Abstract
The temporal dynamics of foraging, diet, and use of space is essential to understand the ecology of harvester ants. Here, we present an account of the foraging ecology of Pogonomyrmex naegelii in Brazilian cerrado savanna. Nests occur on bare ground and contain 166–580 workers (N = 3 colonies). Colony activity is unimodal year-round and peaks around the middle of the day. Foragers leave the nest independently and individually search for food in all directions. Ants ventured up to 15 m from nests, with most foraging occurring within 2 m of nests. Colonies tended to have larger home ranges in the dry/cold (April–September) than in the wet/warm season (October–March). P. naegelii has a generalist and season-dependent diet comprised of many seed species and arthropod prey, and pieces of plant and animal matter. Foragers collected seeds from 34 plant species, predominantly grasses (genera Gymnopogon, Axonopus, Aristida). Over 6,700 seeds can be stored in nest granaries. Ants and termites were the main animal prey retrieved by P. naegelii. The proportion of seeds and arthropods foraged by P. naegelii changes year-round: in the dry/cold season, the diet is predominantly granivorous, whereas in the wet/warm season, seeds and arthropods are retrieved in more balanced proportions. Although food availability was not assessed, year-round diet of P. naegelii matches the pattern of seasonal abundance of grass seeds and arthropod prey in cerrado. Data on harvester ants come mostly from arid habitats; this study is a first assessment of the ecology of a Neotropical Pogonomyrmex from a moderately moist savanna environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability with which animals use space and food resources determines to a great extent their survival, growth, and reproduction (Alcock 2009). Since ecosystems are not spatially or temporally homogeneous, successful organisms must be able to cope with environmental variability by responding appropriately to changing conditions (Stephens and Krebs 1987). Ants always bring their forage products (entire or in parts) to the nest for storage, consumption, or feeding the brood, and as central-place foragers they tend to concentrate their foraging activities around the nest location (Carroll and Janzen 1973; Traniello 1989). In tropical environments, primary production normally fluctuates in accordance with seasonal patterns in rainfall and this in turn may markedly affect insect herbivores, including ants (Janzen and Schoener 1968; Wolda 1988; Hölldobler and Wilson 1990). As consumers of plant-derived resources, ants must track primary production in space and time by adjusting their foraging behavior and diet accordingly (e.g., Carroll and Janzen 1973; Crist and MacMahon 1992; Díaz-Castelazo et al. 2004; Pol et al. 2011).
Ants interact with plants in a variety of ways, and indeed the numerous mutualistic and antagonistic relationships between ants and plants have made enormous contributions to our understanding of biological communities (MacMahon et al. 2000; Wirth et al. 2003; Rico-Gray and Oliveira 2007). Ants are usually attracted to plants through plant-derived food resources, and the main rewards directly provided by plants to ants are nectar, food bodies, pollen, fruits, and seeds (see Rico-Gray and Oliveira 2007; and included references). Seeds are rich in lipids and proteins and have a high nutritional value, which make them the preferred food item by a diversity of animals such as mammals, birds, and insects (Janzen 1971; Brown et al. 1979). Seed predation by ants has been widely documented, and the so-called harvester ants may encompass over 150 species worldwide, occurring more frequently in temperate and tropical habitats with semiarid to arid vegetation (Hölldobler and Wilson 1990; Taber 1998). Nearly half of the harvester ant species belong to the genus Pogonomyrmex and most of the knowledge about their natural history, ecology and behavior come from studies of North American species (e.g., Cole 1968; Hölldobler 1974, 1976; Taber 1998; Johnson 2000, 2001; MacMahon et al. 2000; Gordon 2010). Most studied species are strict granivores that harvest large quantities of seeds of a few preferred species (Whitford 1978; Johnson 2000; MacMahon et al. 2000 and included references). Harvester ants, however, may also take live prey and frequently scavenge for dead arthropods (MacKay 1981; Hölldobler and Wilson 1990; Taber 1998).
Studies on the ecology of South American Pogonomyrmex have focused mostly on a few species from Argentina (e.g., Pol and Lopez-de-Casenave 2004; Pirk et al. 2009a, b; Pol et al. 2011) and Colombia (Kugler and Hincapié 1983; Kugler 1984). Kusnezov (1951) was the first to comment about taxonomy and distribution of Pogonomyrmex ants of Argentina, Chile, Bolivia, Paraguay, and Brazil, but indeed, very little is known about the natural history, ecology, and behavior of South American harvester ants. The development of models on ant foraging is hampered by the small amount of field data on basic ecological features and foraging behavior of different ant taxa, and this is especially noticeable in the tropical region where ants are especially diverse and dominant (Brown 2000). The study of the temporal dynamics of foraging activity, diet, and use of space is essential to understand the ecology of harvester ants (e.g., Hölldobler 1976; Crist and MacMahon 1991a; Gordon 1995; Wilby and Shachak 2000). Here, we present a detailed field account of the natural history and foraging ecology of Pogonomyrmex naegelii, which commonly occurs in the Brazilian “cerrado” savanna (Belchior 2010), as well as in the Amazon and Atlantic rain forests (Kempf 1972; Taber 1998). Specifically, we provide data on colony composition and nest structure, and investigate colony activity rhythm, diet, and home range in different seasonal contexts.
