Abstract
Genetic transformation is a tool of special interest for developing new biotechnological strategies for the production of bio-active compounds such as cardenolides, which are exclusively obtained from plants. To date, Digitalis plants are the main economically viable source of cardenolides for the pharmaceutical industry. This study describes the development of efficient plant regeneration and Agrobacterium-mediated genetic transformation protocols for Digitalis purpurea L. First, a plant regeneration procedure starting from leaf segments of in vitro-cultivated plants was established and the minimal inhibitory concentration of G-418 (geneticin) for callus induction was determined. Both leaf segments and callus tissue were sensitive to G-418 70 mg l−1. Afterwards, two Agrobacterium strains were used to test their T-DNA transfer ability on D. purpurea leaf tissues, EHA105 and C58C1RifR (pMP90), both harboring the binary vector pTJK136. Strain C58C1RifR (pMP90) yielded a higher number of transformed plants than EHA105. Successful transformation was confirmed by histochemical β-glucuronidase (GUS) assays of the putative transgenic tissues and PCR analyses using β-glucuronidase (uidA)- and neomycin phosphotransferase II (nptII)-specific primers. Southern blot hybridization confirmed the stable integration of the nptII gene in the transgenic plants. In total, 518 independent transgenic lines were regenerated with an average of 6.91 transgenic lines per initial leaf segment infected with A. tumefaciens strain C58C1RifR (pMP90). To date, only a few studies have been published on the genetic transformation of Digitalis species. The protocols for plant regeneration and genetic transformation described in this paper will contribute to functional studies for a better understanding of cardenolide biosynthetic pathways and the metabolic engineering of cardenolides to develop high-yielding improved genotypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant transgenesis offers new opportunities not only for crop improvement but also to better understand the basic molecular biology of plants. Several studies have described the stable transfer and expression of foreign genes in important medicinal plants, which naturally produce a large number of pharmacologically active secondary metabolites (Canter et al. 2005; Abou-Alaiwi et al. 2012; Gatica-Arias et al. 2012). In most cases, biopharmaceutical products are derived from wild or cultivated plants that have not been genetically altered (Gómez-Galera et al. 2007).
Digitalis purpurea L. (foxglove), a member of the Scrophulariaceae family, contains cardiac glycosides, which are of major interest to the pharmaceutical industry. These compounds have proved to be the most effective drugs to treat heart failure during the past two centuries (Warren 2005; Kuate et al. 2008). In addition, recent findings suggest a regulatory role of cardiac glycosides in several cellular processes, thus highlighting new therapeutic applications for these compounds, especially as anticancer drugs (Prassas and Diamandis 2008; Menger et al. 2012). However, the chemical synthesis of these glycosides has so far not been cost effective due to their complex chemical nature (Pérez-Bermúdez et al. 2010). Thus, plants remain as the sole source for their acquisition, and pharmaceutical industries are still relying on plant material, which contains very low amounts of these glycosides (Patil et al. 2013). In this context, new biotechnological approaches could be developed to enhance the yield of cardenolides.
The current knowledge on the biosynthesis of the steroid part of cardenolides in the genus Digitalis was recently reviewed by Kreis and Müller-Uri (2013). Some genes involved in different cardiac glycoside metabolic steps were described in Digitalis such as 3ß-HSD (AY789453) (Herl et al. 2007), Dop5βr (AJ555127) (Roca-Pérez et al. 2004), P5ßR (AJ310673) (Gavidia et al. 2007), and P5ßR2 (GU062787) (Pérez-Bermúdez et al. 2010). However, no reports on functional analysis of these genes have been published to date, mainly due to the lack of an efficient transformation system in D. purpurea. In addition, minimal impact of biotechnological approaches on the genetic improvement of Digitalis species has been described so far, with very few papers showing advances in the genetic transformation of Digitalis species. Saito et al. (1990) obtained hairy roots of D. purpurea via Agrobacterium rhizogenes, but the regeneration of transgenic plants was not described. Lehmann et al. (1995) reported the first transgenic D. lanata from protoplasts, while Pradel et al. (1997) regenerated D. lanata plants from hairy root cultures established after infection with several wild-type strains of A. rhizogenes. Similarly, Koga et al. (2000) generated D. purpurea transformants using an A. rhizogenes strain harboring the rolC gene. Subsequently, Sales et al. (2003) described a protocol for Agrobacterium-mediated transformation in D. minor. Thereafter, these authors used this protocol as a valuable system to achieve metabolic engineering of the cardenolide pathway in D. minor (Sales et al. 2007). However, Sales et al. (2011) further stressed the significance of developing reliable genetic transformation protocols for those Digitalis species of high economic value such as D. purpurea. Very recently, Li et al. (2014) developed a regeneration protocol through direct organogenesis, and subsequently established an Agrobacterium-mediated genetic transformation protocol starting from D. purpurea mature leaf explants. The authors reported the regeneration of 0.62 kanamycin-resistant shoots per initial explant. In order to efficiently obtain D. purpurea transgenic plants, we studied some factors that significantly affect regeneration and genetic transformation processes. The reproducible protocol described here yielded up to 6.91 transgenic lines per initial leaf segment infected with C58C1RifR (pMP90) A. tumefaciens strain; providing a fast and reliable tool for metabolic engineering of cardenolide production and genetic improvement of D. purpurea.
