Abstract
Salinity is a major problem affecting crop production all over the world. A wide range of adaptation strategies are required to overcome this problem. Endophytic bacteria can build a symbiotic association with their host to improve host plant salt tolerance. In this study, eighteen bacterial endophyte strains were isolated from two native halophytic plants Arthrocnemum macrostachyum and Spergularia marina, and identified as Bacillus, Brevibacillus, Agrobacterium, and Paenibacillus. These endophytic strains exhibit plant growth-promoting activities including phosphate solubilizing, ammonia production, biocontrol of phytopathogen, extracellular enzymatic activities, and indole-3-acetic acid production under normal and salinity stress. A pot experiment was conducted under field conditions to alleviate the harmful effects of soil salinity on bean (Vicia faba L.) by inoculating their seeds with the most potent bacterial isolates Bacillus subtilis (AR5) and Bacillus thuringiensis (BR1). Salinity treatments induced a significant decrease in both growth parameters and metabolic activities, while the activity of antioxidant enzymes and proline content was significantly increased. However, salinity stress induced higher contents of Na+ and decreased contents of N+, P3+, K+, Ca2+, Mg2+, and K+:Na+, it was found that treatment with B. subtilis (AR5) and B. thuringiensis (BR1) individually or in a combination mitigated the effect of salt stress and improved the plant height, shoot dry weights, proline contents, enzymes activities as well enhanced the accumulation of mineral nutrients in shoot plants. Our results concluded that treatment with co-inoculation of B. subtilis (AR5) and B. thuringiensis (BR1) exerted the greatest effect in alleviating the harmful effect of soil salinity stress and can be used as a suitable bio-approach to reclaim salinity-stressed soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many of the important crops around the world are suffering from soil salinity stress which has become a major threat to crop production soon (FAO 2015; Munns and Tester 2008). Around 20% of arable land and 33% of irrigated land is seriously influenced by salinity stress (Ali et al. 2014). Irrigation of crops with saline water, soil erosion, and low precipitation are considered the main factors for increasing soil salinity (Shrivastava and Kumar 2015). Salinity influences irrigated land which generates 40% of the world’s food (Pimentel et al. 2004). Moreover, Jamil et al. (2011) announced that over half of the arable land would be suffering from detrimental levels of salinization by 2050. Salinity influences practically all aspects of plant development and the major biochemical reactions related to photosynthesis, protein synthesis, etc. (Ahmad et al. 2014). It causes ionic toxicity, osmotic stress, nutrient deficiency, and oxidative stress in plants, and accordingly, limits the rate of soil water absorption (Shrivastava and Kumar 2015).
Broad bean is considered one of the most important leguminous crops planted in a large area of the world. Cultivation of broad beans has been recorded to improve the nitrogen contents of the soil (Hungria and Vargas 2000). Thus, many plant scientists are interested to increase its production, especially on marginal lands. In Egypt, broad beans are grown in the largest area although its cultivated area declined in the last ten years from 73,650 to 43,314 hectares (FAO 2015). The development and production of broad bean are affected by various abiotic stresses such as soil salinity. World scientists are looking for alternative solutions to maintain crop yield under stressful conditions. One of these alternative solutions is the use of halophytic bacteria that act as a growth regulator and mitigate the harmful impacts of salinity stress on plants.
Plant growth-promoting endophytic (PGPE) bacteria have been appeared to have many valuable consequences for their host plant, including growth-promoting activity, modulation of plant metabolism, and phytohormone signaling that lead to adaptation to abiotic or biotic stress (Lata et al. 2018; Eid et al. 2021). PGPE are known to enable plants to tolerate salinity stress through several mechanisms including activation of antioxidant enzymes, enhanced synthesis of antioxidant metabolites, ion homeostasis, polyamine biosynthesis, biosynthesis of compatible solutes and osmoprotectants, modulation in phytohormones, and uptake and transport regulation of ions (Hashem et al. 2015; Yaish et al. 2015; Numan et al. 2018). Salt tolerant bacteria (e.g., Rhizobium, Azospirillum, Pseudomonas, Flavobacterium, Arthrobacter, and Bacillus) have a positive impact on crops productivity in stressed environments (Almaghrabi et al. 2014).
Several reports showed that PGPE bacteria can improve developmental aspects in several plants like rice (Oryza sativa) (Jaemsaeng et al. 2018), tomato (Solanum lycopersicum) (Palaniyandi et al. 2014), canola (Brassica napus) (Chen et al. 2007), groundnut (Arachis hypogaea) (Saravanakumar and Samiyappan 2007), mung bean (Vigna radiate) (Ahmad et al. 2011), musli (Chlorophytum borivillianum) (Barnawal et al. 2016), and black pepper (Piper nigrum) (Maxton et al. 2018) under saline conditions. Maize seedlings inoculated with the B. amyloliquefaciens bacterial strain showed enhanced salinity stress tolerance with increased chlorophyll, improved peroxidase and catalase activities along with enhanced glutathione content (Chen et al. 2016).
Increased antioxidant activities have been reported in Capsicum annum, Pisum sativum, Zea mays, Glycine max, etc., upon inoculation with PGPR strains such as Microbacterium oleivorans KNUC7074, Brevibacterium iodinum KNUC7183, Rhizobium massiliae KNUC7586, Planomicrobium sp. MSSA-10, Serratia liquefaciens KM4, Bacillus firmus SW5 subjected to salinity stress (Shahid et al. 2018; El-Esawi et al. 2019). In another study, treatment of salt-sensitive Arabidopsis thaliana plants by the PGPR strain Burkholderia phytofirmans PsJN enhanced the levels of salt tolerance with increased proline content and expression level of genes related to abscisic acid signaling (Pinedo et al. 2015).
Low molecular weight (LMW) antioxidants such as ascorbic acid and glutathione, which are synthesized within the chloroplast, have played a significant role as redox buffers in modulating plant development and growth processes ranging from mitosis and cell elongation to senescence and death (Kasote et al. 2015). In a related manner, these compounds can stimulate gene expression linked with biotic and abiotic stress responses to help sensitive plants defend themselves.
Ascorbic acid (AsA) is a molecule that is produced during aerobic metabolism and is known to be the best molecule for H2O2 detoxification, particularly as a substrate of ascorbate peroxidase (APX), an essential enzyme of the ascorbate–glutathione cycle that is found in most compartments of the plant cell (Smirnoff and Wheeler 2000). Additionally, AsA aids in the regeneration of antioxidant pigments, carotenoids, and -tocopherol.
Glutathione (GSH) is the most prevalent LMW thiol in plants and is a tripeptide (-glutamyl-cysteinyl-glycine) (Noctor et al. 2012). Free radicals can accept an electron from GSH's sulfhydryl or thiol group (as shown by the –SH). GSH contributes significantly to the redox environment due to its abundance and negative redox potential (Schafer and Buettner 2001), helping cells to maintain a healthy decreased redox homeostasis (Noctor et al. 2012).
The objective of this investigation was to identify halotolerant endophytic bacteria isolated from halophyte plants and investigate their ameliorative role in the enhancement of salinity tolerance of broad bean plants.
Materials and methods
Plant sampling
Healthy aerial and root parts of native halophytic plant species Arthrocnemum macrostachyum (Moric) C. Koch and Spergularia marina (L.) Besser were collected from two saline land sites: Barakat Al-Mamur (28° 24′ 16.3″ N, 28° 52′ 22.1″ E) and Al-Tahatnia (28° 21′ 15.3″ N, 28° 51′ 47.8″ E), El-wahat el-Bahariya, Western desert, Giza governorate, Egypt (Fig. 1A, B). Four individual plants from each species were collected per site. The collected plant materials were carefully placed in sterile bags of polyethylene and brought back to the laboratory in a portable cooler maintained at 4 °C using icepacks. The plant specimens were identification at the herbarium of Botany Department, Al-Azhar University, according to (Boulos 2005, 2009).
Bacterial endophytes isolation
Of each plant, the shoot tip and root samples were excised and cleaned using running tap water. Sterilization of leaf and root surfaces were done by soaking the tissues in a series of baths: sterile refined H2O for 60 s, 70% ethyl alcohol for 60 s, 2.5% sodium hypochlorite for 4 min, 70% ethyl alcohol for 30 s, and a final series of three rinses in sterile refined H2O in three different containers. A 0.1 mL aliquot of the last flush water was plated onto nutrient agar plates to affirm the achievement of surface sterilization (Fouda et al. 2021).
The bacterial endophytes resistant to salts were isolated by two methods. Firstly, the sterilized plant leaves and roots were cut into 5 mm segments, and twenty leaf segments per individual plant were put in four Petri dishes (9 cm; five segments/plate) containing supplemental agar media and nystatin (25 μg mL–1) to suppress fungal growth, and 100 mM NaCl. Then, the cultured plates were put in obscurity at 35 ± 2 °C. Secondly, another twenty segments of sterilized leaves and roots per individual plant were together crushed in 100 mL sterile saline solution using a sterile homogenizer, and 1.0 mL of the suspension was sequentially diluted until 10–3 from which a 0.1 mL aliquot was spread onto each of three Petri plates containing supplemental agar medium and nystatin (25 μg mL–1) to suppress fungal growth, and 100 mM NaCl and incubated in obscurity at 35 ± 2 °C (Arora et al. 2014). The cultures were regularly observed for bacterial growth, for a period of 96 h. Bacteria growing from internal tissues or crushed segments were moved to fresh culture plates, streak plated to isolate single colonies, and then stored on inclines at 4 °C until more investigation.
Molecular characterization of endophytic bacteria
Bacterial identification was based on the analysis of 16S rRNA sequence. Genomic DNA of bacterial isolates was extracted according to Miller et al. (1999) with some modification. Briefly, individual colonies from bacterial growth plates were picked up using a sterile toothpick and resuspended in 50 μL of sterile deionized H2O. The cell suspension was set in a water bath at 97 °C and heated for 10 min, the cell lysate was forced (15,000 g, 10 min), and the upper layer containing the DNA was recovered. The content of extracted DNA was calculated by detecting its absorbance at 260 nm using a UV spectrophotometer. A fragment of 16S rDNA was PCR amplified using the bacterial universal primers 27f (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492r (5′-GGTTACCTTGTTACGACTT-3′) (Lane 1991). The PCR tube contained: 1 × PCR buffer, 0.5 mM MgCl2, 2.5 U Taq DNA polymerase (QIAGEN), 0.25 mM dNTP, 0.5 μM primer, and approximately 5 ng of bacterial genomic DNA.
The cycling of PCR conditions was 94 ºC for 3 min, 30 cycles of 94 ºC for 0.5 min, 55 ºC for 0.5 min, and 72 ºC for 1.0 min, a final extension performed at 72 ºC for 10 min. The PCR products were forward and reverse sequenced using the Applied Biosystem’s 3730xl DNA Analyzer technology at sigma company, Cairo, Egypt. The sequences generated in this study are deposited in GenBank under accession numbers MN860026 to MN860043. The 16S rRNA sequences were then compared against the GenBank database using the NCBI BLAST nucleotide search. A multiple sequence alignment was constructed using the ClustalX 1.8 software package (http://www.clustal.org/clustal2) and a phylogenetic tree was constructed using the neighbor-joining method in the MEGA v6.1 software (www.megasoftware.net), with confidence tested by bootstrap analysis (1000 repeats).
Salinity tolerance
The identified endophytic bacterial strains were cultured either without or with different NaCl concentrations (200, 400, 600, 800, and 1000 mM) to detect sublethal doses for each organism. The results were reported as plus (+) or minus (-) according to bacterial ability to grow in the presence/absence of NaCl.
Evaluate the bacterial endophytes as plant growth-promoting (PGP) in the absence and presence of salt stress
Phosphate solubilization
The qualitative and quantitative screening of phosphate solubilization by bacterial endophytic isolates was investigated in the absence and presence of 600 mM NaCl. The qualitative screening was analyzed according to Jasim et al. (2013) as follows. Pikovskaya agar medium [glucose 10 g L–1; Ca3(PO4)2·5 g L–1; (NH4)2SO4 0.5 g L–1; NaCl 0.2 g L–1; MgSO4·7H2O 0.1 g L–1; KCl 0.2 g L–1; FeSO4·7H2O 0.002 g L–1; yeast extract 0.5 g L–1; MnSO4·2H2O 0.002 g L–1; agar 15 g L–1; 1.0 L dH2O] was prepared and bromophenol blue was added as an indicator. The medium was inoculated with endophytic bacterial isolates and incubated for 48 h. Pikovskaya agar medium without bacterial growth was used as a control. The formation of a clear zone around the colony, due to the utilization of tricalcium phosphate, was measured to assess the qualitative ability to solubilize phosphate by bacterial endophytes.
