Abstract
The Mongolian medicinal plant Zygophyllum potaninii has been assessed as an endangered species with regional status. We applied the somatic embryogenesis technique using aseptic in vitro germinants of the plant as an effective propagation technology. The seed germination rate in vitro was 16.5% after 2 weeks of culture. Embryonic calli (EC) and somatic embryos (SEs) were induced using the cotyledon or hypocotyl segments of the germinants. Calli were effectively induced on MS medium supplemented with 0.1 mg/L 2, 4-dichlorophenoxy acetic acid (2, 4-d) and 0.5 mg/L 6-benzylamino purine (BA). The callus was composed of pale yellow or pale green friable cells. SE formed from EC only on Murashige and Skoog medium (MS) with 0.5 mg/L abscisic acid (ABA). Other concentrations of ABA failed to induce SE formation. All SEs germinated in MS medium with different salt levels. However, normal plant conversion was achieved only on half-strength MS medium. The converted plantlets were effectively acclimatized in vitro in sand and transferred to a mixture of sand and perlite (1:1 v/v) in the greenhouse. After 8 weeks of culture, 55.4% of the plants survived. This is a first report of propagating the medicinal desert plant Z. potaninii via somatic embryogenesis and plant regeneration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zygophyllum consists of about 150 species in the family Zygophyllaceae. These plants are distributed in deserts and steppes from the Mediterranean to Central Asia, South Africa, and Australia (Beier et al. 2003). 11 species of Zygophyllum are found in Mongolia, and Zygophyllum potaninii Maxim. is sub-endemic to Mongolia (Urgamal et al. 2014).
Zygophyllum potaninii is an ecologically important species and is widely distributed in arid regions of Southern Mongolia (Figs. 1, 2). This species has been included in the “List of Very Rare Plant Species” in the Compendium of Laws (2009).
Distribution of Zygophyllum potaninii in Mongolia which has been found in Gobi-Altai, Zhungarian Gobi, Trans-Altai Gobi, and Alashaa Gobi. Reprinted from Regional Red List Series (Nyambayar et al. 2011)
Zygophyllum potaninii is characterized by thick stems, leaves with one or two pairs of leaflets, and flat petioles. Flowering occurs in the axils during May and June (Liu and Zhou 2008). In Mongolia, this species grows on debris tailings of mountains and hills, the sandy-pebble bottoms of dry river beds, stony slopes of hills, and pebble deserts (Nyambayar et al. 2011; Grubov 1982; Figs. 1, 2).
Traditional medicine has always played a major role in Mongolia. Z. potaninii has been used in Mongolian medicine to treat hepatitis, hepatocirrhosis, cholecystitis, and ascites (Ligaa et al. 2005). A few studies have evaluated the utilization of Z. potaninii. Bayarmaa et al. (2013) investigated the antioxidant activity of this medicinal herb and showed that Z. potaninii has a possible protective effect against carbon tetrachloride-induced liver injury in rats.
In recent years, there has been renewed interest in natural medicines obtained from plant parts or extracts (Cragg and Newman 2013; Newman and Cragg 2012). Natural products and their derivatives represent > 50% of all drugs in clinical use in the world (Gurib-Fakim 2006). Thus, it is important to preserve and develop valuable and useful medicinal plants, such as Z. potaninii.
Plant proliferation and conservation utilizing biotechnological methods have been applied to a variety of plants and is expected to continue in the future (Rai et al. 2011; Deo et al. 2010; Merkle et al. 2003; Merkle and Nairn 2005). However, micropropagation studies on Z. potaninii are very rare. Organogenesis studies have been performed on its close relative Z. xanthoxylon, but no studies of somatic embryogenesis have been performed in Z. potaninii (Sun et al. 2008).
The objective of this study was to develop an efficient plant production technology using somatic embryogenesis for the rare and endangered Mongolian medicinal plant Z. potaninii. To the best of our knowledge, this is the first study to report plant regeneration via somatic embryogenesis of the species.