Methods
Study site
Field work was carried out between May 2008 and June 2009 in the cerrado reserve (127 ha) at the Clube de Caça e Pesca Itororó, Uberlândia, State of Minas Gerais, southeast Brazil (18°59′S, 48°18′W). The vegetation consists of a scrub of shrubs and trees with a fair amount of herbaceous plants, which corresponds to a cerrado sensu stricto (Oliveira-Filho and Ratter 2002). The climate of the region is the Aw type of Köppen’s system, consisting of well-defined rainy and dry seasons. A wet/warm season occurs from October to March (rainfall, 270 ± 50 mm; temperature, 23 ± 5 °C), and a dry/cold season from April to September (22 ± 20 mm; 19 ± 3 °C; daily data from the climatological station of the Universidade Federal de Uberlândia; additional details in Réu and Del-Claro 2005).
Activity patterns and nest structure
Fifteen nests of Pogonomyrmex naegelii were tagged in the study site (≥4 m apart from each other). Previous field observations indicated that colonies did not present any nocturnal activity, and by 1900 hours the nest entrances were already sealed with twigs and pebbles (Fig. 1). Colony activity rhythms in four nests (colonies # 1–4) were monitored during the dry/cold (June) and wet/warm season (December), from 7.00 to 19.00 h. Samplings consisted of counting continuously all ants exiting or entering the nests during 10 min of every hour (each colony was sampled on a different day, always by the same observer and using all occurrence sample; sensu Altmann 1974). Simultaneously, we recorded the air temperature and humidity (20 cm above the nest entrance).
Vertical section through chambers and connecting galleries of a Pogonomyrmex naegelii nest in the Brazilian cerrado savanna. The profile is based on a nest excavated in August 2008 and shows the typical subterranean architecture. The colony (# 13) contains 479 workers, and a total of 6729 seeds from 18 species were found in the granaries. See also Fig. 5
To obtain data on nest structure and composition, three nests of P. naegelii were excavated in the dry/cold season (colonies # 13–15). All seeds found inside nest chambers were collected and conserved dry in labeled tubes for further identification and were weighted in the laboratory. Dry fruits producing only one seed, such as caryopses or achenes, were considered seeds (see Whitford 1978; Pirk and Lopez-de-Casenave 2006).
Diet and colony home ranges
To evaluate the diet of P. naegelii colonies, 10 tagged nests were each monitored during 1 h per month at the peak of their activity (determined as described above), between June 2008 and May 2009 (total of 120 h of observation). Food items retrieved by workers were collected by removing them from the mandibles of returning foragers and were conserved in 70 % alcohol for further identification. To avoid disturbance of ant foragers, no food item was collected during sessions monitoring the daily activity rhythm of ant colonies (see above).
To determine the foraging ranges of P. naegelii colonies, we visually monitored marked foragers of three of the colonies (# 7, # 8, and # 11) used for the investigation of diet. All individuals encountered outside the nests (26 ± 9 individuals per nest; mean ± SD; N = 3 nests) were individually marked with powdered food coloring (Mix®) on the thorax and gaster, using a distinct color for each tagged colony. Colony foraging ranges were assessed by following marked individuals as they departed from the nest entrance and by recording the maximal distance they had walked before returning to the nest. A small numbered flag was placed at the maximal distances achieved by different ant foragers, and the respective data points were recorded on a gridded map of the study plot. Colony home ranges were assessed based on the cumulative data of maximal distances achieved by the foragers of each monitored colony. Foraging areas were estimated as convex polygons created by connecting the outermost points at which workers were seen. Colonies were monitored on non-consecutive days during the dry/cold (June–July 2008) and wet/warm season (December 2008–January 2009), mainly at the peak hours of their activity (see below). Ant voucher specimens are deposited in the Museu de Biodiversidade do Cerrado of the Universidade Federal de Uberlândia (MBC), and in the Museu de Zoologia da Universidade de São Paulo (MZUSP).
Results
All nests of Pogonomyrmex naegelii were located on bare ground of cerrado savanna, inhabited by scattered woody vegetation and few grass and forb species. Nests presented one or two inconspicuous entrances that are closed at dusk with dry leaves, twigs, or pebbles and opened early morning (Fig. 1). Nest openings were 0.4–1.5 cm in diameter (0.72 ± 0.32; mean ± SD; N = 12); their locations changed by 15–130 cm through time in seven tagged nests. Excavated nests had five to seven interconnected oval chambers located 3–70 cm beneath the ground surface. Upper chambers together contained numerous twigs and over 3,800 seeds per nest (3,833.7 ± 3,444.1, mean ± SD; N = 3); the single queen and immature stages were found in the deepest chamber (Fig. 1). The number of workers in each of the three excavated colonies was 166, 479, and 580, respectively. The workers are monomorphic, with body length of 0.52 ± 0.04 (mean ± SD; N = 30 workers).