On the other hand, quantitative phosphate solubilizations were screened by detecting pH values and P concentration at interval times as follows. Pikovskaya’s broth medium supplemented with 0.5% Ca3(PO4)2 in the absence and presence of 600 mM NaCl were inoculated with bacterial endophytes and incubated at 28 ± 2 °C for 7 days on a rotary shaker at 180 rpm. Five mL samples were taken daily and centrifuged at 10,000 rpm for 10 min. The phosphomolybdate method was used for the determination of available soluble phosphate in the culture supernatant using a spectrophotometer (Jenway 6305 UV) measuring at O.D. 700 nm. The concentration of P was calculated from the slope of the standard curve of P. Also, the pH of the broth medium was measured daily with a digital pH meter.
Ammonia production
The ability of the isolated endophytic bacterial strains to produce ammonia was assessed after growing the bacterial strains in peptone water (peptone 10 g L–1; NaCl 5 g L–1; 1L dH2O) for 72 h at 35 ± 2 °C in the absence and presence of 600 mM NaCl. Peptone water without bacterial inoculation was used as a control. The addition of 1.0 mL of Nessler’s reagent in the peptone liquid medium was used to assess ammonia production. A color change to faint yellow indicated the minimum ammonia production while deep yellow to the brownish color indicated the maximum ammonia production (Singh et al. 2014).
Nitrogen-fixing activity
The qualitative screening for nitrogen-fixing activity was assessed through inoculation of endophytic bacteria on Nitrogen Free Malate Agar (NFMA) media, supplemented with Bromothymol Blue (BTB) as an indicator, in the absence and presence of 600 mM NaCl. The inoculated NFMA plates were incubated at 35 ºC and 50 ºC for up to 24 h. The blue-colored zone-producing isolates were marked as nitrogen fixers in the solid culture conditions. The intensity of the color zone formed around bacterial growth was observed as + , + + , and + + + (Gothwal et al. 2008).
In-vitro antagonistic bioassay
The antagonistic activity of purified bacterial endophytes was evaluated in the absence and presence of 600 mM NaCl against widely prevailing fungal plant pathogens by dual-culture in-vitro assay according to Landa et al. (1997). Four phytopathogenic fungi represented as Alternaria alternata, Fusarium oxysporum, Pythium ultimum, and Verticillium dahlia were obtained from the Plant Pathology Department, Faculty of Agriculture, Zagazig University. For bioassay, each bacterial isolate was spotted at 3 equidistant points along the perimeter of the PDA plate (3 plates per isolates). After 24 h of incubation at 28 °C in the dark, a 5 mm plug from the leading edge of a 7-day-old culture of each phytopathogen on PDA was placed in the center of the plate. Plates without bacteria were used as a control. Plates were incubated at 28 °C for 5 days, after which the relative growth inhibition percent was calculated according to the following equation.
where R1 represented the lengths of hyphal growth toward the bacteria and R2 represented the lengths of hyphal growth on a control plate.
Screening the extracellular enzymatic activities of bacterial endophytes
The production and activity of extracellular enzymes (amylase, cellulase, protease, and xylanase) of isolated bacterial endophytes were assessed in the absence and presence of 600 mM NaCl. For enzymatic activity, the purified isolates were inoculated in a mineral salt (MS) media (NaNO3 5.0 g L–1; KH2PO4 1.0 g L–1; K2HPO4 2.0 g L–1; MgSO4.7H2O 0.5 g L–1; KCl 0.1 g L–1; CaCl2 0.01 g L–1; FeSO4.7H2O 0.02 g L–1; agar 15 g L–1; 1.0 L dH2O) complemented with various additives, depending on the enzyme being tested, as detailed below. Control treatments consisted of the same media in the presence and absence of salt without bacterial inoculation. After incubation for 24–48 h depending on the growth rates of the bacterial endophytes at 35 ± 2 °C, specific reagents were added (see paragraph below), and the size of the clear zone surrounding the bacterial colony was measured, indicating extracellular enzymatic activities (Ismail et al. 2021). All assays were performed in triplicates.
Amylolytic and cellulase activity was assessed by growing the endophytic bacterial isolates on MS agar medium supplemented with 1% soluble starch and 1% cellulose or carboxy-methylcellulose (CMC), respectively in the presence and absence of NaCl. After incubation, the plates were flooded with 1.0% iodine. The pectinolytic activity was determined by growing bacteria in an MS medium containing 1.0% pectin in the presence and absence of NaCl. After the incubation period, the plates were flooded with a 1.0% aqueous solution of hexadecyl trimethyl ammonium bromide. MS agar medium supplemented with 1% xylan from corncobs was used to measure bacterial xylanolytic activity in the presence and absence of NaCl. After the incubation period, the xylanase activity was assessed after flooding with absolute ethyl alcohol to indicate biodegradation.
Quantitative screening for indole-3-acetic acid (IAA) production
The ability of bacterial endophytes to produce IAA in the absence and presence of 600 mM NaCl was assessed in nutrient broth at 35 ± 2 °C for 24 h. Briefly, one mL of each bacterial suspension was added to 20 mL of nutrient broth medium containing 0.0 and 5.0 mg mL–1 tryptophan, in the absence and presence of salt and incubated for 7 days. Controls consisted of nutrient broth media containing 0 and 5 mg mL–1 tryptophan in the absence and presence of salt but without bacterial inoculation. At the end of incubation periods, the bacterial cultures were centrifuged at 10,000 rpm for 10 min at 4 °C. Produced IAA was assessed as follows, one mL of the supernatant was mixed with 1.0 drop of orthophosphoric acid and 2.0 mL of Salkowski’s reagent (300 mL concentrated sulfuric acid, 500 mL distilled water, 15 mL 0.5 M FeCl3). Development of a pink color indicated IAA production. The optical density at 530 nm was measured using a spectrophotometer (Jenway 6305 UV spectrophotometer), and the amount of IAA produced was estimated using a standard curve for authentic IAA (Ismail et al. 2021).
Greenhouse experiment
Experimental design, soil analysis, and culture condition
A pot experiment was conducted during the winter season of 2019 at the farm of the Faculty of Science, Al-Azhar University, Cairo, Egypt to examine the impact of two bacterial endophytes isolates, Bacillus subtilis AR5 and Bacillus thuringiensis BR1 and their mixture on morphological and biochemical characteristics of broad bean plants growing under normal and salinity conditions. Soil texture was determined using a series of sieves: fine gravel (2 mm), coarse sand (0.2 mm), fine sand (0.02 mm), silt (0.002 mm), and clay (sieves base) to evaluate gravel percent and particle size analysis. The soil of the experimental site is classified as sandy loam soil containing 76.8% sand, 10.9% silt, and 12.2% clay in the 0–20 cm soil layer (Table S1, see supplementary data). Soil samples used for plant growth studies were analyzed before and after sanity and bacterial treatments for its various chemical properties (Table S1, see supplementary data). Soil and water were mixed in 1:2.5 ratio; pH and electrical conductivity (EC) was measured with pH meter and conductivity meter (Mettler Toledo, Switzerland). Chloride ions (Cl−) were determined using silver nitrate (AgNO3) and potassium chromate (K2CrO4) solution as indicator. Ammonium acetate buffered at pH 7 was used to determine exchangeable (K+, Ca2+, Na+) cations. After extraction methods, P, K+, Ca2+, and Na+ were determined using an inductively coupled plasma spectrophotometer (Optima 2100 DV, ICP/OES; Perkin-Elmer, Shelton, CT (Table S1, see supplementary data). Endophytic bacterial isolates (adjusted O.D. at 1.0) were added to the nutrient broth and incubated at 35 ± 2 °C for 24 h on a shaker at 180 rpm. The experimental design was a completely randomized design (CRD) which consisted of two groups (The first one was not supplemented with NaCl and the second was supplemented with NaCl), and each group has four treatments for one test plant of Vicia faba L. The treatments included: (i) non-inoculated control (ii) inoculation with AR5 (iii) inoculation with BR1 (iv) co-inoculation with AR5 and BR1. For co-inoculation, equal amounts of two bacterial cultures were mixed and used for the experiment. Five replicates of each of the four treatments were prepared.
Inoculation and growth of faba bean plants under salinity stress
Healthy broad bean (Vicia faba L. cv. Sakha-1) seeds were obtained from the Agricultural Research Center, Field Crop Research Institute, (FCRI), Giza, Egypt. Seeds were surface sterilized by soaking in 2.5% sodium hypochlorite for 3 min and then they were washed with sterile distilled water 5 times. Sterilized seeds were incubated with 50 mL of each bacterial isolate broth medium at room temperature for 24 h. The nutrient broth without bacterial inoculation was used as a control treatment. After 24 h of incubation, the soaked seeds were planted in 35 cm diameter pots filled with 7.5 kg of soil mixture. Each pot was planted with five germinated and treated seeds at a temperature of 25–30 °C. Thinning was achieved after one weak germination leaving three similar plants per pot. Irrigation by applying variable salinity levels increased gradually, 0.0, 50, 100, 150, 200, and 250 mM NaCl. Following 45 days of seed sowing, each pot received 150 mM NaCl, while following 90 days of seed sowing, each pot received 300 mM NaCl.
Measurements of vegetative growth traits
Forty-five- and ninety-day-old bean plants (n = 3) were removed, and shoots were separated from plants, and the following vegetative growth parameters were recorded: plant height was measured, dry weights of shoots were recorded after placing them in an oven at 70 °C until constant weight, and fresh shoot subsamples were kept in a refrigerator for assessment of the plant’s enzymatic activities (Khalil et al. 2021). The photosynthetic pigments were measured as follows: 1.0 g of leaves was cut and grinding with suitable amount of glass powder using 100 mL of acetone solution (80%). The grinding solution was transferred to Buchner filter to extract using Whatman filter paper. The obtained filtrate was transferred to 100 mL conical flask and mixed with 80% acetone to reach 100 mL. The optical density (O.D.) of the previous plant extract was measured at two wavelength of 649 nm and 665 nm. The concentration of chlorophyll a (Chla), chlorophyll b (Chlb), and total chlorophyll (Chla,b) was calculated using the following equations (Ismail et al. 2021):
The concentration of carotenoids was calculated according to the following equation:
where A is the optical density; Chla is the chlorophyll a; Chlb is the chlorophyll b; Car is the carotenoids concentration.
Antioxidant enzymes
Fresh leaf tissue including the terminal buds beside the first and second young leaves (5 g) was macerated in 50 mM sodium phosphate buffer (pH 7.0) containing 1.0% soluble polyvinyl pyrrolidine (PVP). The homogenate was centrifuged at 15,000 rpm for 20 min at 4 °C, and the supernatant was utilized for the enzyme assays. Catalase (CAT, EC 1.11.1.6) activity was evaluated according to the method described by Chen et al. (2000). The reaction mixture with a final volume of 10 mL containing 40 µL enzyme extract was added to 9.96 mL H2O2 phosphate buffer pH 7.0 (0.16 mL of 30% H2O2 to 100 mL of 50 mM phosphate buffer). The rate change of H2O2 absorbance in 60 s was measured by spectrophotometer at 250 nm and was expressed as EU mg−1 protein. Peroxidase (POD, EC 1.11.1.7) activity was measured according to the method described by Kar and Mishra (1976). The assay mixture consisted of 125 μM phosphate buffer (pH 6.8), 50 μM pyrogallol, 50 μM H2O2, and 1.0 mL of the 20 × diluted enzyme extract in a total volume of 5.0 mL. The amount of purpurogallin formed was determined by measuring the absorbance at 420 nm, and the enzyme activity was expressed as EU mg−1 protein. Polyphenol oxidase (PPO, EC 1.10.3.1) activity assayed using 125 µmol of phosphate buffer (pH 6.8), 100 µmol pyrogallols, and 2 mL of enzyme extract. After the incubation period of 5 min at 25 °C, the reaction ended by add 1 mL 5% H2SO4. The blank sample was made using very well-boiled enzyme extract, the developed color was read at 430 nm and the enzyme activity was expressed as the changes in the optical density/gram fresh weight/hour (Kar and Mishra 1976). Superoxide dismutase (SOD, EC1.15.1.1) was estimated as described by Bayer and Fridovich (Beyer and Fridovich 1987) following the photoreduction of nitroblue tetrazolium (NBT). The activity of SOD was expressed as enzyme unit (EU) mg−1 protein. The enzyme ascorbate peroxidase (APX, EC1.11.1.11) was measured using the Nakano and Asada method (Nakano and Asada 1981). 1.0 mL reaction buffer [potassium phosphate (pH 7.0) with 0.1 mM EDTA, 0.5 mM ascorbate, 0.1 mM H2O2, and 0.1 mL enzyme extract] was added to the reaction mixture. APX activity was measured as a decrease in ascorbate absorbance at 290 nm, and it was expressed as a unit mg−1 protein.
Assessments of glutathione and ascorbic acid contents
The glutathione (GSH) content was determined according to Griffith (1980) technique. Fresh leaf tissue (50 mg) was homogenized in 2.0 mL of 2.0 percent (v/v) metaphosphoric acid, followed by centrifugation at 17,000 g for 10 min. 0.6 mL of 10% (w/v) sodium citrate was used to neutralize the supernatant (0.9 mL). For each sample, three replicates were evaluated. Each test (1.0 mL) was made up of 700 mL of 0.3 mM NADPH, 100 mL of 6 mM 5,5′-dithio-bis-2-nitrobenzoic acid, 100 mL distilled water, and 100 mL of extract, which was stabilized at 25 °C for 3–4 min before adding GSH reductase (10 µ of 50 Units mL–1). Absorbances were read at 412 nm to calculate GSH contents from a standard curve.