Materials and methods
Plant material
Z. potaninii seeds were collected in 2011 from Gobi-Altai aimag, Erdene sum, Oasis of Shar Khuls, Trans-Altai Gobi, Mongolia (N43°18,231, E097°46,655; h 1754 m; Col: B. Oyumtsetseg) and maintained at 4 °C until use. The seeds were rinsed and soaked in distilled water for 2 h, surface sterilized with 70% ethanol for 30 s, treated with 4% sodium hypochloride for 5 min under gentle agitation, and rinsed four times with sterile distilled water. The surface disinfected seeds were germinated on MS (Murashige and Skoog 1962) basal medium supplemented with 3% sucrose and solidified with 0.7% agar. The pH of the culture medium was adjusted to 5.8 before agar was added, followed by autoclave sterilization at 121 °C for 15 min. We also tested the germination efficiency of Z. potaninii seeds on MS medium containing 1.0 mg/L gibberellic acid (GA3).
Callus and embryogenic callus (EC) induction
Cotyledons and hypocotyls of 2-week-old in vitro germinants were used as the source of explants. Calli were induced on callus induction medium (CIM), which consisted of MS medium supplemented with auxins [0.5 mg/L 1-naphthaleneacetic acid (NAA), 0.5 mg/L indole-3-acetic acid (IAA), 0.1 mg/L 2,4-dichlorophenoxyacetic acid (2,4-d)], cytokinins [0.5 mg/L 6-benzylaminopurine (BA), and 0.5 mg/L kinetin], 1.0 g/L l-glutamine, and 3% (w/v) sucrose (Sigma, St. Louis, MO, USA). The pH of the medium was adjusted to 5.7 before adding 0.3% (w/v) gellan gum (Phytagel; Sigma) and was autoclaved at 120 °C and 1 kg·cm−2 pressure for 20 min. Subsequently, 25 mL aliquots were dispensed into plastic 87 × 15 mm Petri dishes. 10–15 explants (about 5 mm thick segments of cotyledons or hypocotyls) were inoculated onto each of five Petri dishes with the cut surface facing down into the medium. The cultures were maintained at 25 °C in a dark culture room.
Somatic embryo induction
Somatic embryo (SE) induction was evaluated using two embryogenic lines derived from the cotyledon or hypocotyl. MS medium containing different levels of ABA (0, 0.05, 0.3, and 0.5 mg/L) was used (Table 2). Cultures were kept at 25 °C for 4 weeks under a 16-h photoperiod at 40 µmol·m−2·s−1 provided by cool-white fluorescent lamps. The number of somatic embryos induced was counted after 4 weeks of culture (Table 2).
Somatic embryo germination and plant conversion
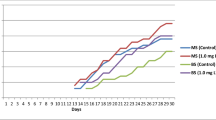
Early cotyledonary stage SEs were cultured on MS medium containing different salt concentrations (1/4, 1/2, and MS) to induce the germination of SEs and convert to the plantlets. A total of 20–25 SEs were cultured on each plate with ≥ 10 replicate plates per experiment. SE germination and plant conversion were determined based on the criteria suggested by Merkle et al. (2003). Briefly, germination was considered when a root emerged from the SE, and plant conversion was considered when normally growing emblings appeared after germination. The frequencies of germination and plant conversion were evaluated after 4 and 8 weeks of culture, respectively (Tables 3, 4; Fig. 3).
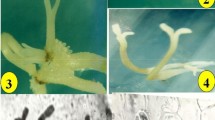
Somatic embryogenesis and plant regeneration of Z. potaninii. a Embryogenic callus induced from cotyledon explants showing pale yellow and green in color. b Microscopic observation of the embryogenic callus showing actively divided cells. c Several somatic embryos formed on embryogenic callus. d Microscopic observation of the bi-polar somatic embryo. e Germinating somatic embryo showing well developed cotyledons and hypocotyl. f Normally converted plantlets derived from somatic embryos
Transplantation and acclimatization
Preliminary experiments revealed that all SE-derived plants were dead when they were transferred directly to soil. Therefore, we tried a two-step procedure. First, we acclimatized in sand under in vitro conditions. Then, we transferred the plants to a mixture of sand and perlite (1:1 v/v) in the greenhouse. A glass bottle (80 × 130 mm) was filled with sand up to 8 cm. Four to six plants per bottle were transplanted and irrigated with 1/4 MS liquid medium and 1% sucrose. After 8 weeks of culture, the plants were transferred to a mixture of sand and perlite (1:1, v/v) and cultured in the greenhouse at a relative humidity of 90% and temperature of 25–30 °C. Plant survival was determined after 4 and 8 weeks (Fig. 4).