Colony activity pattern in Pogonomyrmex naegelii is typically diurnal with a clear unimodal distribution of daily foraging movements throughout the year (Fig. 2). The peak of external activities, however, shifts from 1100 to 1400 hours in the dry/cold season to 1300–1600 hours in the wet/warm period. In both seasons, the activity rhythm was positively associated with temperature (Dry season: r s = 0.59; p < 0.01; Wet season: r s = 0.69; p < 0.01) and negatively associated with humidity (Dry season: r s = -0.50; p < 0.01; Wet season: r s = −0.46; p < 0.01) (see Fig. 2). Colony activity normally increased on days following a rain because more workers engaged in digging activity (removal of soil particles from the nest), and dead arthropods can be more easily found in the vicinity of the nest.
Daily and seasonal variation in the foraging activity of ground-dwelling Pogonomyrmex naegelii colonies in the Brazilian cerrado savanna. The activity of four ant colonies was evaluated once during a the dry/cold season (June) and once during b the wet/warm season (December). Air temperature and humidity were registered simultaneously with the ant activity measurements. Data are means ± 1 SE (N = 4)
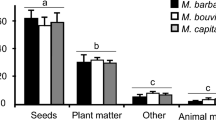
Foraging activities of P. naegelii were exclusively on the ground, irrespective of the presence of leaf litter or plant cover. Data from 10 monitored nests indicate that P. naegelii has a generalist and season-dependent diet comprised of many seed species and arthropod prey (dead and alive), as well as pieces of plant and animal matter (Table 1). Altogether, ant foragers collected seeds (N = 780) from a total of 27 plant species (seed weight 0.1–5.2 mg), with a high proportion of the grasses Gymnopogon spicatus, Axonopus barbiger, and Aristida riparia (seed weight 0.1–0.7 mg, N = 639). These three grass species accounted for 73 % of the food items collected in the dry/cold season (N = 742 items), and only 24 % in the wet/warm season (N = 404 items). Species richness of seeds in the diet, however, was similar throughout the year; 18 seeds species were collected in the dry period against 21 in the wet period. Ants and termites were the main arthropods captured by P. naegelii, comprising respectively 54 and 15 % of the animal prey brought to the nests as entire food items or as insect parts (N = 273; Table 1). In the dry/cold season, ants and termites comprised together 9 % (64/742) of the food brought by ant foragers, rising to 31 % (125/404) in the wet/warm season (Table 1). Overall, the proportion of seeds and arthropods collected by P. naegelii foragers changed throughout the year, with a marked predominance of seeds in the dry/cold season and a more balanced diet in the wet/warm period (G = 216.83; df = 3; p < 0.0001; see Fig. 3). This marked seasonal change from a predominantly granivorous diet to a mixed diet of seeds and arthropods is illustrated for four individual colonies in Fig. 4.
Frequency distribution of different types of food items retrieved by foragers of ten colonies of Pogonomyrmex naegelii from June 2008 to May 2009, in the Brazilian cerrado savanna. Each colony was monitored during 1 h per month. The relative proportions of different food types differ significantly between seasons (G = 216.83; df = 3; P < 0.0001). See also Fig. 4
Relative proportion of seeds and arthropod prey among the food items retrieved by foragers of Pogonomyrmex naegelii colonies from June 2008 to May 2009, in the Brazilian cerrado savanna. Each colony was monitored during 1 h per month. Non-seed plant items and unidentified animal prey were retrieved in very small quantities and are not shown. See also Fig. 3
The data from two colonies (# 13, # 14) excavated in the dry/cold season revealed large quantities of seeds from the grass species Axonopus barbiger and Melinis minutiflora, and lower numbers of several other seed species (Fig. 5). A third excavated colony of 166 workers (colony # 15) contained in its chambers only 25 seeds of the grass Panicum cervicatum. Taken as a whole, pooled data of seed-harvesting foragers (Table 1) and of stored seeds in excavated colonies (Figs. 1,5) produced a total of 34 seed species collected by P. naegelii over 1 year. Inside the nests we found grass caryopses with and without bracts. Harvester ants may keep the seeds intact in the nest until consumption, when bracts are removed and the seeds eaten (see also Pirk and Lopez-de-Casenave 2006). No seed species collected by P. naegelli possessed an elaiosome (i.e., nutritious seed appendage typical of specialized ant-dispersed species known as myrmecochores; see Beattie 1985).
