Abstract
The rice genome contains 13 Flowering Locus T-Like (FT-Like) genes, among which, OsFTL2/Hd3a and OsFTL3/RFT1 are characterized as florigen-encoding genes, promoting rice flowering under short-day and long-day conditions, respectively. However, the functions of the other FT-Like members are still unknown in rice. In this study, we characterized and identified a member of FT-Like family, OsFTL8 gene. The investigation of photoperiodic expression pattern showed that OsFTL8 transcription was mainly induced under short-day conditions. The qRT-PCR and GUS staining assays indicated that OsFTL8 was broadly expressed in various organs, including stems, leaves and panicles. OsFTL8 overexpression transgenic lines and its knockout mutants displayed no obvious change in their heading date, compared with the wild type. However, the pollen viability and seed setting rate were decreased in the overexpression transgenic lines. OsFTL8 localizes in both nucleus and cytoplasm. And we found that OsFTL8 does not interact with OsFD1 in vivo, suggesting that OsFTL8 may not be the component of florigen activation complex (FAC). In summary, our results indicate that OsFTL8 is associated with pollen viability and not involved in regulating rice flowering time, implying functional divergence of FT-Like members.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flowering, a remarkable transition from vegetative growth to reproductive growth of higher plants, is mainly induced by environmental signal such as photoperiod and regulated by the expression of endogenous florigen genes (Song and Luan 2012). Florigen is a kind of small globular protein, belonging to the phosphatidylethanolamine-binding protein (PEBP) family (Chardon and Damerval 2005). The PEBP genes are conserved among various plant species, from monocots to dicots (Karlgren et al. 2011; Lorenzo et al. 2020; Sun et al. 2018).
Rice is a facultative short-day plant, and its flowering is promoted and repressed by short-day and long-day, respectively (Cai et al. 2019). There are 19 PEBP genes in rice genome, which are further divided into FT-like (FTL), TF-like (TFL) and Mother of FT (MFT) subfamilies, based on the deduced protein similarity, which include 13 (OsFTL1 ~ OsFTL13), 4 (RCN1 ~ RCN4) and 2 (OsMFT1 and OsMFT2) members, respectively (Kabayashi et al. 1999; Chardon and Damerval 2005; Karlgren et al. 2011).
The functions of several rice PEBP genes have been characterized, most of which are involved in flowering regulation. For example, OsFTL2/Hd3a and OsFTL3/RFT1 are florigen genes under short-day and long-day conditions, respectively (Kojima et al. 2002; Tamaki et al. 2007; Komiya et al. 2008; Du et al. 2017). OsFTL2/Hd3a and OsFTL3/RFT1 control rice flowering through the OsGI-Hd1-Hd3a pathway and rice unique Ghd7-Ehd1-Hd3a/RFT1 pathway (Komiya et al. 2009; Itoh et al. 2010; Zhang et al. 2017; Lee and An 2015; Xue et al. 2008; Zheng et al. 2019). Hd3a protein is expressed in companion cells of vascular tissue in leaf blade, and then transported to the shoot apical meristem (SAM) through phloem (Taoka et al. 2011). In the cytoplasm of SAM cells, Hd3a protein interacts with 14–3-3 protein to form hetero-tetramer, which then enters the nucleus and interacts with OsFD1 protein to form hetero-hexamer, generating the florigen activation complex (FAC). The FAC binds to the promoter of floral meristem identity genes, OsMADS15, inducing its transcription and thus promoting the floral initiation (Taoka et al. 2013). Several residues in Hd3a protein (D62, M63, R64, F66, P96, T98, T99, F103, and R132) were predicted to be localized on its interacting surface with 14-3-3, and were thought important for the FAC formation (Taoka et al. 2013).
RCNs have been identified as anti-florigen genes. Overexpression of RCN1 and RCN2 delayed heading and increased the number of branches per panicle (Nakagawa et al. 2002). All four RCN proteins were expressed in vascular cells and transported into apical meristem cells to compete the components of FAC, delaying floral transition (Kaneko-Suzuki et al. 2018). For MFT subfamily, overexpression of OsMFT1 delayed flowering and increased the number of branches and spikelets per panicle (Song et al. 2018). Hd3a, RFT1, and all of RCNs could interact with OsFD1, suggesting that interacting with OsFD1 may be the prerequisite for their flowering regulation.
Among FTLs, the double RNAi lines of OsFTL2/Hd3a and OsFTL3/RFT1 did not flower in 300 days, suggesting that OsFTL2/Hd3a and OsFTL3/RFT1 are indispensable for promoting flowering (Komiya et al. 2008). Recent studies showed that overexpression of OsFTL10 promoted flowering through the formation of FAC. Overexpression of OsFTL10 also improved tolerance to drought stress (Fang et al. 2019), suggesting that FTLs may be involved in abiotic stress tolerance. Till now, the functions of the rest 10 FTLs remain to be elucidated. In this study, we characterized the expression pattern and investigated the function of OsFTL8 gene. Our results showed that OsFTL8 was expressed in various organs. Either knockout or overexpression of OsFTL8 did not affect rice flowering, whereas OsFTL8-overexpression plants exhibited reduced pollen viability and decreased seed setting. OsFTL8 protein was localized in both the nucleus and cytoplasm. OsFTL8 did not interact with OsFD1 in vivo, implying that it may not be involved in the formation of FAC. Our results demonstrated that the function of OsFTL8 is divergent from flowering regulation.