The Mukherjee and Choudhari (1983) method was used to determine ascorbic acid (AsA) content. Extraction was performed in 10 mL of 6% (w/v) tri-chloroacetic acid, and the extract was mixed with 2% (w/v) dinitrophenylhydrazine, and then one drop of 10% (w/v) thiourea in 70% (v/v) ethanol was added. Using a water bath, the mixture was boiled for 15 min. After cooling, 5 mL of 80% (v/v) H2SO4 was added and absorbances were measured at 530 nm to calculate the contents of AsA from a standard curve.
Detection of proline
The content of proline was evaluated based on Bates et al. (1973) method. Leaf tissue (1.0 g) was crushed in 10 mL of aqueous sulphosalicylic acid (3%) using mortar and pestle and centrifuged at 11.000 rpm for 10 min. The supernatant was taken out, and the volume was maintained to 10 mL with sulphosalicylic acid. The reaction was started by taking 2 mL of the extract in a test tube having 2 mL acid ninhydrin (prepared by warming 1.25 g of ninhydrin in 30 mL of glacial acetic acid and 20 mL of 6 mol orthophosphoric acid with agitation until dissolved) and 2 mL glacial acetic acid. The above mixture was boiled in a water bath at 100 °C for 30 min until a brick red color developed. The reaction was stopped by cooling in an ice bath, and the reaction mixture was extracted with 4 mL of toluene. The extract obtained was moved to a separating funnel, and the upper layer was collected after mixing vigorously for 15–20 s. The chromophore containing toluene was aspirated from the aqueous phase and warmed to room temperature, and the absorbance was read at 520 nm using toluene for a blank. The concentration of proline samples was determined according to the standard curve plotted with known concentrations of L-proline.
Determinations of N, P, K+, Ca2+, Na+ contents and K+/Na+ ratio
Concentration of N was determined in powdery dried material of plants by Orange-G dye colorimetric method according to Hafez and Mikkelsen (1981). The wet digestion of 0.1 g of fine dried material of plants was conducted using a sulphuric and perchloric acid mixture. The content of P (%) was calorimetrically determined using the chlorostannusmolybdo-phosphoric blue color method in the sulphuric acid system. The concentration of Ca2+ was determined using a Perkin-Elmer Model 3300 Atomic Absorption Spectrophotometer. The concentrations of K+ and Na+ were determined using a Perkin-Elmer Flame photometer. The ratio of K+/Na+ was calculated from the determined contents of K and Na.
Statistical analysis
Data were statistically analyzed using SPSS v17 (SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) was used to compare the bacterial isolates for In-vitro antagonistic bioassay, extracellular enzymes production, IAA, and ammonia production, P-solubilization ability, and the effect of these endophytes on Vicia faba growth performance. A posteriori multiple comparisons were done using Tukey’s range tests at p ≤ 0.05. All results are the means of three to six independent replicates, as specified above.
Results and discussion
Isolation and identification of halotolerant bacterial endophytes
In this study, eighteen isolates of endophytic bacteria were separated and identified from the leaves and root system of the two native halophytic plants in the presence of 100 mM NaCl (Table 1). Eleven bacterial isolates were separated from A. macrostachyum: three bacterial strains characterized as Bacillus lentus, B. amyloliquefaciens, and B. thuringiensis were separated from leaves, while eight strains identified as Brevibacillus agri (2 strains), Agrobacterium sp. (1 strain), Bacillus megaterium (1 strain), Bacillus sp. (1 strain), Bacillus subtilis (2 strains), and Bacillus brevis (1 strain) were isolated from roots. Seven isolates were separated from S. marina and classified as follows: three isolates from leaves were Bacillus sp., Brevibacillus brevis, and Paenibacillus barengoltzii, while four isolates separated from roots were B. subtilis (2 strains), B. amyloliquefaciens, and Brevibacillus agri. This is the first report concerning the isolation of putative bacterial endophytes associated with the halophytic S. marina plant. Sequences of 16S rRNA of eighteen bacterial strains with sequence similarity percentages were represented in Fig. 2 and Table 1. Recently, Bacillus and Brevibacillus spp. were identified as endophytic strains isolated from two medicinal plants Fagonia mollis and Achillea fragrantissima that showed different plant growth-promoting activities (ALKahtani et al. 2020).
Phylogenetic analysis of 16S rRNA sequences of bacterial strains with reference sequences from NCBI. Bacterial strains with code A refer to 16S rRNA sequences of bacterial isolates from A. macrostachyum plants, whereas bacterial strains with code B are the sequences from isolates from S. marina. Identities of the bacterial isolates are available in Table 2. The analysis was performed in MEGA 6
Characterization of halotolerant bacterial isolates under salinity stress
In the present investigation, all identified bacterial isolates exhibit normal growth in media that contain no NaCl. The endophytic isolates were grown at gradually increasing NaCl concentration to detect sub-lethal salt concentrations (Table S2, see supplementary data). All identified bacterial isolates exhibited normal growth at 200, 400, and 600 mM NaCl. The data showed that elevation of NaCl concentrations above 600 mM reduced bacterial growth to weak or very weak growth. Therefore, the concentration of 600 mM NaCl was applied as a sub-lethal dose in further experiments. In accordance with our results, Pirhadi et al. (2016), who isolated salt-tolerant Bacillus sp. from sugarcane leaves was able to grow on nutrient agar media stressed with 600 mM NaCl. Isolated bacteria from inside plants under salt stress have a different strategy to tolerate stress besides plant growth-promoting traits, therefore they have potential candidates to ameliorate stress (Shrivastava and Kumar 2015). One of these strategies is the osmoprotectants accumulation in the cytoplasm (Mishra and Sharma 2012). This phenomenon demonstrates the power of endophytic bacteria to alleviate salt stress through the production of exo-polysaccharides which restrict sodium adsorption by plants (Milošević et al. 2012) or indirectly by auxin production (Yaish et al. 2015).
Phosphate solubilization in the absence and presence of 600 mM NaCl
Phosphorus is an essential element for plants and its deficiency retards plant growth. Most phosphorus in soil is not accessible to plants because it is fixed in organic and inorganic compounds (Kalayu 2019). Certain bacterial strains are able to solubilize phosphate compounds converting insoluble phosphorus compounds to soluble ones that are taken by plants. The qualitative assays of phosphate solubility on Pikovskaya’s agar medium revealed that 12 bacterial isolates (67% of total isolates) had the power to dissolve inorganic forms of phosphate under saline and non-saline conditions (Table 1). The clear area produced along the bacterial colonies might be attributed to the generation of organic acids, polysaccharides formation, or activation of enzymes responsible for phosphate solubility in bacterial isolates (Paul and Sinha 2015).
The ability of twelve endophytic isolates (A1, AS2, AS3, AR1, AR3, AR4, AR5, ARS7, B1, B2, BR1, and BR2) to dissolve the phosphate was confirmed by quantitative estimation tests. The content of phosphate released due to quantitative solubility TCP in Pikovskaya’s broth media is presented in (Figure S1, see supplementary data) in salt and non-salt conditions. Phosphate content increased with incubation time irrespective of strains. In presence of 600 mM NaCl, the isolate BR1 enhanced the P solubility by 18.59%, recording 211.58 mg/L, while the isolate AR5 boosted the P solubility by 12.15%, recording 182.48 mg/L (p ≤ 0.05) (Figure S1, see supplementary data).
The P released under saline and non-saline conditions cause a gradual decrease in pH values until it reached its maximum drop on the fifth day and then increased again on the sixth and seventh incubation day. Therefore, we concluded that the increased acidity of the media caused the increased level of P available. Acidification seemed to be the main strategy utilized by these strains for solubilizing phosphate. The formation of organic acids with a low molecular weight that causing soil acidification is considered one of the most important phosphate solubilization mechanisms in endophytic bacteria (Khan et al. 2019). These organic acids can coat the cation around the phosphate with their active groups. Consistent with the present data, the Pseudomonas and Bacillus bacterial endophytes had moderate to high phosphate solubilizing capacities (∼400–1300 mg L−1), and were accompanied by a decline in the pH value because of Gluconic acid formation in broth culture media (Otieno et al. 2015). The efficiency of phosphate dissolving ability is correlated with the type and strength of acids (Kalayu 2019). Common acids produced during phosphate solubilization are citric, gluconic, lactic, oxalic, 2-ketogluconic, acetic, glyconic, fumaric, malic, succinic, malonic, tartaric, glutaric, butyric, propionic, adipic, and glyoxalic acid (Selvi et al. 2017; Kumar et al. 2018).
Ammonia production in absence and presence of 600 mM NaCl
Ammonia promoted plant growth through the synthesis of nitrogen-containing biomolecules. Our data showed that 16 isolates selectively generated ammonia either without or with 600 mM salinity stress and accordingly developed a brownish coloration after adding Nessler’s reagent to the supernatant of peptone water culture broth. Only the isolates AS3 and B2 were unable to generate ammonia (Table 1). These results show that bacterial endophytic isolates have the ability to form ammonia which potentially provides plants with nitrogen and also increases the plant protection against pathogen attack. Also, ammonia-producing microbes can help satisfy the nitrogen demand to host plants (Banik et al. 2019), and in excess reduce the attack of plants by pathogens (Rodrigues et al. 2016).
Nitrogen fixation in absence and presence of 600 mM NaCl
Nitrogen is the most important nutrient essential for the growth aspects of all living organisms including plants and bacteria. Farmers add nitrogenous fertilizers to soils that suffer from nitrogen deficiency to build up necessary plant requirements for maximum yield (Aczel 2019). Nitrogen gas [N, which represents about 78% of the earth’s atmosphere, is not readily accessible for plant assimilation until it is first converted to ammonia.
In the present investigation, almost all endophytic strains could fix atmospheric nitrogen. In addition, 33% of isolated endophytes (6 isolates) demonstrated the maximum zone of coloration on nitrogen-free malate agar media without salt stress, while 16% of endophytic isolates (3 isolates) coded AR2, AR5, and BR1 exhibited maximum coloration under salinity stress (Table 1).
Leite and co-authors successful to isolate both endophytic and rhizospheric bacteria from the sugarcane’s root and rhizosphere, respectively in the culture media provided with NaCl (5%) and without NaCl at Pernambuco, Brazil (Leite et al. 2014). They investigated the salinity tolerance degree beside other growth-inducing characters like biological nitrogen fixation, indole acetic acid synthesis, solubilization of inorganic phosphate, and genetic diversity of isolated endophytic bacteria. They observed that 72.5% of isolates were potential atmospheric nitrogen fixers.
In-vitro antagonistic bioassay in the absence and presence of 600 mM NaCl
Generally, all bacterial strains have an enormous capacity for phytopathogen growth inhibitions. Interestingly, this capacity was increased at salt stress. Data represented in Table (S3, see supplementary data) revealed that the average growth inhibition percentages of A. alternata, F. oxysporum, V. dahlia, and P. ultimum in presence of 600 mM NaCl were 44.85%, 42.45%, 34.03%, and 16.37%, respectively, and significantly (p ≤ 0.05) different from those recorded in absence of salinity which was 42.38%, 40.07%, 37.09%, and 15.67%, respectively (p ≤ 0.05). Consistent with our results, Abla et al. (2015) isolated bacterial endophytes belonging to Bacillaceae and Pseudomonadaceae from Mentha Rotundifolia L. with in-vitro antagonistic activity against the phytopathogenic fungi Fusarium oxysporum, Aspergillus niger with inhibition percent 70%, and Botrytis cinerea with inhibition percent 60%. On the other hand, bacterial endophytes are associated with nodules of soybean (Glycine max L.) and belonging to Enterobacter, Acinetobacter, Pseudomonas, Ochrobactrum, and Bacillus have an inhibitory effect on the phytopathogen Phytophthora sojae with inhibition percent up to 70% (Zhao et al. 2018).
In this study, the highest inhibition percentages were achieved due to treatment with Bacillus sp. AR5 and Bacillus thuringiensis BR1 (Table S3, see supplementary data).
Till now, few published studies inspect the antagonistic activity of bacterial endophytes against fungal pathogens under salinity stress conditions. Therefore, the inoculation of plants by endophytic bacterial strains that have antagonistic activity will increase their efficacy to resistant the invasion by phytopathogens in the presence and absence of stresses. In this respect, Egamberdieva et al. (2017a) isolated bacterial endophytes Bacillus cereus, Achromobacter xylosoxidans, Bacillus thuringiensis, and Bacillus subtilis from root nodules of chickpea (Cicer arietinum L.), which had antagonistic activity against F. oxysporum, F. solani, F. culmorum, A. alternata, and Botrytis cinerea in media stressed with 2% NaCl.
