Abstract
The single-copper protein azurin from Pseudomonas aeruginosa has attracted great interest as an anti-cancer therapeutic agent or as a fuel cell catalyst for energy conversion. In this work, we obtained transgenic tobacco plants transformed with the chloroplast expression vector harboring the mature azurin polypeptide fused to psbA 5′UTR element, confirmed the integration of site-specificity into the tobacco chloroplast genome through homologous recombination by Southern hybridization analysis, and also identified the maternal inheritance. Northern hybridization analysis showed the polycistronic transcription expression pattern of the azurin gene. In addition, post-transcriptional processing of azurin monocistron was observed, which may be due to the endonucleolytic and intercistronic cleavage of the psbA mRNA 5′UTR element. Also, we examined the azurin expression levels depending on leaf maturity, showing a high expression level of 5.7 % of total soluble protein (TSP) in young leaves, in contrast to a low expression level of 0.72 % TSP in fully mature leaves. In addition, the copper level of transplastomic chloroplasts increased by twofold compared with that of non-transplastomic chloroplasts. These results suggest that the increased copper level may be due to the production of azurin in transplastomic chloroplasts, representing the formation of active azurin with copper ions in active sites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Azurin, a member of the cupredoxin family of redox proteins, is a type I single-copper-containing soluble protein (10–14 kDa) that serves as an electron-transfer agent in denitrifying bacteria (Adman 1991; van de Kamp et al. 1990). The presence of copper ions in the polypeptide chain contributes to azurin stability (Pozdnyakova and Wittung-Stafshede 2001). Thus, azurin is known as highly stable in nature. Azurin has greatly interested biomedical researchers as an anticancer therapeutic agent which enters human breast cancer cells and induces apoptosis without any adverse effects in the cancer patients (Punj et al. 2004; Yamada et al. 2002). Recently, there have been reports about the utilization of the cupredoxin azurin in industrial and fuel cell research. The reduction potential of the cupredoxin azurin is over a 700 mV range, surpassing the highest and lowest reduction potentials reported for any mononuclear cupredoxin, which means that azurin has the potential for applications such as artificial photosynthetic centers or fuel cell catalysts for energy conversion (Marshall et al. 2009).
Plant molecular farming is a new branch of plant biotechnology, in which plants are engineered to produce recombinant pharmaceutical and industrial proteins in large quantities. Plants have been considered as an economic, safe (owing to low risk of product contamination with human pathogens and/or endotoxins) and easily scalable production system (Twyman et al. 2003). The first recombinant plant-derived pharmaceutical protein (human growth hormone) was expressed in transgenic tobacco (Barta et al. 1986). Since then, much effort has been carried out to produce the recombinant proteins with pharmaceutical, industrial and agricultural interest in transgenic plants. Meanwhile, it was reported that a Bacillus thuringiensis toxin protein was accumulated to extraordinarily high levels in plastids by chloroplast transformation (Kota et al. 1999). This result showed that plants could be used as biofactories for large-scale production of recombinant proteins. Besides the potential for high-level production of foreign protein, chloroplast transformation has several advantages compared to nuclear transformation (Bock 2001). Transgenes are integrated into the plastid genome via homologous recombination, which is neither affected by position effects nor by epigenetic gene-silencing mechanisms. Furthermore, several transgenes can be expressed at the same time by a single promoter, because the prokaryotic organization of plastid genomes allows the expression of polycistronic mRNAs from operons. Another important point is that chloroplast genes are inherited uniparentally, maternally to the next generation (Day and Ellis 1984). Therefore, chloroplast transformation ensures much higher ecological safety than nuclear transformation. Hence, many recombinant therapeutic proteins and antibiotics have been produced in plastids by chloroplast transformation.
This study aims to produce the transplastomic plant expressing a high level of active azurin with copper ions in the active sites. To accomplish this, we cloned the mature azurin polypeptide from Pseudomonas aeruginosa and constructed the chloroplast expression vector harboring the mature azurin gene fused to the psbA 5′UTR element for enhancing the protein efficiency. Our results show that the azurin accumulated to 5.7 % TSP in young leaves and the copper level of transplastomic chloroplasts increased by twofold compared with that of non-transplastomic chloroplasts.