Histological observations
ECs and SEs after different culture periods were fixed in a solution containing 2.5% glutaraldehyde and 1.6% paraformaldehyde buffered with 0.05 M phosphate buffer (pH 6.8) at 4 °C for 24 h. The samples were dehydrated in a graded alcohol series and then embedded in Technovit 7100 (Kulzer, Hanau, Germany) according to a method published previously (Yeung 1995, 1999). Serial sections (3 µm thickness) were cut with a glass knife and a Reichert-Jung 2040 Autocut rotary microtome (Reichert Technologies, Depew, NY, USA). The sections were stained with Toluidine Blue-O (Yeung 1999), examined with a Leica DMR light microscope, and photographed with a digital camera (Leica DC 300F; Leica, Wezlar, Germany) using IM-50 software. At least ten replications were processed and examined for each time point.
Experimental design and data analysis
Experiments were performed using a completely randomized design strategy. Each treatment was repeated three times in five dishes with 10–15 explants per dish. Data were subjected to Duncan’s multiple range test using the SAS program (SAS Institute 1989). A p value < 0.05 was considered significant.
Results
Seed germination
Seed germination began 4 days after in vitro planting. Germination frequencies after 9 and 14 days of planting were 10.96 and 16.5%, respectively. Among those that germinated, 1.32% were abnormal, as they showed only a tap root without cotyledons (Table 1). Treatment with GA3 failed to increase germination efficiency but induced the abnormal growth characteristics to some extent (data not shown).
Callus induction
Calli were induced from cotyledon and hypocotyl explants of 2-week-old in vitro germinants. Explants began to swell and started to form a callus from the cut surface of explants in CIM after 4 weeks of culture. These calli were subcultured on fresh CIM at 4-week intervals. The calli were friable and slightly damp and were pale yellow or pale brown, regardless of the explant source. Calli gradually spread into all segments over the culture period. Callus formation and growth appeared to be dependent on the plant growth regulator (PGR) treatment. Calli formed well on media containing 2,4-D, IAA, or NAA, and combinations with BA (data not shown); however, no callus was initiated from MS basal medium or MS with BA alone. A combination of 0.1 mg/L 2, 4-d and 0.5 mg/L BA was the most efficient medium tested to proliferate calli from cotyledon and hypocotyl explants.
Somatic embryo induction
The embryogenic callus (EC) was yellow in color during the early stage of culture on SIM and was very wet. These calli gradually turned light green and then hardened into a dark green color. Most of the pale yellow calli became hardened and died gradually with a brown color. After 1 week of culture, the dark green ECs were swollen and many protruding structures had formed around the rugged callus. Most SEs seemed to form in these protruded tissues (Fig. 5c). After 4 weeks of culture, torpedo-stage SEs were observed at the surface of these protruded calli (Fig. 5c, d). After 8 weeks of culture, many SEs developed to the torpedo stage; however, early-stage SEs (globular or heart-shaped SEs) were not observed clearly.
In vitro acclimatized plantlets and survival. In vitro acclimatized plantlets (b) were transferred to soil in greenhouse and cultivated for 4 (c) and 8 (d) weeks, respectively. a and b In vitro acclimatized plantlets in sand treated with aqueous 1/4 MS medium and 1% sucrose. c and d Normally survived plants and their growth in a mixture of sand and perlite (1:1, v/v) in greenhouse
The SE induction rates from embryogenic calli are shown in Table 2 and Fig. 5. The rate differed markedly depending on the ABA concentration. SEs were induced exclusively on MS medium supplemented with 0.5 mg/L ABA (Table 2). There was no change in color of the EC and no protruding tissue was observed at other concentrations of ABA or in ABA-free medium.