The antagonistic activity of microorganisms may be related to different ecological mechanisms, including pathogenesis, competition within the ecological niche, and the generation of compounds that inhibit their growth and development (Chebotar et al. 2015). Interestingly, in the current investigation, the bacterial endophytes isolated from the halophytic plant had the power to produce IAA, ammonia, plant cell wall degrading enzymes, and nitrogen fixation. Those traits, singly or together, may result in enhanced root growth, nutrient availability to the plants, and limitation of pathogen infection.
Extracellular enzymatic activities of bacterial endophytes in absence and presence of 600 mM NaCl
Endophytic bacteria provide the plants with additional beneficial resources such as secondary metabolites, enzymes, and nutrients, which help the host plant to tolerate and/or resist diverse biotic and abiotic stresses. In this examination, all endophytic bacteria isolated from halophytes S. marina plants showed positive enzymatic activities for amylase, pectinase, cellulase, and xylanase which were detected through agar-based methods either with or without salt stress except bacterial strain BR2 which exhibited activities for only two enzymes at salinity stress (Table S4, see supplementary data).
In harmony with our results, four Bacillus endophytic strains isolated from mango roots grown in saline-sodic soils exhibit extracellular amylase, cellulase, protease, and lipase enzyme activity in 3 M NaCl stressed media (Kannan et al. 2015). The symbiotic relationship between endophytes and their host plant may be attributed to hydrolytic actions of extracellular enzymes secreted by endophytes that help the endophyte to penetrate plant tissues. Moreover, the enzymatic activities of endophytes protect their host plant cell against phytopathogens through hydrolysis of the pathogen’s cell walls (Glick 2012). Endophytes give up their nutrients to the host plant through enzyme secretion and these extracellular enzymes promote plant nutrition and are utilized in plant senescence by protein and polysaccharides degradation (Choi et al. 2005). In another view regarding biotechnology, amylolytic and proteolytic enzymes of endophytes are being investigated to improve industrial processes for polysaccharides and protein biodegradation (Zaferanloo et al. 2013).
The efficacy of endophytic bacterial strains to producing indole-3-acetic acid (IAA) in the absence and presence of 600 mM NaCl
Indole-3-acetic acid (IAA) is the most common plant growth regulator that controls several plant growth processes such as stem elongation, cell division, response to light and gravity as well cell differentiation (Dhungana and Itoh 2019). IAA is produced by plants and by various microorganisms. Besides this hormone enhance plant growth, it also encourages the relationship between plants and microbes (Lin et al. 2009). In the present investigation, all tested bacterial isolates have the ability to produce IAA at different concentrations without adding tryptophan to the fermentation broth media. Analysis of variance revealed that variation in IAA production by different endophytic bacterial isolates was significantly increased compared with control (p ≤ 0.001). The maximum IAA content (21.40 and 19.91 µg/mL) was detected in BR1 and AR5 isolates, respectively (p ≤ 0.001). All bacterial isolates showed osmotic stress tolerance by growing in fermentative media supplemented with 600 mM NaCl. Therefore, bacterial IAA production increased significantly in salinity stressed culture media (p ≤ 0.002), with the maximum IAA production (29.75 and 27.02 µg/mL) reported for the isolates of BR1 and AR5, respectively (Fig. 3).
IAA production by endophytic bacterial isolates in the presence and absence of salt stress. A denotes IAA production in fermentative media without tryptophan. B denotes IAA production in fermentative media supplemented with 5 g/L of tryptophan. Bars with the same letter for each bacterial isolate did not differ significantly at a significant level of (p ≤ 0.05). Small case letters for comparison at the presence of salinity, while large case letters for comparison without salinity. Error bars indicate means ± SE by Tukey’s test (n = 6)
In our previous study, endophytic strains isolated from Asclepias sinaica were able to produce IAA in the absence and presence of 1, 2, and 5 mg/mL tryptophane as a precursor for IAA. The obtained results revealed that the maximum IAA productivity was achieved in presence of 5 mg/mL of tryptophane (Fouda et al. 2015). Therefore, the power of bacterial endophytes isolated from halophytic plants to build IAA was assessed in presence of 5 mg/mL tryptophane after 7 days. Our data exhibited that bacterial isolates BR1 and AR5 kept the highest IAA productivity which is significantly increased by adding tryptophane in comparison with growth media without tryptophan (p ≤ 0.001). IAA synthesis in salt-free fermentative media supplemented with 5 mg/mL of tryptophane was 48.03 and 39.57 µg/mL for isolates BR1 and AR5, respectively (Fig. 3).
Interestingly, IAA production increased by adding tryptophane to salt-fermentation broth media. The results showed that IAA production increased to 68.43 and 61.49 µg/mL for isolates BR1 and AR5, respectively by adding tryptophan to the salt-stressed growth media (Fig. 3).
Endophytic bacteria can be utilized to promote plant growth directly or indirectly with different and unique mechanisms like the formation of phytohormones, increment of nutrient uptake and stress tolerance, bio-control of plant pathogens (Mei et al. 2014). In the present examination, Bacillus sp. (AR5) and Bacillus thuringiensis (BR1) have a higher capacity for biocontrol, extracellular enzyme production, phosphate solubilizing activity, ammonia production, nitrogen fixation, and IAA production. Therefore, these two endophytic bacterial isolates were used to study their effect on broad bean plant growth performance in the presence and absence of salinity stress.
Ameliorative role of AR5 and BR1 endophytic bacteria on salt-stressed broad bean plants growing under greenhouse pot conditions
A greenhouse pot investigation (Fig. 4) was performed to examine the impact of Bacillus subtilis (AR5) and Bacillus thuringiensis (BR1) PGPR isolated from halophytic plants, individually or in a combination on developmental aspects and metabolic activities of non-halophytic broad bean plants grown without or with salinity stress at two harvested stages (45 and 90 days of planting). After 45 days of planting each pot received 150 mM NaCl, while after 90 days of planting each pot received 300 mM NaCl.
Effect of endophytic inoculation on growth properties of Vicia Faba: A Control plant without salinity and without bacterial inoculation, B endophytic bacterial treatment without salinity, C control plant with salinity and without bacterial inoculation, D bacterial treatment with salinity, 1; isolate AR5- Bacillus subtilis, 2; isolate BR1- Bacillus thuriengensis, 3; bacterial consortium (AR5 + BR1)
Effect of inoculated bacteria on physicochemical properties of the soil
The physical and chemical properties of soil are closely related to crop growth. After 90 days of planting the faba bean at 300 mM NaCl, the K+ content was lower than original soil (an decrease of 46.38%), however, the pH, EC, Na+, Cl−, Ca2+, and P content were higher than original soil. After 90 days of inoculation with the mixed of B. subtilis (AR5) and B. thuringiensis (BR1) isolated bacteria, the chemical properties of soil were altered. The pH (an increase of 2.37%) and EC (an increase of 7.41%) of uninoculated salted soil were higher than those of inoculated soil. The exchangeable K (an increase of 10.60%), Ca2+ (an increase of 25.34%), and P (an increase of 27.27%) were higher in the inoculated soil than those in the uninoculated soil. While the Na+ content (an decrease of 3.14%) and Cl− content (an decrease of 1.8%) were lower in the inoculated soil than those in the uninoculated soil (Table S1 (see supplementary data). The physical and chemical characteristics of saline soil can be improved by B. subtilis (AR5) and B. thuringiensis (BR1) strains. PGPB are the primary biological factors governing plant growth through ion exchange, nutrient transformation, and the availability of nutrients to plants (Schloss and Handelsman 2006). As a preliminary analysis of PGPR’s effect on rhizosphere soil, this study demonstrates that the application of microbial agents seems to be a successful approach for significantly reducing soil pH and salinity, boosting the management of saline-alkali soil, and improving plant survival in saline-alkali soil (Mbarki et al. 2017). In addition to having a rapid impact on plant growth, this sort of biological management is sustainable, low-cost, and does not cause pollution. As a result, the employment of microbial agents to cultivate plants in saline-alkali soil has enormous potential (Nabti et al. 2015).
Improvement of growth parameters, chlorophyll contents, enzymatic and non-enzymatic antioxidants accumulation in salt-stressed broad bean plants inoculated with AR5 and BR1 endophytic bacteria
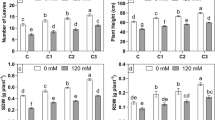
Salinity stress caused a significant reduction in height of plants and the dry weight of shoots of faba bean plants at the two harvested stages (45 and 90 days from seed sowing). Inoculation of the plants with a mixture of two bacterial isolates caused a great improvement in such parameters under stress and control conditions. The maximum improvement in plant height was recorded in faba bean plants inoculated with two bacterial isolates by 31.18% and 46% under control and salinity conditions, respectively 45 days after sowing. The mixed bacterial isolates significantly improved plant height by 24.91% and 26.73% under normal and saline conditions, respectively 90 days after sowing (Table S5, see supplementary data).
Faba bean plants inoculated by the two bacterial isolates in the mixture caused a maximum increase of plant biomass with or without salinity stress at both harvested stages. Inoculation with the mixed bacterial isolates led to maximum improvement in dry weight of the shoot by 45.49% and 85.18% without or with salinity stressed conditions, respectively contrasted with normal plants at the first harvest stage. Also, the two bacterial isolates caused a maximum increase in dry weight of the shoot by 24.39% and 27.41% with or without salinity stressed, respectively contrasted with normal plants at the second harvest stage (Table S5, see supplementary data).
Moreover, the photosynthetic pigments was significantly increased after 90 days of sowing compared to pigments content after 45 days (Fig. 5). Inoculated plants in absence of salinity demonstrated improved chlorophyll concentration which is significantly increased with time. Total chlorophyll was increased by 66.81%, 66.23%, and 63.44% for isolates AR5, BR1, and Mix after 90 days of sowing compared to the total chlorophyll after 45 days (p ≤ 0.001). Smilarly, carotenoids increased by 37.69%, 46.35%, and 47.57% for isolates AR5, BR1, and Mix after 90 days of sowing compared to the total chlorophyll after 45 days. Wheras, the chlorophyll significantly decreasd in salinity affected plants and the impact of salinity on plants was increased by time (Fig. 5).
The results about the impact of treatment with B. subtilis (AR5) and B. thuringiensis (BR1) bacterial isolates singly or in the mix on the accumulation of catalase (CAT), peroxidase (POD), polyphenol oxidase (PPO), superoxide dismutase (SOD), and ascorbate peroxidase (APX) antioxidant enzymes as well as the activities of non-enzymatic antioxidants; glutathione (GSH) activity, ascorbic acid (AsA), and total proline in broad bean leaves at two harvested stages (45 and 90 days) under saline and normal conditions are illustrated in Tables 2 and S5.
A notable increment was detected in CAT (35.46% and 19.71%), POD (21.25% and 31%), PPO (21% and 26.78%), SOD (20.82% and 37.57%), APX (20.95% and 27.55%), GSH (59.29 and 50%), and AsA (32.82% and 43.52%) of broad bean plants irrigated with NaCl alone at two harvested stages, respectively. Plants treated with BR1 bacterial isolate showed a maximum increase of 14.34% and 8.20% in CAT at two harvested stages, respectively at normal conditions. Our results showed that BR1 isolate conferred significant increases in CAT activity in plants treated with NaCl that reached 27.31% and 13.3% at two harvested stages, respectively (Table S5, see supplementary data).
Our data exhibited that plants inoculated by BR1 isolate showed the greatest value in POD activity of 22% and 23.61% at two harvested stages, respectively at normal conditions in comparison with control and rest treatments. Our results pointed out that POD activity increased to the highest level of 44.4% and 31.5% in presence of BR1 isolate at two harvested stages, respectively under salinity stress (Table 2).
At 45 days after sowing, the plants treated by BR1 isolate showed maximum improvement in PPO activity of 27.78 and 35% at unstressed and stressed conditions, respectively compared to control plants. However, the BR1 isolate showed the greatest increase in PPO activity reached 14.64% and 17.3% at unstressed and stressed conditions, respectively in comparison with rest treatments and control plants after 90 days from seed sowing (Table 2).
The increase in SOD activity of broad bean plants inoculated with the mix of bacteria reached 23.65% in comparison with rest treatments and control plants after 45 days after sowing at unstressed conditions. However, the results reported that the maximum increase in SOD activity reached 53.26% in plants inoculated with BR1 isolate in comparison with rest treatments and control plants under NaCl stress. The SOD content significantly increased by 13% and 17.23% for BR1 inoculated plants at unstressed and stressed conditions, respectively in comparison with rest treatments and control plants after 90 days from seed sowing (Table 2).