Materials and methods
Chloroplast expression vector
The 390-bp sequence of the modified Azurin gene (GenBank:M30389) without a putative transit peptide was amplified by PCR from Pseudomonas aeruginosa strain PAO1 genomic DNA and the 90-bp sequence of the psbA 5′UTR (GenBank: Z00044, plastome position 1594–1680) without the promoter was amplified by PCR from N. tabacum plastid DNA and then inserted into pCR2.1-topo vector, respectively. The primers used were as follows; AzuF (5′-TAACCATGGCCGAGTGCTCGGTGGACATC-3′; AzuR (5′-CATCACTTCAGGGTCAGGGTGCCCTTC-3′); UTRF (5′-TCGCGAGGCCTAAAAAGCCTTCCATTTTC-3′); UTRR (5′-CCATGGTACGTAGTAAAATCTTGGTTTATTTA-3′). These primers contain the NcoI site (underlined). The azurin gene fragment (~2.0 kb) was excised with NcoI and then was ligated into the NcoI site at the 3′ end of the psbA 5′-UTR. The EcoRI fragment (psbA 5′-UTR/Azurin) was inserted into the EcoRI site at the 3′ end of the aadA of the pLD vector (Daniell et al. 1998) to generate the pLDUTRAzurin.
Chloroplast transformation
Sterile leaves of N. tabacum cv. Petit Havana were placed on MS basal medium containing 3 % sucrose and 0.4 % phytagel and then bombarded using a Bio-Rad biolistic PDS-100/he device fitted with a 1100-psi rupture disc, as described previously (Lee et al. 2003). Five micrograms of DNA were coated with the golden particles (2.5 mg, 0.6 μm diameter) using 2.5 M CaCl2 and 0.1 M spermidine. Bombarded leaves were incubated at 25 ± 1 °C for 2 days under dark conditions and then cut into small pieces and transferred to an RMOP medium (Svab et al. 1990) supplemented with 500 mg L−1 of spectinomycin. After two cycles of shoot regeneration on the selection medium, including spectinomycin (500 mg L−1). Shoots were transferred to an MSO medium containing spectinomycin (500 mg L−1) for rooting, and then transferred to pots in the greenhouse.
Southern hybridization analysis
The genomic DNA was extracted from leaves (transformed and untransformed plants) by phenol–chloroform method (Kang and Yang 2004). Total DNA (10 μg) was digested with BglII. DNA fragments were separated overnight by electrophoresis (30 V) in a 0.8 % agarose gel, and then transferred to nylon membranes (Hybond N+; Amersham, Buckinghamshire, UK) under alkaline conditions (0.4 N NaOH). DNA fragments were revealed by hybridization with 32P-labeled probes. The azurin probe was amplified by PCR with the primers described above, while the 0.81-kb flanking probe (containing the trnI and trnA gene) was generated by digesting pLD-CtV with BamHI and BglII. First, the azurin probe was labeled with (32P) CTP by the oligolabeling procedure (Ready-to-go; Amersham) at 37 °C for 1 h. After exposure on X-ray film, the azurin probe on the membrane was removed by boiling in 0.1 % SDS solution for 5 min, and then the membrane was rehybridized with the flanking probe.
RT-PCR and Northern hybridization analysis
Total RNA was isolated from the leaves of transgenic tobacco plants using the Plant RNA Purification Reagent (Invitrogen, Carlsbad, CA, USA). For RT-PCR, complementary DNA (cDNA) was synthesized from 2 μg of total RNA using the SuperScript™ First Strand Synthesis Kit (Invitrogen). One microliter of cDNA from the reverse transcription reaction was amplified using the same primer used to amplify the azurin gene. As an internal control, the actin gene (GenBank: X69885) from N. tabacum was amplified using the primers Tac9-F (5′-CCCTCCCACATGCTATTCT-3′) and Tac9-R (5′-AGAGCCTCCAATCCAGACA-3′). For northern hybridization, about 10 µg of total RNA was denatured with sample buffer (2.2 M formaldehyde, 50 % formamide, and 0.5 × MOPS buffer) for 10 min at 65 °C, and then fractionated on 1.5 % (w/v) formaldehyde agarose gel electrophoresis in MOPS buffer for 4 h at 80 V, before being transferred onto nylon membranes (Hybond N+; Amersham) under capillary transfer using the sodium citrate (20× SSC) buffer. After transfer, RNA was hybridized with (α-32P) dCTP-labeled azurin gene cDNA fragment.
