Picea abies. J Chem Ecol 26:1701–1720
Sulivan BT, Berisford CW (2004) Semiochemicals from fungal associates of bark beetles may mediate host location behavior of parasitoids. J Chem Ecol 30:703–717
Švestka M, Hochmut R, Jančařík V (1996) Praktické metody v ochraně lesa (practical methods in forest protection, in Czech). Silva Regina, Prague. ISBN 80-902033-0-3
Tømmerås BÅ (1985) Specialization of the olfactory receptor cells in the bark beetle Ips typographus and its predator Thanasimus formicarius to bark beetle pheromones and host tree volatiles. J Comp Physiol A 157:335–341
Tømmerås BÅ, Mustaparta H (1987) Chemoreception of host volatiles in the bark beetle Ips typographus. J Comp Physiol A 16:705–710
Van Den Dool H, Kratz PD (1963) A generalization of the retention index system including linear temperature programmed gas–liquid partition chromatography. J Chromatogr 11:463–471
Vrkočová P, Valterová I, Vrkoč J, Koutek B (2000) Volatiles released from oak, a host tree for the bark beetle Scolytus intricatus. Biochem Syst Ecol 28:933–947
Wallin KF, Raffa KF (2000) Influences of host chemicals and internal physiology on the multiple steps of postlanding host acceptance behavior of Ips pini (Coleoptera: Scolytidae). Environ Entomol 29:442–453
Wood DL (1982) The role of pheromones, kairomones, and allomones in the host selection and colonization behavior of bark beetles. Ann Rev Entomol 27:411–446
Zhao T, Solheim H, Långström B, Borg-Karlson A-K (2011) Storm-induced tree resistance and chemical differences in Norway spruce (Picea abies). Ann For Sci 68:657–665
Acknowledgments
Grants FRVŠ 2954/G4, CIGA CZU 3109, NAZV QH81136, MA CR 0002070203, and financial support provided by the Academy of Sciences of the Czech Republic (subvention for development of research organization RVO: 61388963) are gladly acknowledged. We thank to J. Titzenthalerová for her skillful assistance in GC-EAD experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Jarmo Holopainen.
Rights and permissions
About this article
Cite this article
Kalinová, B., Břízová, R., Knížek, M. et al. Volatiles from spruce trap-trees detected by Ips typographus bark beetles: chemical and electrophysiological analyses. Arthropod-Plant Interactions 8, 305–316 (2014). https://doi.org/10.1007/s11829-014-9310-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-014-9310-7