Somatic embryo germination and plant regeneration
The SE germination and plant regeneration rates in MS medium with different salt concentrations are shown in Table 3. Germination was rapid. Most SEs germinated after 2 weeks of culture. SEs formed cotyledons and roots after 3–4 days. The hypocotyl was elongated after 1 week of culture. The shoot grew over 1.5 cm in length after 4 weeks of culture, and the cotyledons and stems turned dark green in color. The roots with many root hairs grew > 2 cm long. All SEs germinated regardless of salt concentration in the medium; however, 100% plant conversion was achieved only in the 1/2 MS medium (Table 3; Fig. 3).
Transplantation and acclimatization
All plants converted from SEs were acclimatized in glass bottles containing sand in vitro (Table 4) and then transplanted into a soil mixture (sand + perlite, 1:1 v/v) for growth in the greenhouse. The plants formed multiple shoots and some showed browning leaves after 4 weeks of culture. At this time, the roots grew straight with four or five roots that were short, dense, and fluffy. Tangled multiple shoots grew > 10 cm and older browning leaves dropped. 4 weeks later, > 60% plants had survived and grew rapidly with darker green leaves (Table 4; Fig. 4c). About 55% plants survived and grew obliquely after 8 weeks (Table 4; Fig. 4d).
Discussion
It is well-known that diverse and complex factors affect the induction of SE tissues in plants (Deo et al. 2010). Young, dividing, and undifferentiated cells generally form embryogenic cells. Depending on the explant source, immature embryos and reproductive organs, such as anthers or pollen, and leaves, petioles, stems, and corms, are commonly used to induce somatic embryogenesis (Deo et al. 2010). In this study, we used cotyledon and hypocotyl explants from in vitro germinants to induce EC. In vitro raised plants have the advantage of inducing the callus or SE directly without surface sterilization, and several studies have used in vitro plants (Conger et al. 1987). Callus induction did not differ significantly in cotyledons and hypocotyl, but embryogenic capacity was somewhat better in cotyledon explants. These results seemed to be related to physiological differences according to the explant’s position, which have been reported in many other plants (Mohajer et al. 2012).
Somatic embryogenesis is also strongly influenced by PGRs. Auxins, such as 2, 4-d, are generally used alone or in combination with cytokinins, such as BA (Jiménez 2001). In our study, among various PGR combinations, the combination of 0.1 mg/L 2, 4-d and 0.5 mg/L BA resulted in the best embryogenic cell induction and proliferation. However, success initiating the EC, SEs, and subsequent recovery of viable plants is not readily achievable in many species (Williams and Maheswaran 1986). In fact, induction may demand long and complex treatments or procedures for non-embryogenic cells are induced into an embryogenic state. These procedures include treatment with PGRs, various chemicals, and manipulations of light, temperature, and pH. The required conditions must be determined empirically by manipulating many factors that affect the plant culture response to determine the most effective conditions for somatic embryogenesis in different species (Jiménez 2001; Jiménez and Bangerth 2001a, b).
SEs are usually induced in vitro in two steps. First, a high concentration of auxin is added to the EC induction medium. Second, SEs are induced on auxin-free or lower concentration medium (Merkle and Nairn 2005; Jiménez 2001). ABA is particularly important for normal EC development and SE maturation. ABA has been frequently used in various coniferous and broad-leaved species (Lema-Ruminska et al. 2013; Rai et al. 2011). Baskaran and Jayabalan (2009) induced embryonic tissue by applying ABA and fostered subsequent development and germination of Psoralea corylifolia SEs. In the present study, we tested four concentrations of ABA to induce SEs. Interestingly, SEs from the two explants were only induced in 0.5 mg/L ABA, and no SEs formed at other ABA concentrations. On the other hand, Stasolla et al. (2002) reported that combined treatment of ABA and PEG promotes development of the embryogenic callus in a number of coniferous species. A future study is needed to determine whether a combination of ABA and PEG promotes EC development of Z. potaninii.
SE induction or SE germination in some species depends on the salt concentration in the medium (Samson et al. 2006). Groll et al. (2002) reported that development and germination of Cassava SEs are excellent in half-strength MS medium and full strength MS medium compared to those in 1/4 MS medium. They showed that SE induction efficiency can be increased by controlling the osmotic potential of the medium thorough adjusting the concentrations of inorganic salts and vitamins (Groll et al. 2002). These results demonstrate that optimizing the medium salt concentration is an important factor for normal SE formation and development.