Materials and methods
Callus induction and plant regeneration
In vitro plants of Digitalis purpurea cv. Berggold were cultured on solid medium as previously described (Pérez-Alonso et al. 2009). For callus induction, leaf segments (1.0 cm2 adaxial surface to the medium) from in vitro plants (fourth–-seventh subculture) were cultured on basal medium containing MS salts (Murashige and Skoog 1962) supplemented with thiamine HCl 4.0 mg l−1, myo-inositol 100 mg l−1, sucrose 30 g l−1 and Gelrite 3.0 g l−1 (Duchefa, Netherlands). The effects of 2, 4-dichlorophenoxyacetic acid (2,4-D) 4.5 or 14.0 µM combined with 6-benzyladenine (6-BA) 0, 2.22 or 4.4 µM were tested. The pH was adjusted to 5.8 with 0.5 N KOH or 0.5 N HCl prior to autoclaving at 1.1 kg cm−2 and 121 °C for 20 min. The cultures were incubated in darkness at 27 ± 2 °C for 4 weeks. Data on callus- forming leaf segments per treatment were recorded. The best medium resulting from this experiment will be referred to as callus induction medium (CIM).
Thereafter, calli were cut into pieces by fresh weight (FW) (~0.6 g each piece) and subcultured twice for 4 weeks on CIM. For plant regeneration, callus pieces weighing 0.5–0.6 g FW were cultured on MS medium supplemented with combinations of indole-3-acetic acid (IAA) 0, 0.57 or 2.83 µM and 6-BA 0 or 4.4 µM. Cultures were incubated in a growth chamber at 27 ± 2 °C under a 16-h photoperiod from cool white fluorescent lamps at a photosynthetic photon flux density of 70 µmol m−2 s−1. Callus pieces with clearly differentiated shoots and leaves, and approximately 1.0–2.0 cm in length, were scored as regenerating callus. After 8 weeks, the number of regenerated shoots per leaf segment was recorded. Developed shoots were transferred to jar flasks containing 30 ml of MS medium solidified with Gelrite 2.5 g l−1.
Callus induction, proliferation and plant regeneration were carried out in 15 90-mm Petri dishes. Five replicates were done for each treatment (five Petri dishes with four segments each = 20 segments). Each experiment was repeated four times.
Geneticin (G-418) sensitivity
Callus induction from D. purpurea leaf segment was studied under different levels of G-418 (Duchefa) to determine the sensitivity of segments and the optimal concentration for the selection of transformed cells. Leaf segments were cultured on the best CIM supplemented with different concentrations of G-418 (0, 30, 40, 50, 60, 70 ,and 80 mg l−1), filter-sterilized prior to addition to the autoclaved medium. After 2 weeks, the leaf segments were transferred to fresh CIM with the same antibiotic concentration. Survival frequency was scored after 4 weeks culture, and the number of leaf segments with calli and the number of calli formed per leaf segment were recorded. All results are the mean of three independent experiments each with 100 leaf segments per treatment (n = 3 × 100).
Bacterial strain and plasmid
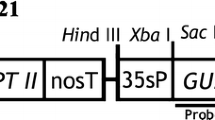
Two C58 chromosomal background strains of Agrobacterium tumefaciens were used to test the T-DNA transfer ability, EHA105 (Hood et al. 1993) and C58C1RifR containing the helper plasmid pMP90 (Koncz and Schell 1986). Plant material was infected with these strains harboring the binary vector pTJK136 (Kapila et al. 1997). The T-DNA of pTJK136 contains the neomycin phosphotransferase II (nptII) gene under control of the nopaline synthase (nos) promoter and the octopine synthase 3′ processing and polyadenylation signals, as well as the Escherichia coli β-glucuronidase (uidA) gene (Jefferson et al. 1987) with the potato st-ls1 intron (Vancanneyt et al. 1990) under the control of the CaMV 35S promoter and the nos 3′ processing and polyadenylation signals.