The foraging home ranges of the three monitored colonies of P. naegelii are shown in Fig. 6. Ants ventured up to 15 m from nests, with mean foraging distances ranging from 0.8 to 2.3 m and greatest foraging activity (>80 %) within 2 m of nests (Fig. 6). The foraging maps are presented separately for each season and also overlapping to better illustrate temporal variation in the foraging areas used by the colonies. All colonies tended to have larger home ranges in the dry/cold than in wet/warm season, and the direction of the foraging terrains could also change considerably across seasons (Fig. 6). There is no evidence of recruitment among workers of P. naegelii. Foragers left the nest independently and individually searched for food in all directions. On rare occasions, two workers were observed interacting during transportation of a large food item.
Seasonal variation of the home ranges of three Pogonomyrmex naegelii colonies in the Brazilian cerrado savanna. Maps are based on maximal distances achieved by individual foragers (filled circles) relative to the nest entrance (cross). The convex polygons for each colony were drawn by connecting the outermost points at which workers were seen (open circles). Estimated values of foraging areas are in bold; mean (±1SE) foraging distances and ranges are also shown for each colony in each season. Overlaps illustrate the seasonal variation in the foraging areas of the colonies
Discussion
The daily activity rhythm is regarded as a distinctive trait among species of ants (Hölldobler and Wilson 1990). The terrestrial locomotion of ant foragers and their tiny bodies make them vulnerable to rapid heat loss, and outside the nest, their body temperature is almost entirely dictated by the physical environment (Heinrich 1993). By deciding when to be active, ants can adjust their foraging requirements with their physiological limitations. In addition to temperature (Cerdá et al. 1998), other factors such as relative humidity (Briese and MacAuley 1980), soil moisture (Levings and Windsor 1984), and food availability (Bernstein 1974) are also regarded as important in mediating foraging costs and causing diurnal and seasonal shifts in ant activity schedules (Carroll and Janzen 1973; Traniello 1989).
All harvester ants forage during the day, but some species may also hunt at night (Taber 1998). The extreme temperatures of arid regions cause severe environmental stress in harvester ants (Whitford and Ettershank 1975). Thus, the commonest daily activity pattern in hot and dry climates is bimodal, with one peak in the morning and another in the afternoon or early evening (Hölldobler and Wilson 1990; MacMahon et al. 2000). This pattern, however, may change seasonally depending on temperature and humidity. For instance, the desert Argentine species Pogonomyrmex pronotalis and P. rastratus (Pol and Lopez-de-Casenave 2004) and the North American P. occidentalis of Wyoming shrub-steppes (Crist and MacMahon 1991b) show a bimodal activity pattern during summer (morning and afternoon) and a unimodal pattern during spring and autumn months (midday). Even tropical species that forage year-round may alter markedly their activity patterns following seasonal changes in surface temperatures: with the onset of the rainy and cooler season in Colombia, Pogonomyrmex mayri shifts summer bimodal rhythm toward a unimodal activity peak at midday (Kugler 1984).
The year-round unimodal foraging activity of Pogonomyrmex naegelii around midday in cerrado savanna confirms field accounts of other harvester species living in cooler and moister habitats (e.g., Onoyama 1982). As opposed to cold temperate or hot arid environments whose extreme climatic conditions constrain ant activity (see Hölldobler and Wilson 1990), seasonality patterns in the tropical cerrado savanna are not severe enough to interrupt external activities by the ants (Marques and Del-Claro 2010). Indeed, P. naegelii colonies actively foraged year-round at daytime periods of mean air temperatures ranging from 22 to 34 °C in the wet/warm season, and from 22 to 29 °C in the dry/cold season (Fig. 2), as also documented for other ant species in cerrado savanna (e.g., Yamamoto and Del-Claro 2008). This pattern differs markedly from Pogonomyrmex species in Argentina desert that alternate unimodal (midday) and bimodal (morning and afternoon) activity patterns (Pol and Lopez-de-Casenave 2004).
Rains are seasonally discontinuous in the cerrado savanna and produce marked temporal heterogeneity in primary production and plant-derived food resources (Batalha and Mantovani 2000; Franco 2002). Indeed, species-specific patterns in plant phenology associated with rainfall levels can markedly affect year-round abundance and consumption patterns by insects in cerrado, including ants (Del-Claro and Oliveira 1999; Marquis et al. 2002; Yamamoto and Del-Claro 2008; Marques and Del-Claro 2010; Silva and Oliveira 2010). Most seed-harvesting ants, however, are also excellent hunters and almost all are even better scavengers (Hölldobler and Wilson 1990; Taber 1998). Vertebrate carcasses, arthropods (dead and alive, entire or in parts), snails, aphid honeydew, and feces are among the many sorts of animal food consumed by Pogonomyrmex ants (Taber 1998; and included references). Our results show that the diet of P. naegelii is flexible enough to allow year-round high foraging activity levels in cerrado savanna, with a clear seasonal influence on the assortment of retrieved food. While in the dry/cold season colonies maintained a mostly granivorous diet with seeds comprising about 80 % of retrieved food items, in the wet/warm period P. naegelii turned more generalist and consumed both seeds and arthropod prey in more balanced proportions (Figs. 3,4). Seasonal variation in the diet entailing the consumption of non-seed items has been observed in a number of harvester species, including Pogonomyrmex (e.g., Whitford 1978; MacKay 1981; Mehlhop and Scott 1983; Pirk and Lopez-de-Casenave 2006; Pol et al. 2011). Few studies, however, have shown quantitatively such a marked seasonal shift in granivory and carnivory as recorded for P. naegelii (Tevis 1958; Whitford et al. 1976; Kugler and Hincapié 1983; Pirk et al. 2009a). Our study is the first to show such a clear seasonal change in diet for a harvester species living in a moderately moist Neotropical savanna (see Oliveira-Filho et al. 1989).