Western hybridization analysis
Transformed and untransformed leaves of transgenic tobacco plants (100 mg) were ground in liquid nitrogen and re-suspended in 1 ml of protein extraction buffer [0.1 M Tris–HCl (pH 8.0), 0.01 M MgCl2, 18 % sucrose, and 40 mM 2-mercaptoethanol]. The protein was determined by the DC protein assay kit (Bio-rad, Hercules, CA, USA). Leaf extracts were boiled in 5× SDS-PAGE sample buffer [150 mM Tris–HCl (pH 6.8), 30 % glycerol, 1.2 % SDS, 1.8 % bromophenol blue, and 15 % 2-mercaptoethanol] and electrophoresed (50 V) in a 15 % polyacrylamide gel. Separated proteins were either stained with Coomassie Brilliant Blue G-250 (0.05 % Coomassie Brilliant Blue R-250, 10 % glacial acetic acid, and 30 % methanol) or transferred to a PVDF membrane (Bio-Rad) for immunoblotting at 50 V for 4 h, using a transfer buffer (25 mM Tris base, 150 mM glycine and 20 % methanol). The primary antibody (rabbit polyclonal anti-azurin; Takara) was used at a 1:5000 dilution, followed with horseradish peroxidase conjugated rabbit IgG (Pierce, Rockford, IL, USA) at a 1:10,000 dilution. Stable peroxide solution (SuperSignal West Pico Chemiluminescent Substrate; Pierce) was used for detection.
ELISA
ELISA was used to quantify the azurin expression levels in transgenic lines. Leaves (untransformed and transformed plants) were ground with liquid nitrogen. Total soluble protein was extracted in 5 volumes (w/v) of bicarbonate buffer (15 mM Na2CO3, 35 mM NaHCO3, and 0.1 % Tween 20, pH 9.6) and incubated overnight at 4 °C. Diluted sample (1:100) was added to each well (100 μl/well) of the 96-well plate and incubated overnight at 4 °C. Bicarbonate buffer was used as blank. After three washes with washing buffer (PBS and 0.05 % Tween 20), rabbit anti-azurin (polyclonal antibody) diluted 1 μg/ml in antibody dilution buffer (0.1× PBS, 0.05 % Tween 20 and 0.3 % BSA) was added into each well (100 μl/well) and incubated for 2 h at 37 °C. The wells were washed three times and incubated with 1:5000 goat anti rabbit IgG-horseradish peroxidase conjugate (100 μl/well) in antibody dilution buffer for 2 h at 37 °C. After three washes, the plate was developed with the SuperSignal ELISA Pico Chemiluminescent Substrate (Pierce) and was read at 425 nm using a luminometer (Victor3; PerkinElmer, Waltham, MA, USA). For a standard curve, purified commercially available azurin (Sigma-Aldrich, St. Louis, MO, USA) was diluted with bicarbonate buffer to concentrations between 3 and 50 ng/ml and processed as above. Total soluble plant protein concentration was determined using the DC protein assay kit (Bio-rad). Azurin expression levels were calculated as a percentage of the total soluble protein.
Isolation of intact chloroplasts
Plants were grown in a growth chamber at 25 °C under long day conditions (16 h light, 8 h dark, fluorescent light 150 μmol/m2 s). Then 5 g of fresh leaves were cut into small (1–2 cm) pieces, and then blended with 2–3 strokes (within 3–5 s) in 20 ml of homogenization buffer [0.2 M sucrose in 10 mM Tricine-NaOH buffer (pH 7.6) and 4 mM MgCl2]. The speed of the blender was regulated to avoid foaming. The chopped mash was filtered through 4 layers of cheesecloth. The filtrate was transferred to a 50-ml centrifuge tube and the chloroplasts were pelleted at 1,000g for 7 min (fixed angle rotor). The chloroplast pellet was resuspended in 0.5 ml of chloroplast suspension (CS) buffer [0.35 M sucrose in 1× Tricine-NaOH buffer (pH 7.6)] by finger tapping. The resuspended sample was carefully loaded onto three Percoll density gradients (top to bottom: 10 ml 20 % sucrose, 10 ml 40 % sucrose, 10 ml 60 % sucrose) prepared in 1× Tricine-NaOH buffer (pH 7.6). The sucrose gradients were centrifuged for 1 h at 4,500 rpm (swing rotor). Broken chloroplasts (light green) accumulated at the 20–40 % interface, while intact chloroplasts (dark green) formed a band at the 40–60 % interface. The fractions containing intact chloroplasts were collected and were washed twice with three volumes of CS buffer by centrifugation at 1,700g for 1 min. Pellets were resuspended in 0.5 ml of CS buffer.