In the present study, normal SE induction and plant regeneration were achieved in 1/2 MS medium supplemented with ABA, suggesting that this condition might be important for SE induction in this plant. Some regenerated plants formed a white callus at the lower hypocotyl part of the plantlets and revealed hyperhydration regardless of medium salt concentration. Therefore, it was necessary to select healthy plants to transplant into the sand bed for effective acclimatization.
Successful soil transplantation of SE-derived plants is an important factor for practical application of micropropagation technology (Merkle and Nairn 2005). The target plant of the present study was a desert plant, so it appeared to be recalcitrant to acclimatization in soil. An earlier report suggested that sand is an important substrate for the acclimatization of Zygophyllum species (Sun et al. 2008). They found that 81% of in vitro raised Z. xanthoxylon plantlets survived in sand but gradually died in perlite. This was also observed in the present study. In our preliminary experiment, in vitro raised plantlets did not survive when transplanted into an artificial soil, such as peat moss, perlite, and vermiculite (data not shown). Thus, we used sand for pre-acclimatization and then transferred the plantlets into a mixture of sand and perlite. This finding is thought to be due to the characteristics of desert plants. Z. potaninii is a very important conservation and restoration species in Mongolia (Ligaa et al. 2005). Our established protocol could be used in a conservation and rehabilitation program in the native area.
Conclusion
In the present study, plant regeneration via somatic embryogenesis was achieved for the Mongolian desert plant Z. potaninii, which has high medicinal and conservation value. We effectively regenerated plants via somatic embryogenesis using cotyledon and hypocotyl segments of in vitro germinants. Plant acclimatization and soil transplantation were accomplished by a two-step treatment: first, plantlets were acclimatized in sand under in vitro conditions and then they were transferred to a soil mixture of sand and perlite (1:1 v/v) in the greenhouse. To our best knowledge, this is the first report of efficient Z. potaninii regeneration via somatic embryogenesis.
References
Baskaran P, Jayabalan N (2009) In vitro propagation of Psoralea corylifolia L. by somatic embryogenesis in cell suspension culture. Acta Physiol Plant 31:1119–1127
Bayarmaa J, Ambaga M, Myagmar L (2013) Effect of Zygophyllum potaninii Maxim on histo-pathological and enzymatic changes in experimental liver injury of rats. Oriental Tradit Med 5:103–109
Beier BA, Chase MW, Thulin M (2003) Phylogenetic relationships and taxonomy of subfamily Zygophylloideae (Zygophyllaceae) based on molecularand morphological data. Plant Syst Evol 240:11–39
Compendium of Laws (2009) A Mongolian Citizens Reference Book. Asia Foundation of Mongolia, p 353
Conger BV, Novak FJ, Afza R, Erdelsky K (1987) Somatic embryogenesis from cultured leaf segments of Zea mays. Plant Cell Rep 6(6):345–347
Cragg GM, Newman DJ (2013) Natural products: a continuing source of novel drug leads. Biochim Biophys Acta 1830:3670–3695
Deo PC, Tyagi AP, Taylor M, Harding R, Becker D (2010) Factors affecting somatic embryogenesis and transformation in modern plant breeding. South Pac J Nat Appl Sci 28:27–40
Groll J, Mycock DJ, Gray VM (2002) Effect of medium salt concentration and maturation of somatic embryos of cassava (Manihot esculenta Crantz). Ann Bot 89:645–648
Grubov VI (1982) Key to the Vascular Plants of Mongolia (with an atlas). Nauka, Leningrad, p 444 (in Russian)
Gurib-Fakim (2006) Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol Aspects Med 27:1–93
Jiménez VM (2001) Regulation of in vitro somatic embryogenesis with emphasis on the role of endogenous hormones. Rev Bras Fisiol Veg 13:196–223