Seasonal dietary shifts in harvester ants are normally attributed to fluctuations in the abundance of preferred seed species; when these become scarce, the ants turn to a more generalist diet and consume less desirable seeds and non-seed food items, including invertebrate prey (Tevis 1958; Davidson 1982; Crist and MacMahon 1992; Pol et al. 2011). Although we did not evaluate food availability across seasons, there is evidence that the year-round pattern of food retrieval by P. naegelii matches the seasonal abundance of grass seeds and arthropod prey in cerrado savanna. A predominantly granivorous diet in the dry/cold season corresponds with the peak of grass seed production in cerrado, particularly by the taxa most consumed by P. naegelii—Gymnopogon, Axonopus, and Aristida (Batalha and Mantovani 2000; see Table 1). A mixed granivorous/carnivorous diet in the wet/warm period, on the other hand, corresponds with the increased abundance of soil-dwelling insects in our study site, including ants and termites that encompass the most foraged prey by P. naegelii (Marques and Del-Claro 2010; see Table 1).
Our data from returning foragers and stored seeds in excavated nests of P. naegelii (Table 1, Fig. 5) corroborate numerous other studies reporting that grasses head the list of most consumed species by harvester ants (Whitford et al. 1976; Taber 1998; MacMahon et al. 2000; Pirk et al. 2009a; Pol et al. 2011; and included references). Indeed, a number of attributes may account for the prevalence of grass seeds in the diet of harvester ants, including their predictability in space and time, absence of secondary compounds, as well as adequate morphology and small size facilitating transport (see Carroll and Janzen 1973; Pulliam and Brand 1975; Crist and MacMahon 1992; MacMahon et al. 2000; Pirk and Lopez-de-Casenave 2006).
The preponderance of ants and termites among the prey retrieved by P. naegelii in cerrado corroborates other studies on more generalist harvester species (Tevis 1958; Whitford et al. 1976; Kugler and Hincapié 1983; Pirk et al. 2009b). Indeed, ants and termites are among the most dominant arthropods of tropical forests and savannas (Fittkau and Klinge 1973; Dejean et al. 1986), and are among the most abundant ground-dwelling insects of the cerrado savanna (Gontijo and Domingos 1991; Andrade et al. 2007). Although P. naegelii was capable of subduing small injured arthropods, individual foragers were usually timid toward live prey (see also Kugler and Hincapié 1983). The scavenging of arthropod corpses was especially frequent after rains, when increased numbers of dead prey (mostly ants and termites) were found on the floor of cerrado (see also Hölldobler and Wilson 1990; Taber 1998).
Spatial foraging patterns recorded in P. naegelii were similar to those documented for other species of Pogonomyrmex: ants ventured 0.4–15 m from nests, with most activity occurring within 2 m of nests (Fig. 6). North American harvester ants have been recorded up to 40 m away from nests, but greatest foraging normally takes place within 10 m of nests (Hölldobler 1976; Rissing 1981; Crist and MacMahon 1991a; Gordon 1992; MacMahon et al. 2000). Likewise, although Argentine harvester species may travel up to 20 m to gather food, over 80 % of the foragers concentrate within 7 m around the nest (Pol et al. 2011).
Harvester ants may change their home range on a seasonal basis or in subsequent years (see Hölldobler 1974, 1976; Davidson 1977; Gordon 1995, 2010). Because the distribution of food resources on the ground can change widely in space and time, ground-dwelling ants should increase their foraging efficiency by continual sampling adjacent areas instead of concentrating entirely on the most recent successful foraging location (Stephens and Krebs 1987; Traniello 1989). For now, we can only infer that the tendency of P. naegelii colonies to decrease their foraging areas in the wet/hot season may be related with a greater abundance of nearby arthropod prey (especially ants) in this period (see Tizo-Pedroso and Del-Claro 2007; Marques and Del-Claro 2010). It is also possible that the large numbers of seeds stored in P. naegelli nests (Figs. 1, 5) may help maintain the colonies at times of short seed supply in cerrado, as shown for other harvester ants (Rissing 1986; Pirk et al. 2009a).