The treatments with B. subtilis (AR5) and B. thuringiensis (BR1) bacterial isolates singly or in combination significantly increased the activities of APX, GSH, and AsA in faba bean plants under salinity stress at two harvested stages (Table 2; Table S5, see supplementary data). Our data displayed that the highest values of APX, GSH, and AsA antioxidant activities reached (39.99% and 31.25%), (31.51% and 8.89%) and (48.35% and 36.29%) after 45 and 90 days from seed sowing, respectively for BR1 inoculated plants at salinity stressed conditions in comparison with non-inoculated stressed plants (Table 2; Table S5, see supplementary data). Our results pointed out the most effective application under salinity conditions was the inoculation of faba bean plants with the BR1 bacterial isolate.
Proline content increased by a percentage of 124% and 131% at two harvested stages, respectively in non-inoculated stressed plants. After 90 days of sowing, the maximum percentage (68%) of proline was observed for plants inoculated with AR5 isolate as compared to other treatments and control plants. Under salinity stress, the plants treated by BR1 isolate recorded maximum increases in proline amount reached to 98% in comparison with rest treatments and the non-inoculated stressed plants (Table S5, see supplementary data).
The main challenges for the growth and productivity of plants are ion toxicity with severe absorb of Cl− and Na+, as well as, nutrients imbalance caused by disturbed uptake of the essential mineral nutrients (Hu and Schmidhalter 2005). As shown in Table S5, studied growth features of faba bean plants treated by 150 and 300 mM NaCl reported great reductions in comparison with plants irrigated by normal water. These results were in accordance with that recorded in several important crops (Abeer et al. 2014; Dawood et al. 2014; Egamberdieva et al. 2017a). This might be related to the harmful impact of salt stress or enhanced osmotic stress level to a dangerous degree at which the salinity-treated plants do not have the ability to take water that is resulted in a decrease in many physiological aspects.
Plant growth-promoting bacteria (PGPB) can help their host plants in adapting to various stresses (Eid et al. 2019; Fouda et al. 2019). PGPB that produce ACC deaminase and phytohormones belong to variable bacterial genera that are known to improve the growth of many crops (Khan et al. 2016) and enhance plant tolerance to various stresses (Khan et al. 2019) including salinity.
Application of AR5 and BR1 bacterial endophytes either individually or in mixture had a remarkably alleviated role against salinity stress. This is in line with several studies supporting our obtained results, Irizarry and White (2017) reported that IAA-producing Curtobacterium oceanosedimentum endophytic bacteria-induced cotton growth under salinity stress. Similarly, IAA-producing bacteria also increased root and shoot length of chickpea and Medicago plants in a saline environment (Bianco and Defez 2009). In other studies, it was observed that fluorescent Pseudomonas induced the growth of root and shoot systems of sunflower under salinity stress (Tewari and Arora 2016). Previous studies reported that salt-tolerant PGPR such as Klebsiella, Agrobacterium, Pseudomonas, and Ochrobactrum enhanced salt-tolerance in groundnut plants (Sharma et al. 2016).
Recently, Khan et al. (2019) reported that SAK1 bacterial endophyte isolated from Artemisia princeps promoted soybeans growing under salinity stress through the production of ACC deaminase and phytohormones. Ullah et al. (2013) show that phytohormones, e.g., IAA and Gas have enhanced shoot length, root length, and several root tips that improved nutrient uptake and hence improved plant growth at stress conditions. Furthermore, co-inoculation of B. japonicum USDA 110 and salt-tolerant P. putida TSAU1 improved growth parameters, protein content, N and P uptake, and root system architecture of salted-stressed soybean (Egamberdieva et al. 2017a, b). Similarly, Ahmad et al. (2011) found that the combination of salt-tolerant Rhizobium and Pseudomonas resulted in improved mung bean productivity under salt-stressed conditions.
Salinity stress triggers plant cells to form reactive oxygen species (ROS) within plant cells that can cause oxidative damage to plant proteins, nucleic acids, and membrane lipids (Lata et al. 2018). To prevent ROS accumulation and mitigate oxidative damage, the plant develops enzymatic and non-enzymatic antioxidant defense systems (Bharti et al. 2016). Endophytes offer the potential to reduce the effect of salinity stress in plants through the aggregation of antioxidant enzymes. These compounds combined with osmotic adjustment, stabilize cell components and free radical scavengers. The current results show that B. subtilis (AR5) and B. thuringiensis (BR1) bacterial isolates and their combinations could have a positive impact on the production of CAT, POD, PPO, SOD, and APX antioxidant enzymes as well as glutathione (GSH) and ascorbic acid (AsA) in broad bean plants grown under non-salinity or salinity conditions. In line with our results, Abd-Allah et al. (2018) reported an increment in CAT, POD, SOD, and glutathione (GR) antioxidant enzymes in chickpea plants inoculated with B. subtilis endophyte grown under salinity and non-salinity conditions. It seems that B. subtilis inoculation stimulated the antioxidant system, leading to the accelerated elimination of toxic ROS radicals (Agami et al. 2016). In contrast to our results, a recent study on soybean plants treated by Curtobacterium sp. SAK1 endophyte bacteria exhibited that both PPO and POD were considerably reduced compared to untreated plants growing under variable levels of salinity (Khan et al. 2019).
Up-regulation of CAT, POD, PPO, SOD, and APX protected plants from free radical-induced membrane dysfunction. The acceleration of POD activity in endophyte inoculated plants resulted in improved salinity stress tolerance caused by enhancement of the biosynthesis of lignin and other related protective compounds for reducing oxidative stress (Boerjan et al. 2003). Increased plant antioxidants due to the addition of bacterial endophytes extracted from halophyte agree with the report of (Joe et al. 2016) who isolated two salt-tolerant endophytes Acinetobacter sp. and Bacillus sp. and found ascending SOD and PPO activity in the inoculated Phyllanthus amarus plants grown under 160 mM salinity stress. Other studies explained that increased activity of CAT and POD for B. subtilis inoculated plants can help in plant growth control through protecting delicate organelles such as chloroplasts wherein key metabolic processes occur (Hashem et al. 2016).
CAT plays a crucial role during stress especially in the elimination of H2O2, a signaling molecule that rapidly diffuses through membranes, to prevent membrane and organelle damage (Bienert and Chaumont 2014). Increased CAT activity in broad bean plants grown under salinity stress could be attributed to decreased production of H2O2 apoplasts (Mutlu et al. 2009). Mittal et al. (2012) demonstrated that higher CAT activity in Brassica juncea led to tolerance against high salinity. Habib et al. (2016) observed that treatment of okra plants with PGPR increased the expression of antioxidant coding genes, leading to enhanced salt stress tolerance. Similarly, PGPR-induced enhancement of antioxidant enzymes has been reported (Younesi and Moradi 2014).
The results demonstrated that the activity of APX, glutathione (GSH), and ascorbic acid (AsA) antioxidant activities significantly increased with rising levels of salinity stress. However, the activities of APX, GSH, and AsA significantly increased in the inoculated faba bean plants with B. subtilis (AR5) and/or B. thuringiensis (BR1) bacterial isolates under salinity conditions compared to non-inoculated plants. The superior values were assigned for the inoculation with B. thuringiensis (BR1) bacterial isolate (Table 2; Table S5). Inoculation with BR1 bacterial isolate significantly enhanced the activities (CAT, POD, PPO, SOD, APX, GSH, and AsA) for detoxifying ROS in stress-induced plants compared to non-inoculated stressed plants (Saravanakumar et al. 2011). Increasing ROS scavengers in stressed plants is a crucial parameter for mitigating salinity negative impacts.
The effect of NaCl on GSH and AsA contents was investigated in the present study either alone or in combination with bacterial strain inoculation. Our findings revealed that bacterial inoculations play an important role in the activation of non-enzymatic antioxidant content in faba bean plants; these findings were evident on the effective role of B. subtilis (AR5) and/or B. thuringiensis (BR1) in ascorbate–glutathione redox cycle enhancement. This finding is in accordance with the findings of (Hidri et al. 2016). This could be because AsA is a powerful antioxidant that reacts with H2O2, as well as O2−, OH−, and lipid hydro-peroxidases. Furthermore, it has been linked to a variety of biological functions in plants, including as an enzyme co-factor, an antioxidant, and a donor/acceptor in electron transport at the plasma membrane or the chloroplasts (Skłodowska et al. 2009). The results display evidence for a beneficial impact of inoculation with PGPR in improving salinity tolerance in faba bean by adjusting the antioxidants activity under salinity stress conditions (Tiwari et al. 2016).
Endophytes reduce the harmful effects of salt stress in plants through the accumulation of osmolyte compounds that are combined with osmotic adjustment and stabilize cell components. In agreement with the present data, two endophytic strains, Arthrobacter sp. and Bacillus sp. were found to stimulate the accumulation of proline content in pepper (Capsicum annuum L.) plants under osmotic stress (Sziderics et al. 2007). They found also that leaves of bacterial-inoculated plants accumulate a larger amount of proline than non-inoculated plants. In addition, bacterization also regulated stress-inducible genes in the pepper plants, suggesting that both bacterial strains alleviated osmotic stress conditions. A PGPB Pseudomonas simiae AU (MTCC-12057) successfully colonized soybean and mungbean plant roots under saline conditions. Vaishnav and Choudhary (2019) reported that P. simiae AU bacterial strain-induced synthesis of proline and total soluble sugar and expression of their respective genes in soybean plants growing under drought stress.
A compatible osmolyte such as proline, glycine, and betaine conferred plant tolerance to stress factors through osmotic adjustment (Hashem et al. 2015). In the current investigation, broad bean plants supplemented with the endophytes B. subtilis (AR5) and B. thuringiensis (BR1) and their combinations showed an increase in the content of synthesized proline with or without stress (Table S5). In agreement with the present data, Abd-Allah et al. (2018) investigated the impact of the endophytic bacterium Bacillus subtilis BERA 71 on salted stressed chickpea plants. They reported that inoculated plants contained higher antioxidant enzymes, non-enzymatic anti-oxidants, ascorbic acid contents in comparison with non-inoculated plants.
Damodaran et al. (2014) showed increased proline in gladiolus plant inoculated with the endophyte Bacillus pumilus planted in sodic soil. Pandey et al. (2012) performed an experiment in which the wheat endophytic bacterium Pseudomonas aeruginosa PW09 was added to cucumber to check cross-species bacterial mediated stress alleviation. Application of the P. aeruginosa PW09 strain-induced accumulation of free proline contents and also increased activity of defense related enzymes like polyphenol oxidase, phenylalanine ammonia lyase and SOD enzyme under NaCl stress and pathogen Sclerotium rolfsii inoculation.
The results of this investigation suggest that increments in proline content in inoculated broad beans could be considered an excellent mechanism to decrease the osmotic potential in stressed bean plants. This supports the assumption that proline is a part of the physiological response of plants to intense stress (Rabie and Almadini 2005). An important consideration is that bacterial osmolytes act synergistically with plant osmolytes accelerating osmotic adjustment. Thus, proline synthesis increased in abiotically stressed plants inoculated with beneficial bacteria such as Burkholderia (Ait Barka et al. 2006).
Our results suggested that a combination of B. subtilis (AR5) and B. thuringiensis (BR1) bacterial endophytes have an ameliorative effect on the vegetative growth of the faba bean plant grown under salt stress. The results also indicate that bacteria could ameliorate the negative effects caused by NaCl salt stress on bean plants by increasing the antioxidant enzymes and the non-antioxidant osmolytes. Therefore, the application of B. thuringiensis (BR1) may be a promising technique to decrease the deleterious impacts of salinity stress.
Enhancement of leaf nutrient contents and sodium in salt-stressed broad bean plants inoculated by AR5 and BR1 endophytic bacteria
The amount of plant nutrient elements (PNE) in plant shoots treated by PGPR strains may provide important information about the impact of bacterial inoculation in PNE uptake. In our investigation, we found that bacterial treatments increased the PNE concentration of unstressed and stressed broad bean shoots. Salinity stress led to a significant decrease in the percentage of leaf nitrogen content by 48.64% and 51.6% for the stressed plants compared with non-stressed control plants at two harvested stages, respectively. Plants inoculated with a combination of the two bacterial isolates resulted in a maximum increase in leaf nitrogen content by 23.44% and 25.49% 45 days after sowing and 42.16% and 39.2% 90 days after sowing for unstressed and stressed treatments, respectively compared with rest treatments (Table 3).
The results showed that salinity caused a nonsignificantly decrease in leaf phosphorus content for the stressed plants compared to non-stressed control plants at two harvested stages. Plants inoculated by two bacterial isolates together resulted in maximum elevation (0.190% and 0.193%), in percentage leaf phosphorus 90 days after sowing without or with salinity, respectively compared with control plants. However, BR1 endophyte strain exerted a greater impact on NaCl–treated plants through an increase of the percentage leaf phosphorus by 0.239% comparison with rest applications and un-stressed control plants 45 days after sowing (Table 3).
Generally, bacterial inoculation enhanced leaf potassium content in broad bean plants, and a great increase (23.19% and 19.14%) was recorded for mixed bacterial endophytes at unstressed and stressed conditions, respectively comparison with rest applications and control plants. after 45 days from seed sowing. 90 days after sowing, also the combination between two tested endophytes caused a maximum increase in leaf potassium content by 24.1% and 48.65% at unstressed and stressed conditions, respectively (Table 3).