Materials and methods
Plant materials
The wild type Zhonghua11 (Oryza sativa, spp. Japonica) and transgenic lines were grown in the fields in Tianjin, China (117.14° E, 39.06° N, NLD). Plants for circadian expression analysis were grown in growth chambers, whose temperature was set as 28 ℃ and day length was set as 9 h light/15 h dark for short-day conditions and 15 h light/9 h dark for long-day conditions.
Cloning of OsFTL8 and sequences alignment
The annotated sequence of OsFTL8 was obtained from the Rice Genome Annotation Project database (https://rice.plantbiology.msu.edu). Coding sequence of OsFTL8 was cloned from cDNA template of Zhonghua11 and Nipponbare (Oryza sativa, spp. Japonica) with primers pFTL8-OX-F and pFTL8-OX-R. The PCR fragments were sequenced, and the deduced protein sequences were aligned with those of Hd3a, RFT1, OsFTL10, RCN1 ~ RCN4, OsMFT1, AtFT and AtTFL on the platform https://espript.ibcp.fr/ESPript/ESPript/ according to the procedures described in (Robert and Gouet 2014).
Vectors construction
For construction of OsFTL8 overexpression vector, the 543 bp coding sequence of OsFTL8 was amplified from the cDNA of Zhonghua11 with primers pFTL8-OX-F and pFTL8-OX-R, and then sequenced and cloned into binary vector pCAMBIA2300 with double CaMV35S promoter.
For construction of promoter::GUS vectors, 2 044 bp promoter sequence upstream of OsFTL8 start codon was amplified with primers pFTL8-2044-F and pFTL8-GUS-R, 1 068 bp truncated promoter fragment of OsFTL8 promoter was amplified with primers pFTL8-1068-F and pFTL8-GUS-R; 422 bp truncated promoter fragment was amplified with primers pFTL8-422-F and pFTL8-GUS-R. These three fragments were cloned into the binary vector pCAMBIA1391Z to generate p2044-GUS, p1068-GUS and p422-GUS recombinant vectors, respectively.
For construction of CRISPR-Cas9 recombinant vector, the 543 bp coding sequence of OsFTL8 gene was analyzed on the platform E-CRISP (https://www.e-crisp.org) to screen the target sites and a 20 bp sequence 5′-GTCGGCGAACAGCCTGGTGC-3′ was selected as the target site to ligase into CRISPR-Cas9 empty vector to produce recombinant CRISPR-Cas9 vector.
Obtainment the transgenic plants
For obtainment of the overexpression transgenic plants, CRISPR-Cas9 gene editing transgenic plants and promoter::GUS transgenic plants of OsFTL8, the recombinant plasmids were introduced into Agrobacterium EHA105 via electroporation transformation method, respectively. The transgenic plants were obtained using the Agrobacterium-mediated transformation method described previously (Hiei et al. 1994). Briefly, the seeds without glumes of Zhonghua11 variety were sterilized with 75% alcohol and then cultured on the solid N6 medium in petri dishes in darkness for 2 weeks to induce calli. The induced calli were transferred to new mediums and subcultured for 1 week. After 3 rounds of subculturing, the vigorous calli were co-cultivated with A. tumefaciens EHA105 with recombinant plasmid for 2 days. Then, the co-cultivated calli were subcultured on the selection medium with corresponding antibiotic for 3 subculturing rounds. Finally, the calli were transferred under light to induce the differentiation of seedlings in differentiation medium. After root emerged, the seedlings were transplanted to field for seed collection.
Gene expression analyses
For analyzing the transcriptional changes with diurnal rhythm, leaves of the plants grown in a chamber were collected every 3 h within 24 h from 60-day-old plants. For gene expression assay in different organs, samples including roots, stems, leaves, SAM, and panicles of different length (P1–P4), were collected. Total RNAs were extracted using Trizol solution (Invitrogen, USA). cDNAs were synthesized from 1 µg of total RNA. One micro liter of cDNA was used for real-time PCR analysis using SYBR Green PCR master mix (Vazyme biotech, China) and the gene specific primer pair of pFTL8-ex-F and pFTL8-ex-R. Real-time PCR was performed in a LightCycler480 system (Roche, USA). OsActin1 gene (Gui et al. 2014) was used as an internal control using primers pActin1-F and pActin1-R. The amplification conditions were set as follows: 5 min at 95 ℃, 40 cycles of 10 s at 95 ℃, 30 s at 60 ℃ 15 s at 95 ℃ 1 min at 60 ℃ and 0 s at 95 ℃; 30 s at 40 ℃. Three replicates of each reaction were performed. Data were analyzed by the LightCycler480 Software system according to the instruction manual. Relative expression levels were calculated according to 2−ΔΔCt method described previously (Livak and Schmittgen 2001).