Determination of chlorophyll contents
The procedure for chlorophyll determination was based on the work of Mackinney (1941) on the absorption of light by aqueous acetone extracts of chlorophyll. Ten microliters of the chloroplast suspension was added in 1 ml of an 80 % acetone solution and mix well. The suspension was centrifuged for 2 min at 3,000g and then transferred the suspension in a new tube. Chlorophyll contents were measured the absorbance of the solution in a spectrophotometer (GENESYS™20; Thermo Scientific, Pittsburgh, PA, USA) at 652 nm. The 80 % acetone solution was used as a blank. Chlorophyll concentration was calculated as follows the equation [mg chlorophyll/ml of chloroplast suspension = (A652 × 100)/36].
Estimation of copper contents
Levels of copper were measured by the QuantiChrom™ Copper Assay Kit (BioAssay Systems, Hayward, CA, USA). Chloroplast suspension (100 μl) was transferred into a 1.5-ml tube, and then 35 μl of trichloroacetic acid was added. After mixing thoroughly, the suspension was centrifuged for 2 min at 14,000 rpm and the clear supernatant was assayed according to the manufacturer’s manual. The intensity of the color was measured at 359 nm using a plate reader (Victor3; PerkinElmer). For a blank, 100 μl of dH2O was used as a substitute of sample (chloroplast suspension).
Results
Azurin chloroplast expression vector
The single-copper protein azurin from Pseudomonas aeruginosa is a pre-protein with a signal peptide of 19 amino acid residues at the N terminus followed by the 128 amino acid mature azurin protein (Arvidsson et al. 1989). We amplified the azurin gene from Pseudomonas aeruginosa strain PAO1 genomic DNA by PCR, corresponding to mature azurin protein without its signal peptide. A translation start codon was added at the N-terminus of the mature azurin, giving a polypeptide of 129 amino acids with a predicted molecular weight of 14 kDa. To enhance the expression, the amplified azurin gene was fused at 3′-terminal of the psbA 5′UTR, which has several sequences for ribosomal binding that act as a scaffold for the light-regulated proteins (Fernandez-San Millan et al. 2003; Roh et al. 2006). This fusion gene was inserted into the pLD vector (Fig. 1a), which contains the homologous recombination sequences trnI and trnA, that allowed site-specific integration into the chloroplast genome as described previously (Daniell et al. 1998). In addition, this construct contains the promoter of the 16S-rRNA gene and the psbA 3′UTR, shown to increase the stability of the transcript (Ebil et al. 1999). The aadA (aminoglycoside 3′adenyltransferase) gene was used to select transplastomic plants.
Chloroplast transformation vector and analysis of transgene integration into the chloroplast genome by Southern hybridization analysis. a Schematic map of pLDUTRAzurin vector. The aadA and azurin sequences were driven by the tobacco rrn16 promoter. The 3′ region of the psbA gene was used as a terminator for the dicistronic messenger. Arrows within genes indicate the direction of transcription. Southern blot analyses of three spectinomycin resistant shoots (1–3): 10 μg of total genomic DNA was digested with BglII and the blot was probed with the probe 1 (b) and the probe 2 (c) shown in (a). trnV Val-tRNA; rrn16 16SrRNA; trnI Ile-tRNA; trnA Ala-tRNA; 23S 23SrRNA; Prrn 16SrRNA promoter; aadA aminoglycoside 3′-adenylyltransferase gene for spectinomycin resistance; 5′psbA 5′UTR of psbA gene; TpsbA 3′UTR of psbA; WT wild-type; P positive control (pLDUTRAzurin vector)
Analysis of site-specific transgene integration
Tobacco plants (N. tabaccum L. cv. Petite Havana) were transformed by particle bombardment using the pLDUTRAzurin vector and several primary shoots appeared from each bombarded leaf as a result of independent transformation events. Confirmed transformants were subjected to a second round of spectinomycin selection to achieve homoplasmy and transferred to a greenhouse for flowering and harvesting of the seed.
Southern blot analysis was performed to select and confirm stable maintenance of integrated transgenes in the transplastomic T0 lines (Fig. 1b, c). The flanking probe (0.81 kb) represented a 4.5-kb fragment in the non-transgenic plant (WT) as expected, while transplastomic genome releases a higher size fragment (~6.3 kb) included the flanking region (4.5 kb) and integrated azurin expression cassette (1.8 kb) (Fig. 1b). To confirm that the 6.3-kb fragment contained the azurin gene, the same blot was reprobed with the azurin probe. As expected, the hybridization fragment was detected only in the chloroplast transgenic lines and no fragment was observed in the wild-type plant (Fig. 1c).