Leaf calcium content was increased for all inoculated plants, where the greatest values (112.6% and 125%) were recorded in plants inoculated by AR5 after two harvested stages, respectively at unstressed conditions compared to control plants. Under salinity stress, the maximum percentage of leaf calcium content reached 85.4% and 124.56% in plants treated by mixed bacterial isolates at two harvested stages, respectively, compared to unstressed plants (Table 3).
Our results showed that plants treated with the mixed isolates recorded the highest percentages in leaf magnesium content ranged from 42.23% to 124.25% at two harvested stages at unstressed and stressed conditions compared with control plants (Table 3).
Leaf sodium content in stressed non-inoculated plants significantly increased by 131.5% and 177.5% at two harvested stages, respectively compared to unstressed control plants. The current study showed that treatment with endophytes resulted in accumulation of leaf sodium content in broad bean plants and the maximum value (14.28%) was recorded for plants inoculated with the combined endophytes comparison with control plants after 45 days from seed sowing. On the other hand, the leaf sodium content of inoculated plants was significantly reduced by 25.19%, 27.24%, and 29.94% for the isolates of AR5, BR1, and their mixed, respectively compared with the unstressed control plant. Ninety days after sowing, endophytic bacterial inoculation led to a slight increase in the percentage of leaf sodium content in plants inoculated with the endophytes isolates, and the largest value (15.9%) was found in plants treated with the mixed bacterial isolates in comparison with control plants (Table 3).
A remarkable reduction in K/Na ratio reached 70% and 79.5% for stressed plants at two harvested stages, respectively comparison with unstressed control plants. After 45 days from seed sowing, the K/Na ratio was increased by a percentage of 6.8%, 7.78%, and 8.09%, respectively for the isolates of AR5, BR1, and their mixed when comparing with unstressed control plants. However, the K/Na ratio significantly increased for inoculated stressed plants. The highest ratio of K/Na (68.75%) was found in plants treated with the mixed isolates comparison with rest applications.
A slight increase in the ratio of K/Na of 6.82% was found in bacterial inoculated plants comparing with non-inoculated control plants under normal conditions 90 days after sowing. However, bacterial inoculation improved the K/Na ratio by 50.7%, 55%, and 65.2% for plants inoculated by AR5, BR1, and their mixed isolates, respectively at salinity stress conditions comparison with non inoculated control plants. (Table 3).
Salinity affecting the quality of vegetative and reproductive organs through reduces availability, mobility, and transfer of Ca2+ and K+ ions to the growing parts of plants. Moreover, many studies pointed out that a high level of NaCl in the soil solution may increase the ratios of Na+/Ca2+ and Na+/K+ in plants, which would then be more sensitive to osmotic and specific ion toxicity as well as to nutritional disorders that result in reduced yield and quality (Sivritepe et al. 2003). A high level of salinity stress causes over-accumulation of Na+ inside the cell, which may result in ionic imbalances and metabolic toxicity (Takahashi et al. 2007). At the present investigation, plant tissue analysis proved that soil salinity caused an accumulation of Na+ and reduced the uptake of N, P, K+, Ca2+, and Mg2+ in plant shoots (Table 3).
Plants tolerate salt stress via a decrease of their leaf osmotic potential that is linked to Na+ and/or Cl− ion exclusion mechanisms or by the retention of salt ions in roots, preventing the accumulation of Na+ and/or Cl− in the shoot. The exclusion of Na+ ions and a higher K+:Na+ ratio in stressed broad bean plants have been confirmed as important selection criteria for salt tolerance (Abdelhamid et al. 2010). Na+ is the main toxic ion in saline water for most plants and the influx and accumulation of Na+ competes with K+, and there is a lower K+ uptake and increase in Na+ influx in plant cells during salt stress (Serrano and Rodriguez-Navarro 2001). Cuin et al. (2011) concluded that a high K+:Na+ ratio is more important for many species than simply maintaining a low Na+ concentration.
Nitrogen (N) fixing bacterial endophytes appeared as an eco-friendly approach to enhance N content and plant growth. The present data showed that salinity caused an increase in Na+ concentration and a decrease in K+ and Ca2+ regardless of treatment with endophytic bacteria. However, plants with endophyte bacteria reduced the Na+ uptake and/or increased the N, P, K+, Ca2+, and Mg2+ in plant shoots (Table 3) in comparison with the unstressed non-inoculated plants under salt stress, thus increasing the K+:Na+ ratio. In a metagenomic analysis of rice, the endophytic root microbiome having N fixation-related genes, which suggested that these endophytes could be involved in N cycle processes (Sessitsch et al. 2012). Puri et al. (2016) identified the N-fixing isolate Paenibacillus polymyxa P2b-2R, which was able to colonize both rhizospheric and endosphere parts of maize plants and promote growth.
Phosphorus (P) is considered one of the limiting macronutrients for plants and most soil P is in immobilized form and not available for plants. Many isolated micro-organisms have a phosphate-solubilizing activity that allows the more efficient use of P fertilizers. Many investigations have been accounted for that most endophytes from various plant species had the option to solubilize mineral phosphates (Lacava and Azevedo 2013). Dias et al. (2009) recovered two bacterial endophytes, Bacillus subtilis and B. megaterium, from strawberries that were able to solubilize phosphate and enhance plant growth.
Potassium (K) is an activator of numerous enzymes which are necessary for metabolic responses (Salisbury and Ross 1998). Plant cells need to keep up high K+ levels under salt stress to keep up with typical metabolic activities (Sairam and Tyagi 2004). Just as the homeostasis between K+ and Na+ in plants was discovered to be significant for salt tolerance (Horie et al. 2007). The K+ content was lower in broad bean leaves planted at salinity soil than those grown in normal conditions. Previous studies indicate that excessive concentration of K+ in plants growing at salinity stress could ameliorate the deleterious effects of salinity on growth and yield (Giri et al. 2007).
The present data showed that potassium concentration in bean leaves significantly (p ≤ 0.05) increased due to treatment with bacterial endophytes singly or in mixture either at normal or salt stress conditions. Increasing the bioavailability of P and K in soils with inoculation of PGPR such as Bacillus sp., via producing organic acids and other chemicals which may lead to increased K and P uptake was reported by many researchers (Lin et al. 2002; Sheng et al. 2002). Nutrient uptake enhanced by inoculated plants may be attributed to the synthesis of growth regulators by the bacteria at the root interface, which stimulated root development and resulted in better absorption of water and nutrients from the soil (Abbasi et al. 2011).
The decrease in Ca2+ and Mg2+ uptake under salt stress conditions might be due to the suppressive effect of Na+ and K+ on these cations or because of lower transport of Ca2+ and Mg2+ ions. In addition, salinity has an antagonistic impact on the uptake of Ca2+ and Mg2+ which was caused by displacing Ca2+ in the membrane of root cells (Aşık et al. 2009) on wheat.
The reduction level of salinity stress on growth parameters was decreased in the bacterial inoculated plants due to the ability of such bacteria to limit Na+ and Cl− transport into the shoots. The soil-salinity mitigating effect of Bacillus sp (AR5), B. thuringiensis (BR1), and their combination on bean plant growth was confirmed by the reduced uptake of sodium, increased K+:Na+ ratio, and the accumulation of N, P, K+, Ca2+, and Mg2+. In accordance with our study, Abd-Allah et al. (2018) examine the ameliorative role of Bacillus subtilis (BERA 71) endophyte on chickpea plants growing at salinity stress. They observed that the bacterial association increased N, P, K+, Ca2+, and Mg2+ content and reduced sodium accumulation of inoculated plants grown under salinity stress. Moreover, Damodaran et al. (2014) noticed that treatment with isolated rhizospheric and endophytic bacteria increased the uptake of potassium (K+) comparison with sodium (Na+), resulting in a lower Na/K ratio of gladiolus plants growing under sodic soils. Other examinations showed that the rhizospheric bacteria allowed the salt to accumulate in root tissues and presumably partition into the vacuole (Cheng et al. 2007), while the bacterial endophytes limited the content of sodium in plant shoots (Ali et al. 2014).
Sodium concentration was higher in salt-stressed plants, however bacterial endophytes application significantly reduced Na+ concentration in broad bean leaves. Increased K+ concentration and reduced Na+ in leaves may be considered as one of the possible mechanisms of elevated salinity tolerance by bacterial endophytes application in broad bean plants. Our results were in agreement with the data of Ashraf (2004), who observed that bacterial exopolysaccharides bind the Na+ ion in the root, through which the plant’s Na+ accumulation decreases. In that way, bacteria may alleviate salt stress in plants.
Sandhya et al. (2009) reported that exopolysaccharides produced by PGPR exhibit increased plant tolerance to drought stress. Co-inoculation of Phaseolus vulgaris L. with Rhizobia tropici and the PGPR Paenibacillus polymyxa (which produces trehalose) increased plant growth, N content, and nodulation under drought stress (Figueiredo et al. 2008). Singh and Jha (2016) revealed that Bacillus licheniformis HSW-16 interacts with Triticum aestivum, leading to improved systemic acquired resistance against salinity and enhanced nitrogen fixation, ammonium assimilation, and potassium and phosphate uptake.