This study is a first assessment of the foraging dynamics of P. naegelii colonies. Clearly, additional investigation into seasonal availability of plant and animal food, nutritional quality and morphology of seeds, role of seed storage, behavioral interactions between neighboring colonies, and forager movements (linked with vegetation structure) is needed before we can properly understand the mechanisms underlying the foraging ecology of P. naegelii in cerrado savanna. Although a range of direct and indirect effects from harvester ants on North American communities and ecosystems have already been reported (see MacMahon et al. 2000), field work with South American species is hitherto mostly restricted to Argentine desert species (Marone et al. 2000). In cerrado savanna, ant-generated top-down effects have already been evidenced for exudate-feeding (Del-Claro and Torezan-Silingardi 2009), leaf-cutting (Costa et al. 2008), and frugivorous ants (Christianini and Oliveira 2009). The potential effect of seed-harvesting by P. naegelii on vegetation, however, is yet to be assessed.
References
Alcock J (2009) Animal behavior: an evolutionary approach. Sinauer Associates, Inc., Sunderland
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 49:227–267
Andrade T, Marques GDV, Del-Claro K (2007) Diversity of ground dwelling ants in cerrado: an analysis of temporal variations and distinctive physiognomies of vegetation (Hymenoptera: Formicidae). Sociobiology 50:1–14
Batalha MA, Mantovani W (2000) Reproductive phenological patterns of cerrado plant species at the Pé-de-Gigante reserve (Santa Rita do Passa Quatro, SP, Brazil): a comparison between the herbaceous and woody floras. Rev Brasil Biol 60:129–145
Beattie AJ (1985) The evolutionary ecology of ant-plant mutualisms. Cambridge University Press, Cambridge
Belchior C (2010) Ecologia, comportamento e história natural da formiga ceifeira Pogonomyrmex naegelii (Formicidae, Myrmicinae): ritmo biológico, dieta, área de vida, estrutura e demografia dos ninhos. Master’s Dissertation, Universidade Federal de Uberlândia, Brasil
Bernstein RA (1974) Seasonal food abundance and foraging activity in some desert ants. Am Nat 108:490–498
Briese DT, Macauley BJ (1980) Temporal structure of an ant community in semi-arid Australia. Aust J Ecol 5:121–134
Brown WL (2000) Diversity of ants. In: Agosti D, Majer JD, Alonso LE, Schultz TR (eds) Ants: standard methods for measuring and monitoring biodiversity. Smithsonian Institution Press, Washington, DC, pp 45–79
Brown JH, Reichman OJ, Davidson DW (1979) Granivory in desert ecosystems. Annu Rev Ecol Syst 10:201–227
Carroll CR, Janzen DH (1973) Ecology of foraging by ants. Annu Rev Ecol Syst 4:231–257
Cerdá X, Retana J, Cros S (1998) Critical thermal limits in Mediterranean ant species: trade-off between mortality risk and foraging performance. Funct Ecol 12:45–55
Christianini AV, Oliveira PS (2009) The relevance of ants as seed rescuers of a primarily bird-dispersed tree in the neotropical cerrado Savanna. Oecologia 160:735–745
Cole AC Jr (1968) Pogonomyrmex harvester ants: a study of the genus in North America. The University of Tennessee Press, Knoxville
Costa AN, Vasconcelos HL, Vieira-Neto EHM, Bruna EM (2008) Do herbivores exert top-down effects in Neotropical savannas? Estimates of biomass consumption by leaf-cutter ants. J Veg Sci 19:849–854
Crist TO, MacMahon JA (1991a) Individual foraging components of harvester ants: movement patterns and seed patch fidelity. Insect Soc 38:379–396
Crist TO, MacMahon JA (1991b) Foraging patterns of Pogonomyrmex occidentalis (Hymenoptera: Formicidae) in a shrub-steppe ecosystem: the roles of temperature, trunk trails, and seed resources. Environ Entomol 20:265–275
Crist TO, MacMahon JA (1992) Harvester ant foraging and shrub-steppe seeds: interactions of seed resources and seed use. Ecology 73:1768–1779
Davidson DW (1977) Foraging ecology and community organization in desert seed-eating ants. Ecology 58:725–737
Davidson DW (1982) Seed utilization by harvester ants. In: Buckley RC (ed) Ant–plant interactions in Australia. Springer, Berlin, pp 1–6
Dejean A, Masens D, Kanika K, Nsudi M, Gunumina R (1986) Les termites et les fourmis, animaux dominants de la faune du sol de plusieurs formations forestières et herbeuses du Zaïre. Actes Coll Insect Soc 3:273–283
Del-Claro K, Oliveira PS (1999) Ant–homoptera interactions in a Neotropical savanna: the honeydew-producing treehopper, Guayaquila xiphias (Membracidae), and its associated ant fauna on Didymopanax vinosum (Araliaceae). Biotropica 31:135–144