Determination of maternal inheritance
Seeds from T1 progeny of non-transplastomic and transplastomic plant were sown on spectinomycin selection medium (Fig. 2). Seedlings of non-transplastomic plant germinated well, but failed to grow further and were shown to have pigmentation deficiency in leaves (Fig. 2a). However, seedlings of transplastomic plants harboring the spectinomycin resistance aadA gene was shown to have normal growth and green pigmentation in leaves (Fig. 2b–d). All seeds derived from T1 progeny of transplastomic plants were resistant on spectinomycin and no Mendelian segregation was observed. This result demonstrates that the chloroplast transgenic trait is inherited maternally.
Maternal inheritance of a transgene integrated into the chloroplast genome. Surface-sterilized seeds are germinated on spectinomycin-containing medium. Seeds from wild-type plants (a) yield spectinomycin-sensitive (white) seedlings, whereas seeds from three independent transplastomic plants (b–d) gives rise to uniformly resistant (green) progeny without Mendelian segregation
Analysis of transcript expression pattern
RNA was extracted from non-transplastomic and transplastomic tobacco plants to identify the transcript of azurin gene (Fig. 3). Transcripts of about 390 bp were detected only in all the transplastomic lines by RT-PCR using the primers of azurin gene isolation (Fig. 3a). Actin transcripts (internal positive control) were observed at a similar level in the non-transplastomic and transplastomic plants. Northern blot analysis showed a peculiar transcript expression pattern in transplastomic plants. Transcripts of about 707 nucleotides were observed in the transplastomic lines, which contains 5′UTR, the azurin gene, and psbA 3′UTR (Fig. 3b). This mRNA is considered monocistronic (Fig. 3c) and it is the most abundant transcript in all the transplastomic lines. In addition, dicistronic and polycistronic transcripts, arising by read-through transcription from the 16S-rRNA gene promoter of the rRNA operon, were observed in lower abundance in the transplastomic lines (Fig. 3c). Contrary to the azurin transcript, degradation products of the aadA transcript were observed in all transplastomic plants. No transcript was detected in a non-transplastomic plant. A trace showed the overflowing result of the next transcripts.
Transcript expression patterns of transplastomic plants. a RT-PCR analysis of transgenic T0 lines. NtActin was used as an internal positive control. b Northern hybridization analysis of transplastomic T1 lines. Three progenies (1–1 to 3–3) of each the three independent transgenic lines were analyzed. The azurin and the aadA gene was used as a probe, respectively. Identified transcripts are indicated to the right. Known RNA size is marked on the left. c Expected transcripts of azurin integrated into the chloroplast genome. Dotted arrows show a monocistron transcript of 707 nt, a dicistron transcript of 1783 nt and polycistron transcripts of 2562 and 4051 nt. GenBank accession numbers of these genes are: azurin (GenBank: M30389) and NtActin (GenBank: X69885). nt Nucleotide
Azurin expression in transplastomic plants
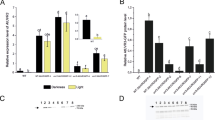
Expression of azurin was examined in protein extracts from mature fully developed leaves of transplastomic lines by western blot analysis. All the independent transplastomic lines of T1 generation were expressing the azurin mature polypeptide, which had a molecular weight of 14 kDa (Fig. 4). No band was observed in wild-type tobacco. To investigate the azurin expression level depending on leaf maturity, we also examined the azurin expression pattern of individual leaves in transplastomic plants (Fig. 5). As shown in Fig. 5b, the azurin accumulation was highly detectable in the young leaves (#6 to #8). Interestingly, it was observed that the azurin expression level sharply dropped immediately after fully mature leaves (#4 to #5) and was hardly detectable in old leaves (#1 to #3), indicating that the azurin protein was almost degraded. Azurin quantities in transplastomic plant were examined by an enzyme-linked immunosorbent assay (ELISA) (Fig. 5c). The young leaves (#7) of transplastomic plants had an expression level of 5.7 % of the total soluble protein (%TSP) and the fully mature leaves (#5) had 0.72 % TSP. The young leaves of the wild-type plant had 0.28 % TSP, regarded as the background level.