For expression analysis of genes involved in pollen viability, total RNAs were extracted from young panicles of the control line and OsFTL8 overexpression transgenic lines, the qRT-PCR experiments were carried out using the same procedures described above. The primer sequences are listed in (Supplementary file 3).
GUS staining assay
To test the activity of 3 fragments of OsFTL8 promoter in promoter::GUS transgenic plants, we performed GUS staining assay. Various organs and tissues, including, stems, leaves, ligules and spikelets of promoter::GUS transgenic lines were harvested at the flowering stage. GUS staining assay was performed according to the procedure described before (Jefferson et al. 1987). Briefly, samples were immersed in the GUS staining solution (100 mM phosphate buffer pH 7.0, 10 mM EDTA, 1 mM potassium ferricyanide, 0.1% Triton X-100 and 2 mM X-Gluc) in a shaker at 30℃ overnight, then washed with 70% ethanol, and subsequently observed under stereoscope.
Cytological observation
At flowering stage, spikelets from the wild type and OsFTL8-OX lines were collected and fixed with Carony’s fixative. Pollen grains were stained with 1% I2–KI solution, and then observed under optical microscope. Pollen viability was determined according to the standard described before (Zhou et al. 2011). More than 30 spikelets and more than 10,000 pollens were investigated for each sample.
Subcellular localization
The cDNA sequence of OsFTL8 without stop codon was cloned into the vector p35S::GFP, which contains the GFP encoding sequence fused at the C terminus of the introduced fragment. The recombinant plasmid p35S::FTL8:GFP was introduced into Agrobacterium strain EHA105 and infiltrated into tobacco leaves. 70 h after infiltration, the epidermal cell layers were observed under fluorescence microscope (Leica, DM5000B, Germany), and images were captured.
Y2H assay
Y2H assays were carried out using the Matchmaker Gold Yeast Two-Hybrid Systems (Clontech) according to the manufactures’ instruction. The cDNA sequence of OsFTL8 was cloned into bait vector pGBKT7 digested with Sma I and Pst I. The coding sequence of OsFD1 was cloned into prey vector pGADT7 digested with Nde I and BamH I. The cDNA sequence of OsFTL2/Hd3a was cloned into pGBKT7 digested with EcoR I and Sal I, which was used as a positive bait control.
Results
Cloning and sequence analyses of OsFTL8 gene
The annotated OsFTL8 gene (LOC_Os01g10590) on Rice Genome Annotation Project database (RGAP) (https://rice.plantbiology.msu.edu/) contains 4 exons and 3 introns, and its coding sequence (CDS) is 510 bp, encoding a protein of 169 amino acids. To study the function of OsFTL8, we cloned the cDNA of OsFTL8 in the rice japonica variety Zhonghua11, according to the annotated ORF. We found that the CDS of OsFTL8 was 543 bp, and the deduced OsFTL8 protein contains 180 amino acids (Fig. 1a, 1b). To verify whether the variation in CDS length of OsFTL8 was caused by diversification among cultivars, we also cloned the cDNA of OsFTL8 from Nipponbare and found it was also 543 bp. Sequence alignment revealed that the first exon of OsFTL8 was 191 bp, rather than 159 bp (Fig. 1a; Supplementary file 1).
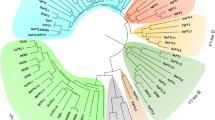
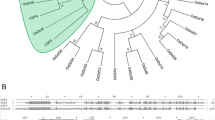
Sequence analyses of OsFTL8. a Comparison of OsFTL8 of annotated in RGAP and cloned by experiment. Boxes indicate exons, horizontal lines indicate introns. b Amino acids alignment of annotated OsFTL8 (OsFTL8-anno) and the cloned OsFTL8 (OsFTL8-clon). c Sequence alignment of OsFTL8 with other functionally characterized PEBPs in rice and Arabidopsis. Three α-helixes (α1–α3) and nine β-sheets (β1–β9) were predicted. Red triangles denote residues predicted on the interacting surface between Hd3a and 14–3-3 protein. Residues were colored according to their physicochemical properties. HKR cyan, DE red, STNQ maroon, AVLIM pink, FYW blue, PG orange, C green
OsFTL8 protein sequence was aligned with those of Hd3a, RFT1, and other functionally characterized PEBPs in rice, as well as FT and TFL in Arabidopsis. As shown in Fig. 1c, the secondary structures, including three α-helixes and nine β-sheets, were indicated; the residues that predicted to be localized on the interacting surface between Hd3a and 14–3-3 (D62, M63, R64, F66, P96, T98, T99, F103, and R132) were highlighted. Among these residues, three in OsFTL8 were substituted by others, which are L58, H59 and Y98. Since these residues may be required for the formation of FAC, the variations in OsFTL8 implies its functional differentiation from Hd3a and other PEBPs.