Inoculation and co-cultivation
Prior to inoculation, Agrobacterium was cultured on selective semi-solid LB medium supplemented with rifampicin 100 mg l−1, spectinomycin 100 mg l−1 and streptomycin 300 mg l−1, and incubated at 28 °C for 24–48 h. Single colonies were cultured in 3 ml selective liquid YEP medium (Bacto yeast extract 10 g l−1, Bacto peptone 10 g l−1, NaCl 5 g l−1, pH 7.5) and incubated at 200 rpm and 28 °C for 16–24 h to an A600 of approximately 1.2 units (Biophotometer; Eppendorf, Germany). One hundred µl of this culture were inoculated in 50 ml selective liquid YEP medium at 200 rpm and 28 °C for 16 h to an A600 of approximately 1.2 units. Cultures were centrifuged at 3214g for 10 min and the pellets were re-suspended in inoculation medium (100 % MS salts, sucrose 20 g l−1, d (+)-glucose 1.98 g l−1, 2-[N-morpholino]ethane sulphonic acid (MES) 3.9 g l−1, pH 5.5) containing 200 µM acetosyringone (AS). Agrobacterium suspensions were adjusted to a final A600 of 0.7. Leaf segments were excised as mentioned above for callus formation and immediately inoculated in Agrobacterium suspensions for 15 min with light manual agitation every 2 min. After inoculation, leaf segments were blotted dry on sterilized filter paper and transferred to co-cultivation medium (CIM supplemented with AS 200 µM, pH 5.6). Co-cultivation was done in darkness at 21 °C for 5 days.
Selection and regeneration of transformants
After co-cultivation, segments were washed with liquid CIM containing cefotaxime 500 mg l−1 and timentin 200 mg l−1 and thereafter blot-dried on sterile filter paper. Then, they were transferred to solid selective CIM containing G-418 at the concentration obtained in the previous experiment and timentin 200 mg l−1. The cultures were maintained for 2 months in the dark at 27 ± 2 °C with bi-weekly subcultures. Transgenic calli were individually subcultured to antibiotic-free regeneration medium as described above. The percentage of segments with calli, the number of calli per segment, and regenerated lines per inoculated segment were recorded. Putative rooted transgenic plants were cultured on MS medium for 4 weeks.
Histochemical β-glucuronidase assays
Transient GUS expression was determined on leaf segments and stable GUS expression were assessed on calli and regenerated plants using the histochemical GUS assay (Jefferson et al. 1987). Samples were incubated overnight at 37 °C in a modified assay solution containing 100 mM phosphate buffer (pH 7.0, 50 mM Na2HPO4 and 50 mM KH2PO4), 10 mM EDTA, 5 mM K-ferricyanide, 5 mM K-ferrocyanide, 0.1 % Triton X-100, and 1 mM X-Gluc. Non-transgenic tissues (leaf segments, calli and regenerated plants) were simultaneously stained in the same way. All the samples were de-stained in 70 % ethanol to remove chlorophylls and other pigments prior to visual analysis and photographing.
DNA extraction and PCR analysis
Genomic DNA was isolated from leaves of putatively transformed plants. One gram of leaf tissue was ground to fine powder in a mortar with liquid nitrogen. Total DNA was extracted following the protocol described by Khayat et al. (2004).
PCR was performed using genomic DNA of each plant as a target. The sequence of primers used for nptII and uidA genes fragment amplification were: nptII forward primer S-nptII-F: 5′-ATGATTGAACAAGATGGATTGCACGC-3′, nptII reverse primer S-nptII-R2 5′-TGATGCTCTTCGTCCAGATCATC-3′, uidA forward primer FW: 5′-TCTCTGCCGACAGTGGTCCCAAAGATGGAC–3′ and uidA reverse primer RV: 5′-GTTTACGCGTTGCTTCCGCCA-3′. PCR products expected to be amplified by these primers were fragments 488 bp for nptII and 1031 bp for uidA gene. PCR amplification reactions were carried out in 25 μl of total volume containing 200 ng genomic DNA, 0.5 µM of each primer, 1.5 mM MgCl2, 200 mM dNTP, 1X Taq polymerase reaction buffer and 0.25 U Taq polymerase (Fermentas, Lithuania). Thermocycling was carried out on Mastercycler programmable thermal control (Eppendorf) starting with denaturation at 94 °C for 3 min, followed by 35 cycles of 94 °C for 30 s, 65 °C for 30 s and 72 °C for 30 or 60 s, with a final extension at 72 °C for 10 min. Amplified fragments were analyzed by electrophoresis at 100 V for 1 h on 1.0 % Tris acetate EDTA agarose gel followed by stained in ethidium bromide (5 µg ml−1) and detection under UV illumination.