Conclusions
In our investigation, endophytic bacterial species associated with two halophytic plants Arthrocnemum macrostachyum and Spergularia marina were isolated and identified using 16S rRNA gene analysis. The obtaining species exhibit plant growth-promoting activities in the absence and presence of salt stress. These activities including phosphate solubilizing, ammonia production, nitrogen fixation, biocontrol of phytopathogens, extracellular enzymatic activities, and IAA production. Irrigation of faba bean plants with 150 and 300 mM NaCl led to significant reductions in shoot length, shoot dry weight, antioxidant enzymes and non-enzymes activities, proline content, and accumulation of N, P, K+, Ca2+, Mg2+ nutrient, Na+, and K+:Na+ ratio. The results of this study revealed that application of B. subtilis (AR5) and B. thuringiensis (BR1) bacterial endophytes singly or in combination significantly improved all the reduced parameters because of salinity stress. The finding of this investigation suggested that inoculation of salt-stressed plants with two bacterial strains could alleviate salinity stress. These two bacterial strains can induce salinity tolerance, enhance nutrient uptake and promote broad bean growth under salinity stress. The combination of bacterial endophytes exerted the strongest effect in alleviating the harmful effect of soil salinity stress. Thus, inoculation with a selected mix of PGPB could serve as a useful tool for alleviating salinity stress in salt-sensitive plants.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Abbasi MK, Sharif S, Kazmi M, Sultan T, Aslam M (2011) Isolation of plant growth promoting rhizobacteria from wheat rhizosphere and their effect on improving growth, yield and nutrient uptake of plants. Plant Biosyst 145:159–168
Abd-Allah EF, Alqarawi AA, Hashem A, Radhakrishnan R, Al-Huqail AA, Al-Otibi FON, Malik JA, Alharbi RI, Egamberdieva D (2018) Endophytic bacterium Bacillus subtilis (BERA 71) improves salt tolerance in chickpea plants by regulating the plant defense mechanisms. J Plant Interact 13:37–44
Abdelhamid MT, Shokr MMB, Bekheta MA (2010) Growth, root characteristics, and leaf nutrients accumulation of four Faba Bean (Vicia faba L.) cultivars differing in their broomrape tolerance and the soil properties in relation to salinity. Commun Soil Sci Plant Anal 41:2713–2728
Abeer H, Abd-Allah E, Alqarawi A, El-Didamony G, Alwhibi M, Egamberdieva D, Ahmad P (2014) Alleviation of adverse impact of salinity on faba bean (Vicia faba L.) by arbuscular mycorrhizal fungi. Pak J Bot 46:2003–2013
Abla E, Souad E, Abdelhadi H, Bazdi O, Khadija O (2015) Antagonistic activity of endophytic bacteria isolated from Mentha rotundifolia L. Int J Sci Technol Res 4:36–39
Aczel MR (2019) What is the nitrogen cycle and why is it key to life? Front Young Minds 7:41. https://doi.org/10.3389/frym.2019.00041
Agami R, Medani R, Abd El-Mola I, Taha R (2016) Exogenous application with plant growth promoting rhizobacteria (PGPR) or proline induces stress tolerance in basil plants (Ocimum basilicum L.) exposed to water stress. Int J Environ Agri Res 2:78
Ahmad M, Zahir ZA, Asghar HN, Asghar M (2011) Inducing salt tolerance in mung bean through coinoculation with rhizobia and plant-growth-promoting rhizobacteria containing 1-aminocyclopropane-1-carboxylate deaminase. Can J Microbiol 57:578–589
Ahmad P, Ozturk M, Sharma S, Gucel S (2014) Effect of sodium carbonate-induced salinity–alkalinity on some key osmoprotectants, protein profile, antioxidant enzymes, and lipid peroxidation in two mulberry (Morus alba L.) cultivars. J Plant Interact 9:460–467
Ait Barka E, Nowak J, Clément C (2006) Enhancement of chilling resistance of inoculated grapevine plantlets with a plant growth-promoting Rhizobacterium, Burkholderia phytofirmans strain PsJN. Appl Environ Microbiol 72:7246
Ali S, Charles TC, Glick BR (2014) Amelioration of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC deaminase. Plant Physiol Biochem 80:160–167
ALKahtani MDF, Fouda A, Attia KA, Al-Otaibi F, Eid AM, Ewais EE-D, Hijri M, St-Arnaud M, Hassan SE-D, Khan N, Hafez YM, Abdelaal KAA (2020) Isolation and characterization of plant growth promoting endophytic bacteria from desert plants and their application as bioinoculants for sustainable agriculture. Agronomy 10:1325
Almaghrabi OA, Abdelmoneim T, Albishri HM, Moussa TA (2014) Enhancement of maize growth using some plant growth promoting rhizobacteria (PGPR) under laboratory conditions. Life Sci J 11:764–772
Arora S, Patel PN, Vanza MJ, Rao G (2014) Isolation and characterization of endophytic bacteria colonizing halophyte and other salt tolerant plant species from coastal Gujarat. Afr J Microbiol Res 8:1779–1788
Ashraf M (2004) Photosynthetic capacity and ion accumulation in a medicinal plant henbane (Hyoscyamus niger L.) under salt stress. J Appl Bot 78:91–96
Aşık BB, Turan MA, Çelik H, Katkat AV (2009) Uptake of wheat (Triticum durun cv. Salihli) under conditions of salinity. Asian J Crop Sci 1:87–95
Banik A, Dash GK, Swain P, Kumar U, Mukhopadhyay SK, Dangar TK (2019) Application of rice (Oryza sativa L.) root endophytic diazotrophic Azotobacter sp. strain Avi2 (MCC 3432) can increase rice yield under green house and field condition. Microbiol Res 219:56–65
Barnawal D, Bharti N, Tripathi A, Pandey SS, Chanotiya CS, Kalra A (2016) ACC-deaminase-producing endophyte Brachybacterium paraconglomeratum strain SMR20 ameliorates Chlorophytum salinity stress via altering phytohormone generation. J Plant Growth Regul 35:553–564
Bates L, Waldren R, Tease I (1973) Rapid determination of the proline for tress studies. Plant Soil 85:107–129
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Bharti N, Pandey SS, Barnawal D, Patel VK, Kalra A (2016) Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci Rep 6:34768
Bianco C, Defez R (2009) Medicago truncatula improves salt tolerance when nodulated by an indole-3-acetic acid-overproducing Sinorhizobium meliloti strain. J Exp Bot 60:3097–3107
Bienert GP, Chaumont F (2014) Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim Biophys Acta 1840:1596–1604
Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annu Rev Plant Biol 54:519–546
Boulos L (2005) Flora of egypt. Al Hadara Publishing, Cairo
Boulos L (2009) Flora of Egypt checklist, revised annotated edition. Al-Hadara Publishing, Cairo, pp 198–201
Chebotar VK, Malfanova NV, Shcherbakov AV, Ahtemova GA, Borisov AY, Lugtenberg B, Tikhonovich IA (2015) Endophytic bacteria in microbial preparations that improve plant development (review). Appl Biochem Microbiol 51:271–277
Chen C, Yu R, Owuor ED, Tony Kong AN (2000) Activation of antioxidant-response element (ARE), mitogen-activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and death. Arch Pharmacal Res 23:605
Chen G, Zhu Y, Wang H-Z, Wang S-J, Zhang R-Q (2007) The metabolites of a mangrove endophytic fungus, Penicillium thomi. J Asian Nat Prod Res 9:159–164
Chen L, Liu Y, Wu G, Veronican Njeri K, Shen Q, Zhang N, Zhang R (2016) Induced maize salt tolerance by rhizosphere inoculation of Bacillus amyloliquefaciens SQR9. Physiol Plant 158:34–44
Cheng Z, Park E, Glick BR (2007) 1-Aminocyclopropane-1-carboxylate deaminase from Pseudomonas putida UW4 facilitates the growth of canola in the presence of salt. Can J Microbiol 53:912–918
Choi Y, Hodgkiss I, Hyde K (2005) Enzyme production by endophytes of Brucea javanica. J Agric Technol 1:55–66
Cuin TA, Bose J, Stefano G, Jha D, Tester M, Mancuso S, Shabala S (2011) Assessing the role of root plasma membrane and tonoplast Na+/H+ exchangers in salinity tolerance in wheat: in planta quantification methods. Plant Cell Environ 34:947–961
Damodaran T, Rai RB, Jha SK, Kannan R, Pandey BK, Sah V, Mishra VK, Sharma DK (2014) Rhizosphere and endophytic bacteria for induction of salt tolerance in gladiolus grown in sodic soils. J Plant Interact 9:577–584
Dawood MG, Abdelhamid MT, Schmidhalter U (2014) Potassium fertiliser enhances the salt-tolerance of common bean (Phaseolus vulgaris L.). J Hortic Sci Biotechnol 89:185–192
Dhungana SA, Itoh K (2019) Effects of co-inoculation of indole-3-acetic acid-producing and -degrading bacterial endophytes on plant growth. Horticulturae 5:17
Dias ACF, Costa FEC, Andreote FD, Lacava PT, Teixeira MA, Assumpção LC, Araújo WL, Azevedo JL, Melo IS (2009) Isolation of micropropagated strawberry endophytic bacteria and assessment of their potential for plant growth promotion. World J Microbiol Biotechnol 25:189–195
Egamberdieva D, Wirth S, Jabborova D, Räsänen LA, Liao H (2017a) Coordination between Bradyrhizobium and Pseudomonas alleviates salt stress in soybean through altering root system architecture. J Plant Interact 12:100–107
Egamberdieva D, Wirth SJ, Shurigin VV, Hashem A, Abd-Allah EF (2017b) Endophytic bacteria improve plant growth, symbiotic performance of chickpea (Cicer arietinum L.) and induce suppression of root rot caused by Fusarium solani under salt stress. Front Microbiol. https://doi.org/10.3389/fmicb.2017.01887
Eid AM, Salim SS, Hassan SE-D, Ismail MA, Fouda A (2019) Role of endophytes in plant health and abiotic stress management. Microbiome in Plant Health and Disease. Springer, pp 119–144
Eid AM, Fouda A, Abdel-Rahman MA, Salem SS, Elsaied A, Oelmüller R, Hijri M, Bhowmik A, Elkelish A, Hassan SE (2021) Harnessing bacterial endophytes for promotion of plant growth and biotechnological applications: an overview. Plants 10:935
El-Esawi MA, Al-Ghamdi AA, Ali HM, Alayafi AA (2019) Azospirillum lipoferum FK1 confers improved salt tolerance in chickpea (Cicer arietinum L.) by modulating osmolytes, antioxidant machinery and stress-related genes expression. Environ Exp Bot 159:55–65
FAO, IFAD, WFP (2015) The state of food insecurity in the World 2015: meeting the 2015 international hunger targets: taking stock of uneven progress. FAO, Rome. Viewed 26 Sept 2016
Figueiredo MVB, Burity HA, Martínez CR, Chanway CP (2008) Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl Soil Ecol 40:182–188
Fouda AH, Hassan SE-D, Eid AM, Ewais EE-D (2015) Biotechnological applications of fungal endophytes associated with medicinal plant Asclepias sinaica (Bioss.). Ann Agric Sci 60:95–104
Fouda A, Hassan SED, Eid AM, El-Din Ewais E (2019) The interaction between plants and bacterial endophytes under salinity stress. In: Jha S (ed) Endophytes and secondary metabolites. Springer International Publishing, Cham, pp 591–607
Fouda A, Eid AM, Elsaied A, El-Belely EF, Barghoth MG, Azab E, Gobouri AA, Hassan SE (2021) Plant growth-promoting endophytic bacterial community inhabiting the leaves of Pulicaria incisa (Lam.) DC inherent to arid regions. Plants 10:76
Giri B, Kapoor R, Mukerji KG (2007) Improved tolerance of acacia nilotica to salt stress by arbuscular mycorrhiza, Glomus fasciculatum may be partly related to elevated K/Na ratios in root and shoot tissues. Microb Ecol 54:753–760
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica 2012:963401. https://doi.org/10.6064/2012/963401
Gothwal R, Nigam V, Mohan M, Sasmal D, Ghosh P (2008) Screening of nitrogen fixers from rhizospheric bacterial isolates associated with important desert plants. Appl Ecol Environ Res 6:101–109
Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106:207–212
Habib SH, Kausar H, Saud HM (2016) Plant growth-promoting rhizobacteria enhance salinity stress tolerance in okra through ROS-scavenging enzymes. Biomed Res Int 2016:6284547
Hafez AA, Mikkelsen DS (1981) Colorimetric determination of nitrogen for evaluating the nutritional status of rice. Commun Soil Sci Plant Anal 12:61–69
Hashem A, Abd-Allah EF, Alqarawi AA, Aldubise A, Egamberdieva D (2015) Arbuscular mycorrhizal fungi enhances salinity tolerance of Panicum turgidum Forssk by altering photosynthetic and antioxidant pathways. J Plant Interact 10:230–242
Hashem A, Abd-Allah EF, Alqarawi AA, Al-Huqail AA, Wirth S, Egamberdieva D (2016) The interaction between arbuscular mycorrhizal fungi and endophytic bacteria enhances plant growth of Acacia gerrardii under salt stress. Front Microbiol. https://doi.org/10.3389/fmicb.2016.01089
Hidri R, Barea JM, Mahmoud OM, Abdelly C, Azcón R (2016) Impact of microbial inoculation on biomass accumulation by Sulla carnosa provenances, and in regulating nutrition, physiological and antioxidant activities of this species under non-saline and saline conditions. J Plant Physiol 201:28–41
Horie T, Costa A, Kim TH, Han MJ, Horie R, Leung HY, Miyao A, Hirochika H, An G, Schroeder JI (2007) Rice OsHKT2; 1 transporter mediates large Na+ influx component into K+-starved roots for growth. EMBO J 26:3003–3014
Hu Y, Schmidhalter U (2005) Drought and salinity: a comparison of their effects on mineral nutrition of plants. J Plant Nutr Soil Sci 168:541–549
Hungria M, Vargas MAT (2000) Environmental factors affecting N2 fixation in grain legumes in the tropics, with an emphasis on Brazil. Field Crop Res 65:151–164
Irizarry I, White J (2017) Application of bacteria from non-cultivated plants to promote growth, alter root architecture and alleviate salt stress of cotton. J Appl Microbiol 122:1110–1120
Ismail MA, Amin MA, Eid AM, Hassan SE, Mahgoub HAM, Lashin I, Abdelwahab AT, Azab E, Gobouri AA, Elkelish A, Fouda A (2021) Comparative Study between exogenously applied plant growth hormones versus metabolites of microbial endophytes as plant growth-promoting for Phaseolus vulgaris L. Cells 10:1059