Expression pattern analyses of OsFTL8 gene
To investigate the diurnal expression pattern of OsFTL8, qRT-PCR assay was carried out with Zhonghua11 plants grown in artificial climate cabinets under short-day and long-day condition. The total RNAs from the leaves was extracted and reverse-transcripted into cDNA for qRT-PCR assay. The results showed that transcription of OsFTL8 was induced strongly under short-day condition but weakly under long-day condition (Fig. 2a). Under short-day conditions, the expression of OsFTL8 peaked at midnight in the dark-period, different from the diurnal expression pattern of OsFTL2/Hd3a, which exhibits peaks after dawn in the light-period and bottoms in the dark-period (Fig. 2b). We also analyzed the expression of OsFTL8 in different organs, including SAMs, roots, leaves, stems and panicles, from the plants grown in field. The qRT-PCR results showed that OsFTL8 was expressed in all of these organs, especially highly in stems (Fig. 2c).
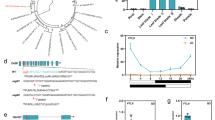
Temporal-spatial expression pattern analyses of OsFTL8 gene. a, b Diurnal expression pattern of OsFTL8 (a) and OsFTL2/Hd3a (b) under short-day (SD) and long-day (LD) conditions, respectively. Wild type plants were grown in artificial chamber under SD and LD conditions. LD condition: 9 h light/15 h dark and 28℃. SD condition: 15 h light/9 h dark and 28 ℃. Leaves were collected for RNA extraction every 3 h within 24 h from 60-day-old plants. OsActin1 was used as internal reference. c Expression of OsFTL8 in different organs. P1–P4, panicles collected at the length of 0–0.5 cm, 1–2 cm, 3–4 cm and 5–6 cm, respectively. d Construction of the truncated promoter of OsFTL8 and GUS reporter gene. 2 044 bp, 1 068 bp and 422 bp upstream sequences from the start codon of OsFTL8 gene were cloned and fused with GUS gene, respectively. e GUS staining assay of different organs from transgenic lines expressing vectors indicated in d
To further investigate the activity of OsFTL8 promoter, several transgenic lines expressing GUS gene driven by different length of OsFTL8 promoter were generated by Agrobacterium-mediated transformation of the recombinant pCAMBIA1391Z plasmids containing 2 044 bp, 1 068 bp or 422 bp upstream sequences of OsFTL8 gene (Fig. 2d). The organs of the transgenic lines, including stems, leaves, spikelets and the sheath with ligules were collected for GUS staining assay. The results indicated that GUS staining was only detected in the transgenic lines p1068-gus and p2044-gus, with higher intensity in the later, but not in the p422-gus and control lines (Fig. 2e), suggesting that the cis-elements in the region from 422 to 2 044 bp upstream sequences of OsFTL8 gene may play important roles for promoting the transcription of OsFTL8.
To reveal the potential cis-element and motifs in the OsFTL8 promoter region, we analyzed the 2044 bp upstream sequence of the start codon (ATG) using the PlantCARE platform (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/) (Lescot et al. 2002). Hundreds of cis-elements are identified in this region, among which, 29 cis-elements are related to gene transcription regulation, 44 cis-elements are related to light response, and 12 cis-elements are related to pollen expression (Supplementary file 2). Most of these cis-elements are located in the region from 422 to 2044 bp, these cis-elements may be responsible for the expression of GUS gene in transgenic plants.
Overexpression of OsFTL8 did not affect the heading date in rice
To investigate the biological function of OsFTL8, we generated the OsFTL8 overexpression transgenic lines. The cDNA sequence of OsFTL8 gene was cloned into the binary vector pCAMBIA2300 under driven by CaMV35S promoter. Transgenic plants were obtained via the Agrobaterium-mediated transformation and characterized by qRT-PCR analysis of OsFTL8 transcriptional levels in T0 generation. Finally, four independent homozygous transgenic lines (OX-L1, OX-L5, OX-L6, and OX-L7) were obtained in T2 generation and used for investigating the phenotypic agronomic traits. The transgenic plants carrying the pCAMBIA2300 empty vector were used as the control.
Firstly, we investigated the transcriptional levels of OsFTL8 in these four homozygous OsFTL8 overexpression transgenic lines. The results showed that OsFTL8 expressed significantly higher in these transgenic lines than that in the control (Fig. 3a), suggesting that the overexpression cassette of OsFTL8 worked efficiently in these transgenic lines. During the reproductive growth stage, several important agronomic traits, including heading date, plant height, panicle length and seed setting rate were investigated. Statistical analysis showed that the heading date of the four transgenic lines were not significantly changed compared with that of the control line (Fig. 3b), but the plant height, panicle length and seed setting rate were significantly reduced in the overexpression lines (Fig. 3c-g). Furthermore, the overexpression lines exhibited abnormal panicle structures, such as the tips of some secondary branches were bald (Fig. 3h). These findings suggested that overexpression of OsFTL8 gene does not affect the heading date, but could result in pleotropic developmental defects.