Southern hybridization
The integration of the T-DNAs was analyzed by Southern blot hybridization. Twenty micrograms of SacII enzyme-digested genomic DNA samples were separated on a 0.8 % Tris-acetate EDTA agarose gel (w/v) and run at 25 V for 12 h. A DIG-labeled marker (molecular weight marker VII; Roche, Vilvoorde, Belgium) was used at 40 ng per lane for size estimation. The DNA was blotted to a Hybond-N + nylon membrane (RPN203B; GE Healthcare) by upward capillarity forces using 20× SSC buffer (3 M NaCl, 0.3 M sodium citrate, pH 7.0) overnight at room temperature. The 488-bp nptII gene fragment, amplified from plasmid pTJK136 with the same primers mentioned above, was labeled with DIG-dUTP using a DIG-High Prime DNA Labeling and Detection Kit (Roche, Germany). Hybridization, washing and detection were performed according to the manufacturer’s instructions (Roche).
Statistical analysis
A completely randomized design was used for all treatments. Data were processed using the computer software SPSS package for Windows v.15. Results were analyzed by the non-parametric Kruskal–Wallis test. In order to distinguish between comparisons a post hoc Mann–Whitney test was performed. Significance was reported at P ≤ 0.05.
Results and discussion
Callus induction and plant regeneration
Within all experimental conditions tested, D. purpurea callus initiation was observed at 7 days of culture. Callus tissue first appeared at the border of segments and the central vein. After 15 days, the segment surface was completely covered by formed callus (Fig. 1a). As shown in Table 1, the highest frequency of callus formation (>90 %) was obtained on the medium supplemented with the lowest concentration of 2,4-D (4.5 µM). The addition of 6-BA to the CIM had a negative effect on callus induction (Table 1) and some sectors of the callus turned brown after 2 weeks of induction. Our results contrast with those reported earlier by Fatima et al. (2009) for leaf segments of D. lanata, in which callus induction was further improved when both auxins and cytokinins were added. In D. thapsi, calli were induced and maintained with subcultures every 4 weeks on medium supplemented with 2.2 µM 2,4-D and 2.87 µM 6-BA (Herrera et al. 1990). It is common to find genotypes that react more readily than others to a particular set of inductive conditions (Jiménez 2005). The endogenous balance of plant growth regulators also differs among plant species and influences the response to exogenous concentrations and type of plant growth regulator needed for best callus induction between Digitalis species.
Callus induction and plant regeneration from leaf segments of Digitalis purpurea. a Callus obtained on MS semisolid medium supplemented with 4.5 µM 2, 4-D after 4 weeks; b green spots developed from callus induced with 2.4-D without 6-BA during first stage. Calli were cultured onto regeneration medium containing 2.8 µM and 4.4 µM 6-BA; c regenerated plants from callus after 8 weeks on same medium as in (b). Bars (a, b) 4 mm, (c) 10 mm
Plant regeneration from callus was strongly influenced by combinations and the concentrations of plant growth regulators used for callus induction (Table 1). Following 7–10 days of culture on regeneration media, calli first became light yellow and later turned green. The highest frequency of green spots was observed for callus previously induced with 2,4-D only (Fig. 1b). In the growth regulator-free medium most green spots developed into roots, although some shoots were also observed. A possible explanation for these findings could be the differences in endogenous growth regulator concentrations as previously reported by Fatima et al. (2009). Our results demonstrated that the percentage of regenerating callus (100 %) and the number of regenerated shoots per segment (16.0) were highest on medium with 0.57 µM IAA and 4.4 µM 6-BA and when calli were induced on 4.5 µM 2,4-D (Table 1; Fig. 1c). Poor or no response was observed for shoot formation on callus induced in the remaining treatments.
According to Verma et al. (2011), shoot regeneration in Digitalis needs a high cytokinin/auxin ratio. The use of growth regulators for callus induction and regeneration has been studied in Digitalis tissue culture starting from different segments. Shoot-forming cultures from D. purpurea leaves were grown in various modifications of MS medium containing 4.4 µM 6-BA and 5.7 µM IAA (Hagimori et al. 1983). Gurel et al. (2011) reported shoot formation using different explant types of D. davisiana on Linsmaier and Skoog (1965) medium supplemented with thidiazuron (TDZ) and IAA. The highest frequency (86.6 %) of organogenic explants was achieved with flamingo-bill explants with 5.9 shoots per explant.