Azurin expression in transplastomic plants: 10 µg of total soluble protein extracts of the non-transplastomic (WT) and two progenies (1–1 to 3–2) of each the three independent transgenic lines (T1) were loaded into a 15 % SDS-PAGE gel; 200 ng of azurin protein sold commercially was used as a positive control (P). Molecular mass standards are shown on the left. WT wild-type, kDa kiloDaltons
Azurin expression pattern in transplastomic plants. a A transplastomic tobacco plant. The leaves are numbered from the basal to the apical. b Expression of azurin on western hybridization analysis was detected using rabbit anti-Azurin. Lane 1 Molecular mass standard (M); lane 2 wild-type (WT); lanes 3–10 10 µg of total soluble protein extracts from the leaves (#1–#8) shown in (a), respectively. Lane 11 Positive control (25 ng of azurin protein). Protein was loaded on a 12 % SDS-PAGE gel. c Azurin quantification by ELISA in non-transplastomic (WT) and transplastomic tobacco leaves (#5, #7) show in (a). The data are presented as the mean ± standard error of three independent lines. kDa kiloDaltons, TSP total soluble protein
Copper assay of intact chloroplasts
We investigated the copper level of transplastomic chloroplasts to ascertain what active azurin (a folded protein with copper ion in the active site) was formed. Intact chloroplasts from non-transplastomic and transplastomic young leaves were isolated using a discontinuous sucrose gradient centrifugation (Fig. 6a). After centrifugation, intact chloroplasts were purified through collecting the dark green band located on the interface of the 40 and 60 % layers in the sucrose gradient buffer. Chlorophyll contents as an index of intact chloroplast quantity were observed at the same level of 26.3 μg both in non-transplastomic and transplastomic chloroplasts (Fig. 6b). Copper level of transplastomic chloroplasts was approximately twofold higher than that of non-transplastomic chloroplasts; the level of copper in 100 μl of chloroplasts suspension of transplastomic leaves was detected as 65 μg while that of non-transplastomic leaves was detected as 33 μg.
Copper assay of intact chloroplasts from transplastomic tobacco leaves. a Intact chloroplast isolation from young leaves using discontinuous sucrose gradient centrifugation. Percentages of sucrose in the buffer are given for the different layers. L light fraction, D dark green band. b Chlorophyll and copper contents of intact chloroplasts collected from dark green band. Chlorophyll contents were examined as an index of chloroplasts quantity of non-transplastomic plants (WT) and transplastomic plants. The data are presented as the mean ± standard error of three independent lines
Discussion
We produced transplastomic plants transformed with the azurin fused to the psbA 5′UTR and confirmed the integration of site-specificity into the tobacco chloroplast genome through homologous recombination by Southern hybridization analysis, and also identified the maternal inheritance of transgene (the spectinomycin resistant aadA gene) co-integrated with azurin showing all seeds of the progeny (T1) of transplastomic plants resistant to selection medium containing the spectinomycin without Mendelian segregation. RT-PCR using the primers for azurin isolation showed a transcript of 390 bp corresponding to the azurin gene as a single band. By contrast, northern hybridization analysis showed the polycistronic transcriptional expression pattern of azurin under the control of the 16S rRNA promoter. In addition, post-transcriptional processing of azurin monocistron was observed as a 707-nt transcript, which may be due to the endonucleolytic and intercistronic cleavage of the psbA mRNA 5′UTR element (Daniell et al. 2009; Fernandez-San Millan et al. 2003; Klaff 1995; Roh et al. 2006). Furthermore, a sharp contrast in transcript level of between the azurin monocistron and the azurin polycistron was shown, showing that the cleavage event of the psbA 5′UTR is highly active. As a result, we can also observe the degradation of aadA transcript not protected by the psbA 3′UTR stabilizing sequences as reported by several researchers (Ebil et al. 1999; Monde et al. 2000; Roh et al. 2006; Zou et al. 2003).
Transplastomic lines express the mature azurin polypeptides. The difference in azurin expression levels depending on leaf maturity is significant. High-level expression of azurin was detected in young leaves while low-level expression of azurin was detected in fully mature and old leaves. This could be related to the degradation of chloroplast DNA during chloroplast development (Rowan and Bendich 2009; Sodmergen et al. 1991; Zoschke et al. 2007). Ortigosa et al. (2010) investigated the 2L21-TD expression levels in young, mature and old leaves (12, 8 and 3 leaf number counted from the bottom of the plant) of transplastomic tobacco plants. They reported that the 2L21-TD protein was highly expressed in young leaves at 6 % TSP corresponding to our results. Birch-Machin et al. (2004) also reported that the amount of the VP6 protein declined as the leaves matured. Unlike these results, it has been reported that expression levels of foreign protein in mature leaves were higher than that in young leaves of transplastomic tobacco plants (Daniell et al. 2009; Koya et al. 2005). They represented the age of the plant, but described the leaves only as ‘young’ or ‘mature’. Without knowing the size of the leaves or which leaves was examined, it cannot be determined whether these leaves are comparable to ours. Thus, it cannot be assessed whether their tobacco leaves were at a similar developmental stage as ours.