Phenotypic analyses of the transgenic lines overexpressing OsFTL8. a Expression levels of OsFTL8 in different transgenic lines of T2 generation. b–e Statistical analyses of heading date, plant height, panicle length and seed setting rate in different transgenic lines. Values shown are mean ± standard deviation (n = 30). DAP, days after planting. *P < 0.05, **P < 0.01. f Full grains and empty grains of per panicle in the control and transgenic lines. g, h Phenotypes of panicles in the transgenic plants and the control. White arrows indicate panicle tips without spikelet development. Bars in g, h = 3 cm
Overexpression of OsFTL8 reduced the pollen viability in rice
To investigate the causation of reduced seed setting rate, spikelets were collected for further developmental observation. We found that the morphology of pistil and stamen in transgenic lines were as normal as that in the control, and pollen grains could be released by anther dehiscence normally. However, when pollen viability was investigated, ~ 94% (n > 10 000) pollen grains of the control line were stained into dark blue-black by I2–KI solution (Fig. 4a), whereas only ~ 63% of OX-L1 (n > 10,000) and ~ 51% of OX-L6 (n > 10,000) pollens could be stained by I2-KI (Fig. 4b, c). The available pollens in these overexpression lines displayed smaller size and irregular shapes (Fig. 4b, c). These results demonstrated that the decreased seed setting rate in OsFTL8 overexpression transgenic lines may be caused by the reduced pollen viability.
Pollen viability and gene expression analyses in OsFTL8 overexpression transgenic lines. a–c Pollen grains stained with 1% I2–KI solution of the control (a) and transgenic lines (b, c). Arrows mark lightly-stained or not-stained pollen grains. Bars = 100 µm. d Expression analysis of genes associated with pollen development and male sterility in OsFTL8 overexpression transgenic lines. OsActin1 was used as internal reference
To investigate the potential mechanisms of the reduced pollen viability, we investigated the expression of several genes that involved in pollen development and male sterility, including Ugp1, Ugp2, DPW, RIP1, OsMADS3 and RTS (Chen et al. 2007; Mu et al. 2009; Shi et al. 2011; Han et al. 2006; Hu et al. 2011; Luo et al. 2006; Guo et al. 2012). We found that the expression of DPW gene was significantly reduced, while the expression of Ugp1, Ugp2, RIP1, OsMADS3 and RST was unchanged (Fig. 4d). DPW gene encodes a fatty acid reductase and plays important role in the pollen wall development (Shi et al. 2011). Therefore, we speculated that overexpression of OsFTL8 may affect the development of pollen by genes associated with pollen development.
Phenotypic analysis of OsFTL8 knockout mutants
To further investigate the biological function of OsFTL8, we also obtained OsFTL8 knockout mutants using CRISPR-Cas9 genome editing system. A target site in the upstream coding sequence of OsFTL8 was selected (Fig. 5a) and constructed into CRISPR-Cas9 vector. Transgenic plants were identified by PCR and sequencing OsFTL8 gene. Three out of seventeen independent transgenic lines were identified as OsFTL8-edited, which carries frame-shift mutation in ftl8-1 and ftl8-3, and fragment deletion in ftl8-2 (Fig. 5b), thus these transgenic lines are OsFTL8 knock out plants. The three mutants were self-fertilized to produce the T2 generation for phenotypic analysis. No significant alterations were found regarding to heading date, plant architecture or seed setting rate. We also investigated the pollen viability of these OsFTL8 knock out plants, the results showed that ~ 93% of ftl8-1 (n > 10,000), ~ 89% of ftl8-2 (n > 10,000), and ~ 91% of ftl8-3 (n > 10,000) pollens could be stained into dark blue-black by I2-KI, displaying no significant changes compared with the wild type. These results indicated that loss-function of OsFTL8 leads to no obvious phenotypic defects, implying that the function of OsFTL8 may be redundant in rice.
Gene editing of OsFTL8 by CRISPR-Cas9 system. a Target site in the genomic region of OsFTL8. The arrow indicates the position of target site. b Alignment of target site of OsFTL8 gene in wild type and mutants. PAM, the protospacer adjacent motif. Red letter means insertion base, and dashes mean deletion bases
Subcellular localization analysis of OsFTL8 protein
To investigate the subcellular localization of OsFTL8 protein, CDS of OsFTL8 without stop codon fused with GFP encoding sequence at C-terminus was transiently expressed in the epidermal cell of tobacco leaves, driven by CaMV35S promoter. Under fluorescence microscope, OsFTL8-GFP were detected in the nucleus with strong intensity; meanwhile, weak signals were also observed in the cytoplasm (Fig. 6), suggesting that OsFTL8 protein may conduct its functions in both the nucleus and cytoplasm.
OsFTL8 did not interact with OsFD1 in vivo
In FTL subfamily, both Hd3a and RFT1 interact with the 14-3-3 and OsFD1 protein to form the FAC; additionally, OsFTL10 protein was also found to interact with OsFD1 (Taoka et al. 2011; Fang et al. 2019). To investigate whether OsFTL8 protein interacts with OsFD1 protein, yeast two-hybrid (Y2H) assay was performed. The ORFs of OsFTL8 and OsFD1 were cloned into pGBKT7 and pGADT7 vectors as the bait and prey, respectively. Hd3a was employed as positive control bait. The results showed that the yeast strain co-transformed with pGBKT7-Hd3a and pGADT7-OsFD1 was able to grow on SD/-Trp-Leu-His-Ade medium, but not the strain co-transformed with pGBKT7-OsFTL8 and pGADT7-OsFD1 (Fig. 7), suggesting that OsFTL8 does not interact with OsFD1 directly. Thus, OsFTL8 may be not involved in FAC formation or controlling the flowering time, which is consistent with the unchanged heading date in OsFTL8 overexpression transgenic plants and its knockout mutants.