Del-Claro K, Torezan-Silingardi HM (2009) Insect–plant interactions: new pathways to a better comprehension of ecological communities in Neotropical savannas. Neotr Entomol 38:159–164
Díaz-Castelazo C, Rico-Gray V, Oliveira PS, Cuautle M (2004) Extrafloral nectary-mediated ant–plant interactions in the coastal vegetation of Veracruz, Mexico: richness, occurrence, seasonality and ant foraging patterns. Ecoscience 11:472–481
Fittkau EJ, Klinge H (1973) On biomass and trophic structure of the central Amazonian rain forest ecosystem. Biotropica 5:2–14
Franco AC (2002) Ecophysiology of woody plants. In: Oliveira PS, Marquis RJ (eds) The Cerrados of Brazil: ecology and natural history of a Neotropical savanna. Columbia University Press, New York, pp 178–197
Gontijo TA, Domingos DJ (1991) Guild distribution of some termites from cerrado vegetation in south-east Brazil. J Trop Ecol 7:523–529
Gordon DM (1992) How colony growth affects forager intrusion in neighboring harvester ant colonies. Behav Ecol Sociobiol 31:417–427
Gordon DM (1995) The development of an ant colony’s foraging range. Anim Behav 49:649–659
Gordon DM (2010) Ant encounters: interaction networks and colony behavior. Primers in complex systems. Princeton University Press, Princeton
Heinrich B (1993) The hot-blooded insects: strategies and mechanisms of thermoregulation. Harvard University Press, Cambridge
Hölldobler B (1974) Home range orientation and territoriality in harvesting ants. Proc Nat Acad Sci USA 71:3274–3277
Hölldobler B (1976) Recruitment behavior, home range orientation and territoriality in harvester ants, Pogonomyrmex. Behav Ecol Sociobiol 1:3–44
Hölldobler B, Wilson EO (1990) The ants. Harvard University Press, Cambridge
Janzen DH (1971) Seed predation by animals. Annu Rev Ecol Syst 2:465–492
Janzen DH, Schoener TW (1968) Differences in insect abundance and diversity between wetter and drier sites during a tropical dry season. Ecology 49:96–110
Johnson RA (2000) Seed-harvester ants (Hymenoptera: Formicidae) of North America: an overview of ecology and biogeography. Sociobiology 36:89–122
Johnson RA (2001) Biogeography and community structure of North American seed-harvester ants. Annu Rev Entomol 46:1–29
Kempf WW (1972) Catálogo abreviado das formigas da região neotropical. Stud Entomol 15:3–344
Kugler C (1984) Ecology of the ant Pogonomyrmex mayri: foraging and competition. Biotropica 16:227–234
Kugler C, Hincapié MDC (1983) Ecology of the ant Pogonomyrmex mayri: distribution, abundance, nest structure, and diet. Biotropica 15:190–198
Kusnezov N (1951) El género Pogonomyrmex Mayr (Hym., Formicidae). Acta Zool Lilloana 11:227–333
Levings SC, Windsor DM (1984) Litter moisture content as a determinant of litter arthropod distribution and abundance during the dry season on Barro Colorado Island, Panama. Biotropica 16:125–131
MacKay WP (1981) A comparison of the nest phenologies of three species of Pogonomyrmex harvester ants. Psyche 88:25–74
MacMahon JA, Mull JF, Crist TO (2000) Harvester ants (Pogonomyrmex spp.): their community and ecosystem influences. Annu Rev Ecol Syst 31:265–291
Marone L, Lopez-de-Casenave J, Cueto VR (2000) Granivory in southern South American deserts: conceptual issues and current evidence. Bioscience 50:123–132
Marques GDV, Del-Claro K (2010) Sazonalidade, abundância e biomassa de insetos de solo em uma reserva de cerrado. Rev Bras Zooc 12:141–150
Marquis RJ, Morais HC, Diniz IR (2002) Interactions among cerrado plants and their herbivores: unique or typical? In: Oliveira PS, Marquis RJ (eds) The Cerrados of Brazil: ecology and natural history of a Neotropical savanna. Columbia University Press, New York, pp 306–328
Mehlhop P, Scott NJ (1983) Temporal patterns of seed use and availability in a guild of desert ants. Ecol Entomol 8:69–85
Oliveira-Filho AT, Ratter JA (2002) Vegetation physiognomies and woody flora of the cerrado biome. In: Oliveira PS, Marquis RJ (eds) The cerrados of Brazil: ecology and natural history of a Neotropical savanna. Columbia University Press, New York, pp 91–120
Oliveira-Filho AT, Shepherd GJ, Martins RF, Stubblebine WH (1989) Environmental factors affecting physiognomical and floristic variations in a cerrado of central Brazil. J Trop Ecol 5:413–431
Onoyama K (1982) Foraging behavior of the harvester ant Messor aciculatus, with special reference to foraging sites and diel activity of individual ants. Jap J Ecol 32:383–393
Pirk GI, Lopez-de-Casenave J (2006) Diet and seed removal rates by the harvester ants Pogonomyrmex rastratus and Pogonomyrmex pronotalis in the central Monte desert, Argentina. Insect Soc 53:119–125