Jaemsaeng R, Jantasuriyarat C, Thamchaipenet A (2018) Molecular interaction of 1-aminocyclopropane-1-carboxylate deaminase (ACCD)-producing endophytic Streptomyces sp. GMKU 336 towards salt-stress resistance of Oryza sativa L. cv. KDML105. Sci Rep 8:1950
Jamil A, Riaz S, Ashraf M, Foolad MR (2011) Gene expression profiling of plants under salt stress. Crit Rev Plant Sci 30:435–458
Jasim B, John Jimtha C, Jyothis M, Radhakrishnan EK (2013) Plant growth promoting potential of endophytic bacteria isolated from Piper nigrum. Plant Growth Regul 71:1–11
Joe MM, Devaraj S, Benson A, Sa T (2016) Isolation of phosphate solubilizing endophytic bacteria from Phyllanthus amarus Schum & Thonn: Evaluation of plant growth promotion and antioxidant activity under salt stress. J Appl Res Med Aromatic Plants 3:71–77
Kalayu G (2019) Phosphate solubilizing microorganisms: promising approach as biofertilizers. Int J Agron 2019:1–7. https://doi.org/10.1155/2019/4917256
Kannan R, Damodaran T, Umamaheswari S (2015) Sodicity tolerant polyembryonic mango root stock plants: a putative role of endophytic bacteria. Afr J Biotech 14:350–359
Kar M, Mishra D (1976) Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol 57:315–319
Kasote DM, Katyare SS, Hegde MV, Bae H (2015) Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int J Biol Sci 11:982–991
Khalil AMA, Hassan SE, Alsharif SM, Eid AM, Ewais EE, Azab E, Gobouri AA, Elkelish A, Fouda A (2021) Isolation and characterization of fungal endophytes isolated from medicinal plant Ephedra pachyclada as plant growth-promoting. Biomolecules 11:140
Khan AL, Al-Harrasi A, Al-Rawahi A, Al-Farsi Z, Al-Mamari A, Waqas M, Asaf S, Elyassi A, Mabood F, Shin J-H (2016) Endophytic fungi from Frankincense tree improves host growth and produces extracellular enzymes and indole acetic acid. PLoS ONE 11:e0158207
Khan MA, Asaf S, Khan AL, Ullah I, Ali S, Kang S-M, Lee I-J (2019) Alleviation of salt stress response in soybean plants with the endophytic bacterial isolate Curtobacterium sp. SAK1. Ann Microbiol 69:797–808
Kumar A, Kumar A, Patel H (2018) Role of microbes in phosphorus availability and acquisition by plants. Int J Curr Microbiol App Sci 7:1344–1347
Lacava PT, Azevedo JL (2013) Endophytic bacteria: a biotechnological potential in agrobiology system. In: Maheshwari DK, Saraf M, Aeron A (eds) Bacteria in agrobiology: crop productivity. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 1–44
Landa BB, Hervás A, Bettiol W, Jiménez-Díaz RM (1997) Antagonistic activity of Bacteria from the chickpea rhizosphere against Fusarium Oxysporum f. sp Ciceris. Phytoparasitica 25:305–318
Lane D (1991) 16S/23S rRNA sequencing. Nucleic acid techniques in bacterial systematics. pp 115–175
Lata R, Chowdhury S, Gond SK, White JF Jr (2018) Induction of abiotic stress tolerance in plants by endophytic microbes. Lett Appl Microbiol 66:268–276
Leite MCBS, Farias ARBD, Freire FJ, Andreote FD, Kuklinsky-Sobral J, Freire MBGS (2014) Isolation, bioprospecting and diversity of salt-tolerant bacteria associated with sugarcane in soils of Pernambuco, Brazil. Revista Brasileira De Engenharia Agrícola e Ambiental 18:73–79
Lin Q-M, Rao Z-H, Sun Y-X, Yao J, Xing L-J (2002) Identification and practical application of silicate-dissolving bacteria. Agric Sci China 1:81–85
Lin L, Li Z, Hu C, Zhang X, Chang S, Yang L, Li Y, An Q (2009) Plant growth-promoting nitrogen-fixing enterobacteria are in association with sugarcane plants growing in Guangxi, China. Microbes Environ 1204160379–1204160379
Maxton A, Singh P, Masih SA (2018) ACC deaminase-producing bacteria mediated drought and salt tolerance in Capsicum annuum. J Plant Nutr 41:574–583
Mbarki S, Cerdà A, Brestic M, Mahendra R, Abdelly C, Pascual JA (2017) Vineyard compost supplemented with Trichoderma Harzianum T78 improve saline soil quality. Land Degrad Dev 28:1028–1037
Mei L, Zhu M, Zhang D-Z, Wang Y-Z, Guo J, Zhang H-B (2014) Geographical and temporal changes of foliar fungal endophytes associated with the invasive plant Ageratina adenophora. Microb Ecol 67:402–409
Miller D, Bryant J, Madsen E, Ghiorse W (1999) Evaluation and optimization of DNA extraction and purification procedures for soil and sediment samples. Appl Environ Microbiol 65:4715–4724
Milošević NA, Marinković JB, Tintor BB (2012) Mitigating abiotic stress in crop plants by microorganisms. Zbornik Matice Srpske Za Prirodne Nauke. https://doi.org/10.2298/ZMSPN1223017M
Mishra IG, Sharma A (2012) Exogenously supplied osmoprotectants confer enhanced salinity tolerance in rhizobacteria. J Ecobiotechnol
Mittal S, Kumari N, Sharma V (2012) Differential response of salt stress on Brassica juncea: photosynthetic performance, pigment, proline, D1 and antioxidant enzymes. Plant Physiol Biochem 54:17–26
Mukherjee SP, Choudhuri MA (1983) Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant 58:166–170
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Mutlu S, Atici Ö, Nalbantoglu B (2009) Effects of salicylic acid and salinity on apoplastic antioxidant enzymes in two wheat cultivars differing in salt tolerance. Biol Plant 53:334–338
Nabti E, Schmid M, Hartmann A (2015) Application of halotolerant bacteria to restore plant growth under salt stress. Halophiles. Springer, pp 235–259
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Noctor G, Mhamdi A, Chaouch S, Han Y, Neukermans J, Marquez-Garcia B, Queval G, Foyer CH (2012) Glutathione in plants: an integrated overview. Plant Cell Environ 35:454–484
Numan M, Bashir S, Khan Y, Mumtaz R, Shinwari ZK, Khan AL, Khan A, Al-Harrasi A (2018) Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: a review. Microbiol Res 209:21–32
Otieno N, Lally R, Kiwanuka S, Lloyd A, Ryan D, Germaine K, Dowling D (2015) Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front Microbiol. https://doi.org/10.3389/fmicb.2015.00745
Palaniyandi SA, Damodharan K, Yang SH, Suh JW (2014) Streptomyces sp. strain PGPA39 alleviates salt stress and promotes growth of ‘Micro Tom’ tomato plants. J Appl Microbiol 117:766–773
Pandey PK, Yadav SK, Singh A, Sarma BK, Mishra A, Singh HB (2012) Cross-species alleviation of biotic and abiotic stresses by the endophyte Pseudomonas aeruginosa PW09. J Phytopathol 160:532–539
Paul D, Sinha SN (2015) Isolation and characterization of a phosphate solubilizing heavy metal tolerant bacterium from River Ganga, West Bengal, India. Songklanakarin J Sci Technol 37:651–657
Pimentel D, Berger B, Filiberto D, Newton M, Wolfe B, Karabinakis E, Clark S, Poon E, Abbett E, Nandagopal S (2004) Water resources: agricultural and environmental issues. Bioscience 54:909–918
Pinedo I, Ledger T, Greve M, Poupin MJ (2015) Burkholderia phytofirmans PsJN induces long-term metabolic and transcriptional changes involved in Arabidopsis thaliana salt tolerance. Front Plant Sci. https://doi.org/10.3389/fpls.2015.00466
Pirhadi M, Enayatizamir N, Motamedi H, Sorkheh K (2016) Screening of salt tolerant sugarcane endophytic bacteria with potassium and zinc for their solubilizing and antifungal activity. Biosci Biotech Res Comm 9:530–538
Puri A, Padda KP, Chanway CP (2016) Evidence of nitrogen fixation and growth promotion in canola (Brassica napus L.) by an endophytic diazotroph Paenibacillus polymyxa P2b–2R. Biol Fertil Soils 52:119–125
Rabie G, Almadini A (2005) Role of bioinoculants in development of salt-tolerance of Vicia faba plants under salinity stress. Afr J Biotech 4:210–222
Rodrigues AA, Forzani MV, Soares RDS, Sibov ST, Vieira JDG (2016) Isolation and selection of plant growth-promoting bacteria associated with sugarcane. Pesquisa Agropecuária Tropical 46:149–158
Sairam R, Tyagi A (2004) Physiology and molecular biology of salinity stress tolerance in plants. Curr Sci 407–421
Salisbury F, Ross C (1998) Plant physiology. Wads Worth Pub Co, Belmont
Sandhya V, Sk ZA, Grover M, Reddy G, Venkateswarlu B (2009) Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol Fertil Soils 46:17–26
Saravanakumar D, Samiyappan R (2007) ACC deaminase from Pseudomonas fluorescens mediated saline resistance in groundnut (Arachis hypogea) plants. J Appl Microbiol 102:1283–1292
Saravanakumar D, Kavino M, Raguchander T, Subbian P, Samiyappan R (2011) Plant growth promoting bacteria enhance water stress resistance in green gram plants. Acta Physiol Plant 33:203–209
Schafer FQ, Buettner GR (2001) Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radical Biol Med 30:1191–1212
Schloss PD, Handelsman J (2006) Toward a census of bacteria in soil. PLoS Comput Biol 2:e92
Selvi K, Paul J, Vijaya V, Saraswathi K (2017) Analyzing the efficacy of phosphate solubilizing microorganisms by enrichment culture techniques. Biochem Mol Biol J 3:1–7
Serrano R, Rodriguez-Navarro A (2001) Ion homeostasis during salt stress in plants. Curr Opin Cell Biol 13:399–404
Sessitsch A, Hardoim P, Döring J, Weilharter A, Krause A, Woyke T, Mitter B, Hauberg-Lotte L, Friedrich F, Rahalkar M, Hurek T, Sarkar A, Bodrossy L, van Overbeek L, Brar D, van Elsas JD, Reinhold-Hurek B (2012) Functional characteristics of an endophyte community colonizing rice roots as revealed by metagenomic analysis. Mol Plant-Microbe Interact 25:28–36
Shahid M, Akram MS, Khan MA, Zubair M, Shah SM, Ismail M, Shabir G, Basheer S, Aslam K, Tariq M (2018) A phytobeneficial strain Planomicrobium sp. MSSA-10 triggered oxidative stress responsive mechanisms and regulated the growth of pea plants under induced saline environment. J Appl Microbiol 124:1566–1579
Sharma S, Kulkarni J, Jha B (2016) Halotolerant rhizobacteria promote growth and enhance salinity tolerance in peanut. Front Microbiol. https://doi.org/10.3389/fmicb.2016.01600
Sheng X-F, He L-Y, Huang W-Y (2002) The conditions of releasing potassium by a silicate-dissolving bacterial strain NBT. Agric Sci China 1:662–666
Shrivastava P, Kumar R (2015) Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci 22:123–131
Singh RP, Jha PN (2016) A Halotolerant bacterium Bacillus licheniformis HSW-16 augments induced systemic tolerance to salt stress in wheat plant (Triticum aestivum). Front Plant Sci. https://doi.org/10.3389/fpls.2016.01890
Singh P, Kumar V, Agrawal S (2014) Evaluation of phytase producing bacteria for their plant growth promoting activities. Int J Microbiol 2014
Sivritepe N, Sivritepe HO, Eris A (2003) The effects of NaCl priming on salt tolerance in melon seedlings grown under saline conditions. Sci Hortic 97:229–237
Skłodowska M, Gapińska M, Gajewska E, Gabara B (2009) Tocopherol content and enzymatic antioxidant activities in chloroplasts from NaCl-stressed tomato plants. Acta Physiol Plant 31:393–400
Smirnoff N, Wheeler GL (2000) Ascorbic acid in plants: biosynthesis and function. Crit Rev Biochem Mol Biol 35:291–314
Sziderics AH, Rasche F, Trognitz F, Sessitsch A, Wilhelm E (2007) Bacterial endophytes contribute to abiotic stress adaptation in pepper plants (Capsicum annuum L.). Can J Microbiol 53:1195–1202
Takahashi R, Nishio T, Ichizen N, Takano T (2007) High-affinity K+ transporter PhaHAK5 is expressed only in salt-sensitive reed plants and shows Na+ permeability under NaCl stress. Plant Cell Rep 26:1673–1679
Tewari S, Arora NK (2016) Fluorescent Pseudomonas sp. PF17 as an efficient plant growth regulator and biocontrol agent for sunflower crop under saline conditions. Symbiosis 68:99–108
Tiwari S, Lata C, Chauhan PS, Nautiyal CS (2016) Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. during drought stress and recovery. Plant Physiol Biochem PPB 99:108–117
Ullah I, Khan AR, Park G-S, Lim J-H, Waqas M, Lee I-J, Shin J-H (2013) Analysis of phytohormones and phosphate solubilization in Photorhabdus spp. Food Sci Biotechnol 22:25–31
Vaishnav A, Choudhary DK (2019) Regulation of drought-responsive gene expression in Glycine max L. Merrill is mediated through Pseudomonas simiae strain AU. J Plant Growth Regul 38:333–342
Yaish MW, Antony I, Glick BR (2015) Isolation and characterization of endophytic plant growth-promoting bacteria from date palm tree (Phoenix dactylifera L.) and their potential role in salinity tolerance. Antonie Van Leeuwenhoek 107:1519–1532
Younesi O, Moradi A (2014) Effects of plant growth-promoting rhizobacterium (PGPR) and arbuscular mycorrhizal fungus (AMF) on antioxidant enzyme activities in salt-stressed bean (Phaseolus vulgaris L.). Agriculture (pol’nohospodárstvo) 60:10–21
Zaferanloo B, Virkar A, Mahon PJ, Palombo EA (2013) Endophytes from an Australian native plant are a promising source of industrially useful enzymes. World J Microbiol Biotechnol 29:335–345
Zhao L, Xu Y, Lai X (2018) Antagonistic endophytic bacteria associated with nodules of soybean (Glycine max L.) and plant growth-promoting properties. Braz J Microbiol 49:269–278
Funding
This work was not supported by any funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by [HAMM], [AF], [AME], [EE-DE], and [SE-DH]. The first draft of the manuscript was written by [HAMM], [AF], [AME], and [SE-DH], and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mahgoub, H.A.M., Fouda, A., Eid, A.M. et al. Biotechnological application of plant growth-promoting endophytic bacteria isolated from halophytic plants to ameliorate salinity tolerance of Vicia faba L.. Plant Biotechnol Rep 15, 819–843 (2021). https://doi.org/10.1007/s11816-021-00716-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-021-00716-y