Yeast two-hybrid analysis of OsFTL8 with OsFD1. Yeast strain co-transformed with pBD-OsFTL8 and pAD-OsFD1 was grown on DDO (SD/-Trp-Leu) and QDO (SD/-Trp-Leu-Ade-His) to check the interaction between OsFTL8 and OsFD1. Yeast strain co-transformed with pBD-Hd3a and pAD-OsFD1 was taken as a positive control, and yeast strain co-transformed with pBD-empty (pGBKT7) and pAD-empty (pGADT7) was taken as a negative control
Discussion
In this study, the expression pattern and biological function of OsFTL8 gene were investigated. OsFTL8 was broadly expressed in various organs. Overexpression of OsFTL8 resulted in reduced plant height, panicle length and seed setting rate, but the heading date was not affected, suggesting that OsFTL8 may be not involved in regulating rice flowering time, whose function is differentiated from that of other FTLs. Moreover, OsFTL8 knockout mutants did not exhibit obvious defects in their agricultural traits when compared with the wild type, implying that OsFTL8 may have functional redundancy with its homologues in rice.
OsFTL8 is not involved in FAC formation and flowering regulation
To date, most PEBP members involved in rice flowering regulation have been indicated to interact with OsFD1. For example, Hd3a interacts with 14-3-3 and OsFD1 to form the FAC (Taoka et al. 2011); RCN proteins, binding with OsFD1, antagonizes florigen by competing its integration into FAC (Kaneko-Suzuki et al. 2018). OsFTL10 was also shown to interact with OsFD1 protein (Fang et al. 2019). In our study, Y2H assay showed that OsFTL8 did not interact with OsFD1 directly, unlike the florigen Hd3a. Similarly, although OsMFT1 gene was involved in flowering time regulation, there was no evidence of its interaction with OsFD1 (Song et al. 2018). Therefore, not all the PEBP members could directly bind with OsFD1, which may be due to the sequence variation. There are 9 residues (D62, M63, R64, F66, P96, T98, T99, F103, R132) in Hd3a were predicted to be localized on the interacting surface between Hd3a and 14-3-3 (Taoka et al. 2013), and these residues were conserved between Hd3a and RFT1 (Fig. 1b). Three and six of these conserved residues in OsFTL8 and OsMFT1, respectively, were substituted by other amino acids, which may be the reason for the loss of direct interaction between OsFTL8 and OsMFT1 with 14-3-3 protein.
The reduced seed setting and pollen viability in OsFTL8 overexpressing lines
Three main factors contribute to the yield of a single rice plant, which are the number of panicles per plant, the number of grains per panicle and the weight of grains (Xing and Zhang 2010). In our study, the numbers of grains per panicle in OsFTL8 overexpression lines were severely reduced due to the altered panicle structure and the reduced seed setting rate, thus resulting in decreased yield. Several PEBPs could affect panicle structure as well as the heading date simultaneously. For example, overexpression of RCN1 and RCN2 delayed heading date and increased secondary branches per panicle, leading to altered panicle structure (Nakagawa et al. 2002). In osmft1 mutants, heading date was advanced and branches per panicle were reduced, leading to reduced spikelets per panicle (Song et al. 2018).
Spikelet sterility markedly reduce the seed setting rate per panicle, which can be divided into male-sterility and female-sterility, which may be caused by the deficiency of the development of stamen and pistil, respectively, with the male-sterility occurs more frequently (Ma 2005; Zhang and Wilsona 2009; Zhang et al. 2011). Male-sterility may occur at several different developmental stages, including stamen primordial formation and development, early anther development and meiosis, and post-meiotic microspore development (Guo and Liu 2012). In our study, pollen grains were normally formed and released by anther dehiscence, but pollen viability was severely reduced in OsFTL8 overexpression transgenic plants, demonstrating that the reduced pollen viability may occur at later stage of stamen development. Gene expression analysis revealed that the transcription level of DPW gene was significantly decreased in OsFTL8 overexpression transgenic lines, indicating that overexpression of OsFTL8 may affect the development of pollen by genes associated with pollen development.
Recent studies from other cereal crops have also shown that FTLs play roles in controlling of spikelet development and seed setting. For example, overexpression of BdFT2 gene reduced spikelet number in B. distachyon, whereas the knockdown of BdFT2 by RNAi resulted in reduced percentage of filled florets (Shaw et al. 2019). In barley, FT-like gene HvFT3 was shown to interact with HvCEN to control spikelet initiation (Bi et al. 2019). These findings, together with our results, suggest that FTLs have diverse functions in plant growth and development, and the future studies of the remaining 9 FTLs in rice will further enrich our knowledge in understanding of functions of FTLs.