Pirk GI, Lopez-de-Casenave J, Pol RG, Milesi FA, Marone L (2009a) Influence of temporal fluctuations in seed abundance on the diet of harvester ants (Pogonomyrmex spp.) in the central Monte desert, Argentina. Austral Ecol 34:908–919
Pirk GI, di Pasquo F, Lopez-de-Casenave J (2009b) Diet of two sympatric Pheidole spp. ants in the central Monte desert: implications for seed–granivore interactions. Insect Soc 56:277–283
Pol R, Lopez-de-Casenave J (2004) Activity patterns of harvester ants Pogonomyrmex pronotalis and Pogonomyrmex rastratus in the central Monte desert, Argentina. J Ins Behav 17:647–661
Pol RG, Lopez-de-Casenave J, Pirk GI (2011) Influence of temporal fluctuations in seed abundance on the foraging behaviour of harvester ants (Pogonomyrmex spp.) in the central Monte desert, Argentina. Austral Ecol 36:320–328
Pulliam HR, Brand MR (1975) The production and utilization of seeds in plains grassland of southeastern Arizona. Ecology 56:1158–1167
Réu WF, Del-Claro K (2005) Natural history and biology of Chlamisus minax Lacordaire (Chrysomelidae; Chlamisinae). Neotrop Entomol 34:357–362
Rico-Gray V, Oliveira PS (2007) The ecology and evolution of ant–plant interactions. University of Chicago Press, Chicago
Rissing SW (1981) Foraging specializations of individual seed harvester ants. Behav Ecol Sociobiol 9:149–152
Rissing SW (1986) Indirect effects of granivory by harvester ants: plant species, composition and reproductive increase near ant nests. Oecologia 68:231–234
Silva DP, Oliveira PS (2010) Field biology of Edessa rufomarginata (Hemiptera: Pentatomidae): phenology, behavior, and patterns of host plant use. Environ Entomol 39:1903–1910
Stephens DW, Krebs JR (1987) Foraging theory. Princeton University Press, Princeton
Taber SW (1998) The world of the harvester ants. Texas A & M University Press, College Station
Tevis L Jr (1958) Interrelations between the harvester ant Veromessor pergandei (Mayr) and some desert ephemerals. Ecology 39:695–704
Tizo-Pedroso E, Del-Claro K (2007) Cooperation in the neotropical pseudoscorpion, Paratemnoides nidificator (Balzan, 1888): feeding and dispersal behavior. Insect Soc 54:124–131
Traniello JFA (1989) Foraging strategies of ants. Annu Rev Entomol 34:191–210
Whitford WG (1978) Foraging in seed-harvester ants Pogonomyrmex spp. Ecology 59:185–189
Whitford WG, Ettershank G (1975) Factors affecting foraging activity in Chihuahuan Desert harvester ants. Environ Entomol 4:689–696
Whitford WG, Johnson P, Ramirez J (1976) Comparative ecology of the harvester ants Pogonomyrmex barbatus (F. Smith) and Pogonomyrmex rugosus (Emery). Insect Soc 23:117–132
Wilby A, Shachak M (2000) Harvester ant response to spatial and temporal heterogeneity in seed availability: pattern in the process of granivory. Oecologia 125:495–503
Wirth R, Herz H, Ryel RJ, Beyschlag W, Hölldobler B (2003) Herbivory of leaf-cutting ants: a case study of Atta colombica in the Tropical Rainforest of Panama. Springer, Berlin
Wolda H (1988) Insect seasonality: why? Annu Rev Ecol Syst 19:1–18
Yamamoto M, Del-Claro K (2008) Natural history and foraging behavior of the carpenter ant Camponotus sericeiventris Guérin, 1838 (Formicinae, Campotonini) in the Brazilian tropical savanna. Acta Ethol 11:55–65
Acknowledgments
We thank A.V.L. Freitas, E.P. Barbosa, A.G. Bieber, C.A. Iserhard, L.A. Kaminski, S.F. Sendoya, P.S.D. Silva, J.C. Santos, H.L. Vasconcelos and P.B. Araújo for discussions and/or suggestions on early drafts of the manuscript. C.B. was supported by a graduate fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). V.L. Belchior helped during fieldwork and with illustrations; R. Romero, J. Nakajima, and J. Tamashiro identified the plants; and R.M. Feitosa identified the ant species. K.D-C. was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG). P.S.O. was funded by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; no. 2011/18580-8), the CNPq, and the Fundo de Apoio ao Ensino, à Pesquisa e à Extensão (FAEPEX).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Jonathan Lundgren.
Rights and permissions
About this article
Cite this article
Belchior, C., Del-Claro, K. & Oliveira, P.S. Seasonal patterns in the foraging ecology of the harvester ant Pogonomyrmex naegelii (Formicidae, Myrmicinae) in a Neotropical savanna: daily rhythms, shifts in granivory and carnivory, and home range. Arthropod-Plant Interactions 6, 571–582 (2012). https://doi.org/10.1007/s11829-012-9208-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-012-9208-1