Azurin is described as a small blue copper protein highly stable in nature. Copper coordination is required for function of azurin (Wittung-Stafshede et al. 1998). Copper is an essential metal and is found in the active sites of proteins that participate in antioxidant defense, cellular respiration and pigment formation (Huffman and O’Halloran 2001). Non-bounded copper ions are toxic due to their ability to catalyze the formation of free radicals and their interference with Fe–S cluster assembly (Macomber and Imlay 2009). To avoid toxicity and to overcome solubility problems of copper ions, the intracellular concentration of copper ions is regulated via dedicated proteins that facilitate their uptake and efflux as well as distribution to target Cu-dependent proteins and enzymes (Festa and Thiele 2011). We observed that transplastomic tobacco plants harboring azurin grew normally and that the copper level of transplastomic chloroplasts increased by twofold compared with that of non-transplastomic chloroplasts. These results suggest that an increased copper level may be due to the production of azurin in transplastomic chloroplasts, representing the formation of active azurin with copper ions in active sites. Purification and testing of the azurin response to p53-dependent cancer cell is currently in progress.
References
Adman ET (1991) Copper protein structures. Adv Protein Chem 42:145–197
Arvidsson RHA, Nordling M, Lundberg LG (1989) The azurin gene from Pseudomonas aeruginosa: cloning and characterization. Eur J Biochem 179:195–200
Barta A, Sommengruber K, Thompson D, Hartmuth K, Matzke MA, Matzke AJM (1986) The expression of a nopaline synthase human growth hormone chimeric gene in transformed tobacco and sunflower callus tissue. Plant Mol Biol 6:347–357
Birch-Machin I, Newell C, Hibbered J, Gray J (2004) Accumulation of rotavirus VP6 protein in chloroplasts of transplastomic tobacco is limited by protein stability. Plant Biotechnol J 2:261–270
Bock R (2001) Transgenic chloroplasts in basic research and plant biotechnology. J Mol Biol 312:425–438
Daniell H, Datta R, Varma S, Gray S, Lee SB (1998) Containment of herbicide resistance through genetic engineering of the chloroplast genome. Nat Biotechnol 16:345–348
Daniell H, Ruiz G, Denes B, Sandberg L, Langridge W (2009) Optimization of codon composition and regulatory elements for expression of human insulin like growth factor-I in transgenic chloroplasts and evaluation of structural identify and function. BMC Biotechnol 9:33
Day A, Ellis THN (1984) Chloroplast DNA deletions associated with wheat plants regenerated from pollen: possible basis for maternal inheritance of chloroplasts. Cell 39:359–368
Ebil C, Zou Z, Kim M, Mullet J, Koop H (1999) In vivo analysis of plastid psbA, rbcL, and rpl32 UTR elements by chloroplast transformation: tobacco plastid gene expression is controlled by modulation of transcript levels and translation efficiency. Plant J 19:333–345
Fernandez-San Millan A, Mingo-Castel A, Miller M, Daniell H (2003) A chloroplast transgenic approach to hyper express and purify human serum albumin, a protein highly susceptible to proteolytic degradation. Plant Biotechnol J 1:71–79
Festa RA, Thiele DJ (2011) Copper: an essential metal in biology. Curr Biol 21:R877–R883
Huffman DL, O’Halloran TV (2001) Function, structure, and mechanism of intracellular copper trafficking proteins. Annu Rev Biochem 70:677–701
Kang TJ, Yang MS (2004) Rapid and reliable extraction of genomic DNA from various wild-type and transgenic plants. BMC Biotechnol 4:20
Klaff P (1995) mRNA decay in spinach chloroplasts: psbA mRNA degradation is initiated by endonucleolytic cleavages within the coding region. Nucleic Acids Res 23:4885–4892
Kota M, Daniell H, Varma S, Garczynski SF, Gould F, Moar WJ (1999) Overexpression of the Bacillus thuringiensis (Bt) Cry2Aa2 protein in chloroplasts confers resistance to plants against susceptible and Bt-resistant insects. Proc Natl Acad Sci USA 96:1840–1845