References
Bi XJ, Esse WV, Mulki MA, Kirschner G, Zhong J, Simon R, Korff MV (2019) Centroradialis interacts with Flowering locus T-like genes to control floret development and grain number. Plant Physiol 180:1013–1030
Cai MH, Chen SH, Wu MM, Zheng TH, Zhou L, Li CN, Zhang H, Wang JC, Xu XY, Chai JT, Ren YL, Guo XP, Zhang X, Lei CL, Cheng ZJ, Wang J, Jiang L, Zhai HQ, Wang HY, Zhu SS, Wan JM (2019) Early heading 7 interacts with DTH8, and regulates flowering time in rice. Plant Cell Rep 38(5):521–532
Chardon F, Damerval C (2005) Phylogenomic analysis of the PEBP gene family in cereals. J Mol Evol 61:579–590
Chen R, Zhao X, Shao Z, Wei Z, Wang Y, Zhu L, Zhao J, Sun M, He R, He G (2007) Rice UDP-glucose pyrophosphorylase1 is essential for pollen callose deposition and its cosuppression results in a new type of thermosensitive genic male sterility. Plant Cell 19:847–861
Du AP, Tian W, Wei MH, Yan W, He H, Zhou D, Huang X, Li S, Ouyang XH (2017) The DTH8-Hd1 module mediates day-length-dependent regulation of rice flowering. Mol Plant 10:948–961
Fang MC, Zhou ZJ, Zhou XS, Yang HY, Li MR, Li HQ (2019) Overexpression of OsFTL10 induces early flowering and improves drought tolerance in Oryza sativa L. Peer J. https://doi.org/10.7717/peerj.6422
Gui J, Liu C, Shen J, Li L (2014) Grain setting defect1, encoding a remorin protein, affects the grain setting in rice through regulating plasmodesmatal conductance. Plant Physio 166:1463–1478
Guo JX, Liu YG (2012) Molecular control of male reproductive development and pollen fertility in rice. J Integr Plant Biol 54(12):967–978
Han MJ, Jung KH, Yi G, Lee DY, An G (2006) Rice Immature Pollen 1 (RIP1) is a regulator of late pollen development. Plant Cell Physiol 47(11):1457–1472
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6(2):271–282
Hu L, Liang W, Yin C, Cui X, Zong J, Wang X, Hu J, Zhang D (2011) Rice MADS3 regulates ROS homeostasis during late anther development. Plant Cell 23:515–533
Itoh H, Nonoue Y, Yano M, Izawa T (2010) A pair of floral regulators sets critical day length for Hd3a florigen expression in rice. Nat genet 42(7):635–639
Jefferson RA (1987) Assaying chimeric genes in plants: The GUS fusion system. Plant Mol Biol Rep 5(4):387–405
Kabayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T (1999) Antagonistic roles in mediating flowering signals. Science 286:1960–1962
Kaneko-Suzuki M, Kurihara-Ishikawa R, Okushita-Terakawa C, Kojima C, Nagano-Fujiwara M, Ohki I, Tsuji H, Shimamoto K, Taoka KI (2018) TFL1-like proteins in rice antagonize rice FT-like protein in inflorescence development by competition for complex formation with 14-3-3 and FD. Plant Cell Physiol 59(3):458–468
Karlgren A, Gyllenstrand N, Källman T, Sundström JF, Moore D, Lascoux M, Lagercrantz L (2011) Evolution of the PEBP gene family in plants: functional diversification in seed plant evolution. Plant Physiol 156(8):1967–1977
Kojima S, Takahashi Y, Kobayashi Y, Monna L, Sasaki T, Araki T, Yano M (2002) Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol 43(10):1096–1105
Komiya R, Ikegami A, Tamaki S, Yokoi S, Shimamoto K (2008) Hd3a and RFT1 are essential for flowering in rice. Development 135(4):767–774
Komiya R, Yokoi S, Shimamoto K (2009) A gene network for long-day flowering activates RFT1 encoding a mobile flowering signal in rice. Development 136:3443–3450
Lee YS, An G (2015) OsGI controls flowering time by modulating rhythmic flowering time regulators preferentially under short day in rice. J Plant Biol 58:137–145
Lescot M, Dehais P, Thijs G, Marchal K, Moreau Y, Peer YV, Rouze P, Rombauts S (2002) PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 30(1):325–327
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods 25:402–408
Lorenzo CD, Garcia-Gagliardi P, Antonietti MS, Sanchez-Lamas M, Mancini E, Dezar CA, Vazquez M, Watson G, Yanovsky MJ, Cerdan PD (2020) Improvement of alfalfa forage quality and management through the down-regulation of MsFTa1. Plant Biotech J 18:944–954
Luo H, Lee JY, Hu Q, Nelson-Vasilchik K, Eitas TK, Lickwar C, Kausch AP, Chandlee JM, Hodges TK (2006) RTS, a rice anther-specific gene is required for male fertility and its promoter sequence directs tissue-specific gene expression in different plant species. Plant Mol Biol 62:397–408