Jiménez VM, Bangerth F (2001a) Endogenous hormone levels in explants and in embryogenic and non-embryogenic cultures of carrot. Physiol Plantarum 111:389–395
Jiménez VM, Bangerth F (2001b) Hormonal status of maize initial explants and of the embryogenic and non-embryogenic callus cultures derived from them as related to morphogenesis in vitro. Plant Sci Lett 160:247–252
Lema-Ruminska J, Goncerzewicz K, Gabriel M (2013) Influence of abscisic acid and sucrose on somatic embryogenesis in Cactus Copiapoa tenuissima Ritt. forma mostruosa. Sci World J 2013:7
Ligaa U, Davaasuren B, Ninjil N (2005) Medicinal plants of Mongolia used in Western and Eastern Medicine. Ulaanbaatar. JCK Printing, p 427
Liu Y, Zhou L (2008) Zygophyllum L. In Wu ZY, Raven PN, Hong DY (eds.) Flora of China, vol 11. Science Press and Missouri Botanical Garden Press, Beijing, St Louis, pp 45–50
Merkle SA, Nairn CJ (2005) Hardwood tree biotechnology. In Vitro Cell Develop Biol Plant 41:602–619
Merkle SA, Battle PJ, Ware GO (2003) Research note factors influencing production of inflorescence-derived somatic seedlings of sweetgum. Plant Cell Tiss Org Cult 73:95–99
Mohajer S, Taha RM, Khorasani A, Yaacob JS (2012) Induction of different types of callus and somatic embryogenesis in various explants of Sainfoin (Onobrychis sativa). AJCS 6(8):1305–1313
Murashige T, Skoog F (1962) A revised method for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:472–497
Newman DJ, Cragg GM (2012) Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod 75:311–335
Nyambayar D, Oyuntsetseg B, Tungalag R (compilers), Ts Jamsran, Ch Sanchir, Bachman S, Soninkhishig N, Gombobaatar S, Baillie JEM, Tsendeekhuu Ts (eds) (2011) Regional Red List Series vol 9. Plants (Part 1). Zoological Society of London, National University of Mongolia, p 52
Rai MK, Shekhawat NS, Gupta AK, Phulwaria M, Ram K, Jaiswal U (2011) The role of abscisic acid in plant tissue culture: a review of recent progress. Plant Cell Tiss Org Cult 106:179–180
Samson NP, Campa EC, Le Gal EL, Noirot EM, Thomas EG, Lokeswari ETS, de Kochko A (2006) Effect of primary culture medium composition on high frequency somatic embryogenesis in different Coffea species. Plant Cell Tiss Organ Cult 86:37–45
SAS Institute (1989) SAS/STAT user’s guide, vers. 6. 4th edn. SAS Inst., Cary
Stasolla C, Kong L, Yeung EC, Thorpe TA (2002) Maturation of somatic embryos in conifers: morphogenesis, physiology, biochemistry, and molecular biology. In Vitro Cell Dev Biol Plant 38:93–105
Sun L, Hou S, Wu D, Zhang Y (2008) Rapid clonal propagation of Zygophyllum xanthoxylon (Bunge) Maxim., an endangered desert forage species. In Vitro Cell Dev Biol Plant 44:396–400
Urgamal M, Oyuntsetseg B, Nyambayar D, Dulamsuren Ch (2014) Conspectus of the vascular plants of Mongolia. Ulaanbaatar, Mongolia. Admon Printing, pp 114–115
Williams EG, Maheswaran G (1986) Somatic embryogenesis: factors influencing coordinated behavior of cells as an embryogenic group. Ann Bot 57:443–462
Yeung EC (1995) Structural and developmental patterns in somatic embryogenesis. In: Thorpe TA (ed) Vitro embryogenesis in plants, pp 205–247
Yeung EC (1999) The use of histology in the study of plant tissue culture systems- some practical comments. In Vitro Cell Dev Biol Plant 35:137–143
Acknowledgements
This work was supported by the Korean Foundation for Advanced Studies to B.G. and the Research Program of Dongguk University, 2017.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bayarmaa, GA., Lee, N.N., Kang, H.D. et al. Micropropagation of the Mongolian medicinal plant Zygophyllum potaninii via somatic embryogenesis. Plant Biotechnol Rep 12, 187–194 (2018). https://doi.org/10.1007/s11816-018-0484-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-018-0484-9