Koya V, Moayeri M, Leppla SH, Daniell H (2005) Plant-based vaccine: mice immunized with chloroplast-derived anthrax protective antigen survive anthrax lethal toxin challenge. Infect Immun 73:8266–8274
Lee SB, Kwon HB, Kwon SJ, Park SC, Jeong MJ, Han SE, Byun MO, Daniell H (2003) Accumulation of trehalose within transgenic chloroplasts confers drought tolerance. Mol Breed 11:1–13
Mackinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140:315–322
Macomber L, Imlay JA (2009) The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci USA 106:8344–8349
Marshall NM, Garner DK, Wilson TD, Gao YG, Robinson H, Nilges MJ, Lu Y (2009) Rationally tuning the reduction potential of a single cupredoxin beyond the natural range. Nature 462:113–116
Monde RA, Greene JC, Stern DB (2000) The sequence and secondary structure of the 3′ UTR affect 3′-end maturation, RNA accumulation, and translation in tobacco chloroplasts. Plant Mol Biol 4:529–542
Ortigosa SM, Fernandez-San Millan A, Veramendi J (2010) Stable production of peptide antigens in transgenic tobacco chloroplasts by fusion to the p53 tetramerisation domain. Transgenic Res 19:703–709
Pozdnyakova I, Wittung-Stafshede P (2001) Copper binding before polypeptide folding speeds up formation of active (holo) Pseudomonas aeruginosa azurin. Biochemistry 40:13728–13733
Punj V, Bhattacharyya S, Saint-Dic D, Vasu C, Cunningham EA, Graves J, Yamada T, Constantinou AI, Christov K, White B, Li G, Majumda D, Chakrabarty AM, Das Gupta TK (2004) Bacterial cupredoxin azurin as an inducer of apoptosis and regression in human breast cancer. Oncogene 23:2367–2378
Roh KH, Shin KS, Lee YH, Seo SC, Park HG, Daniell H, Lee SB (2006) Accumulation of sweet protein monellin is regulated by the psbA 5′ UTR in tobacco chloroplasts. J Plant Biol 49:34–43
Rowan BA, Bendich AJ (2009) The loss of DNA from chloroplasts as leaves mature: fact or artefact? J Exp Bot 60:3005–3010
Sodmergen Kawano S, Tano S, Kuroiwa T (1991) Degradation of chloroplast DNA in second leaves of rice (Oryza sativa) before leaf yellowing. Protoplasma 160:89–98
Svab Z, Hajdukiewicz P, Maliga P (1990) Stable transformation of plastids in higher plants. Proc Natl Acad Sci USA 87:8526–8530
Twyman RM, Stoger E, Schillberg S, Christou P, Fischer R (2003) Molecular farming in plants: host systems and expression technology. Trends Biotechnol 21:570–578
Van de Kamp M, Silvestrini MC, Burunori M, van Beeumen J, Hali FC, Canters GW (1990) Involvement of the hydrophobic patch of azurin in the electron-transfer reactions with cytochrome C551 and nitrite reductase. Eur J Biochem 194:109–118
Wittung-Stafshede P, Hill MG, Gomez E, Bilio AD, Karlsson G, Leckner J, Winkler HB, Gray HB, Malmstrom BG (1998) Reduction potentials of blue and purple copper proteins in their unfolded states: a closer look at rack-induced coordination. J Biol Inorg Chem 3:367–370
Yamada T, Goto M, Punji V, Zaborina O, Chen ML, Kimbara K, Majumdar D, Cunningham E, Das Gupta TK, Chakrabarty AM (2002) Bacterial redox protein azurin, tumor suppressor protein p53, and regression of cancer. Proc Natl Acad Sci USA 99:14098–14103
Zoschke R, Liere K, Börner T (2007) From seedling to mature plant: Arabidopsis plastidial genome copy number, RNA accumulation and transcription are differentially regulated during leaf development. Plant J 50:710–722
Zou Z, Ebil C, Koop HU (2003) The stem-loop region of the tobacco psbA 3′UTR is an important determinant of mRNA stability and translation efficiency. Mol Genet Genomics 269:340–349
Acknowledgments
This study was carried out with the support of Research Program for Agricultural Science and Technology Development (Project No. PJ010075), National Academy of Agricultural Science, Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roh, K.H., Choi, S.B., Kwak, BK. et al. A single cupredoxin azurin production in transplastomic tobacco. Plant Biotechnol Rep 8, 421–429 (2014). https://doi.org/10.1007/s11816-014-0333-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-014-0333-4