Ma H (2005) Molecular genetic analyses of microsporogenesis and microgametogenesis in flowering plants. Annu Rev Plant Biol 56:393–434
Mu H, Ke JH, Liu W, Zhuang CX, Yip WK (2009) UDP-glucose pyrophosphorylase2 (OsUgp2), a pollen-preferential gene in rice, plays a critical role in starch accumulation during pollen maturation. Chinese Sci Bull 54(2):234–243
Nakagawa M, Shimamoto K, Kyozuka J (2002) Overexpression of RCN1 and RCN2, rice TERMINAL FLOWER1/CENTRORADIALIS homologs, confers delay of phase transition and altered panicle morphology in rice. Plant J 29(6):743–750
Robert X, Gouet P (2014) Deciphering key features in protein structures with the new ENDscript server. Nucl Acids Res 42(W1):W320-324
Shaw LM, Lyu B, Turner R, Li C, Chen F, Han X, Fu D, Dubcovsky J (2019) FLOWERING LOCUS T2 regulates spike development and fertility in temperate cereals. J Exp Bot 70(1):193–204
Shi J, Tan H, Yu XH, Liu Y, Liang W, Ranathunge K, Franke RB, Schreiber L, Wang Y, Kai G, Shanklin J, Ma H, Zhang D (2011) Defective Pollen Wall is required for anther and microspore development in rice and encodes a fatty acyl carrier protein reductase. Plant Cell 23:2225–2246
Song YL, Luan WJ (2012) Molecular regulatory network of flowering by photoperiod and temperature in rice. Rice Sci 19(3):169–176
Song S, Wang GF, Hu Y, Liu HY, Bai XF, Qin R, Xing YZ (2018) OsMFT1 increases spikelets per panicle and delays heading date in rice by suppressing Ehd1, FZP and SEPALLATA-like genes. J Exp Bot 69(18):4283–4293
Sun J, Cao PP, Wang LJ, Chen SM, Chen FD, Jiang JF (2018) The loss of a single residue from CmFTL3 leads to the failure of florigen to flower. Plant Sci 276:99–104
Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K (2007) Hd3a protein is a mobile flowering signal in rice. Science 316:1033–1036
Taoka KI, Ohki I, Tsuji H, Furuita K, Hayashi K, Yanase T, Yamaguchi M, Nakashima C, Purwestri YA, Tamaki S, Ogaki Y, Shimada C, Nakagawa A, Kojima C, Shimamoto K (2011) 14-3-3 proteins act as intracellular receptors for rice Hd3a florigen. Nature 476:332–338
Taoka KI, Ohki I, Tsuji H, Kojima C, Shimamoto K (2013) Structure and function of florigen and the receptor complex. Trends Plant Sci 18(5):287–294
Xing YZ, Zhang QF (2010) Genetic and molecular bases of rice yield. Annu Rev Plant Biol 61:421–442
Xue W, Xing YZ, Weng XY, Zhao Y, Tang WJ, Wang L, Zhou HJ, Yu SB, Xu CG, Li XH, Zhang QF (2008) Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet 40(6):761–767
Zhang D, Wilson AZ (2009) Stamen specification and anther development in rice. Chin Sci Bul 54:2342–2353
Zhang DB, Luo X, Zhu L (2011) Cytological analysis and genetic control of rice anther development. J Gene Geno 38:379–390
Zhang Z, Hu W, Shen GJ, Liu HY, Hu Y, Zhou XC, Liu TM, Xing YZ (2017) Alternative functions of Hd1 in repressing or promoting heading are determined by Ghd7 status under long-day conditions. Sci Rep 7:5388. https://doi.org/10.1038/s41598-017-05873-1
Zheng TH, Sun J, Zhou SR, Chen SH, Lu J, Cui S, Tian YL, Zhang H, Cai MH, Zhu SS, Wu MM, Wang YH, Jiang L, Zhai HQ, Wang HY, Wan JM (2019) Post-transcriptional regulation of Ghd7 protein stability by phytochrome and OsGI in photoperiodic control of flowering in rice. New Phytol 224:306–320
Zhou SR, Wang Y, Li WC, Zhao ZG, Ren YL, Wang Y, Gu S, Lin Q, Wang D, Jiang L, Su N, Zhang X, Liu LL, Cheng ZJ, Lei CL, Wang JL, Guo XP, Wu FQ, Ikehashi H, Wang HY, Wan JM (2011) Pollen Semi-Sterility1 encodes a kinesin-1-like protein important for male meiosis, anther dehiscence, and fertility in rice. Plant Cell 23:111–129
Acknowledgements
We thank Mo Wang (Fujian Agriculture and Forestry University) for writing suggestions. This research was supported by the National Nature Science Foundation of China (Grant No. 31770343), the Tianjin Natural Science Foundation of China (Grant No. 17JCYBJC30000), and the Tianjin Rice Industrial Technology System of China (Grant No. ITTRRS2018006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, S., Jin, Y., Hao, H. et al. Characterization and identification of OsFTL8 gene in rice. Plant Biotechnol Rep 14, 683–694 (2020). https://doi.org/10.1007/s11816-020-00644-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-020-00644-3