Moreover, addition of cytokinins promoted the induction of D. purpurea shoot proliferation from several explants including leaf segments (Patil et al. 2013). The authors reported 57.1 % plant regeneration and 7.2 shoots formed per leaf segment incubated on MS medium with 7.5 μM BA.
Li et al. (2014) described 100 % shoot regeneration from mature leaf segments cultivated on MS medium containing 4.5 μM TDZ and 0,54 μM 1-naphthaleneacetic acid (NAA). Unfortunately, data about the number of shoots regenerated per explant are not provided from their study.
Compared to the above-mentioned reports, we described here the highest plant regeneration efficiency for D. purpurea. The overall regeneration efficiency from callus cultures was thus significantly increased in MS medium containing 4.5 µM 2,4-D for the induction stage and 4.4 µM 6-BA combined with 0.57 µM IAA for plant regeneration, resulting in 16.0 shoots regenerated per leaf segment. Consequently, the developed protocol was further used for genetic transformation procedures.
G-418 (geneticin) sensitivity test
Leaf segments were cultured on optimized CIM medium supplemented with different concentration of G-418. A gradual decrease in the number of segments with calli and the number of calli per segment were associated with an increase in G-418 concentration (Fig. 2). The number of segments with calli and the number of calli per segment were reduced drastically in G-418 concentrations higher than 30 mg l−1. At concentrations above 70 mg l−1, no calli were induced and segments were dead within 4 weeks. As a result, 70 mg l−1 would be the minimal inhibitory concentration of G-418 and was considered optimum for the selection of transformants in the subsequent experiments.
G-418 dose response of Digitalis purpurea L. leaf segments on callus induction medium after 4 weeks. Data are means from three independent experiments, each with 100 segments per treatment (n = 300). Values followed by different letters for each variable are significantly different (P ≤ 0.05) based on Kruskal–Wallis/Mann–Whitney tests
Plant transformation protocols generally involve the use of selectable marker genes for the screening of transgenic material (Rosellini 2012). Efficient selection is a necessary prerequisite for the successful production of transgenic plants through Agrobacterium-mediated transformation (Rajesh et al. 2013). One of the most commonly used selectable marker gene is nptII, which confers resistance to kanamycin or G-418 (geneticin). Moreover, this gene is considered safe and commercially available (Rosellini 2012). Sales et al. (2003) reported the use of kanamycin or geneticin in the transformation protocol developed for D. minor. G-418 has been used as selective agent in different transformation systems in plants such as Saccharum officinarum L. (Elliott et al. 1998), Sorghum bicolor L. (Tadesse et al. 2003), Castanea dentata (Marshall) Borkh (Andrade et al. 2009), Triticum aestivum L. (Fahim et al. 2010; Xue et al. 2011), Brassica napus L. (Boszoradova et al. 2011), Musa spp. (Chong-Pérez et al. 2012), and Oryza sativa L. (Nandy and Srivastava 2012). These investigations showed the diversity of minimum lethal concentrations of G-418. Plant susceptibility to antibiotics seems to change broadly among species, genotypes, and plant tissues, and depends on the in vitro experimental conditions of the transformation protocol (Padilla and Burgos 2010).
Selection and regeneration of transgenic shoots
Susceptibility of the plant cell to Agrobacterium infection is the primary requirement for T-DNA transfer. In the present study, transient expression levels in leaf segments inoculated with A. tumefaciens, either strain C58C1RifR (pMP90) or EHA105, were similar (Fig. 3a, b). Untransformed segments used as negative controls did not show GUS expression (Fig. 3c).
Histochemical β-glucuronidase assays. Transient GUS expression on Digitalis purpurea leaf inoculated with EHA105 (pTJK136) (a), C58C1RifR (pMP90) (pTJK136) (b), and non-transformed leaf segment (c); d, e untransformed control without (d) and with (e) G-418 70 mg l−1; f, g recovery of transformed callus after transformation with EHA105 (pTJK136) (f) and C58C1RifR (pMP90) (pTJK136) (g); h–j stable GUS expression in transformed callus (h), plant (i), and root (j) obtained after transformation with C58C1RifR (pMP90) (pTJK136); k–m GUS blue spots were not observed in callus (k), shoot (l), or roots (m) from untransformed segments. Bars (a–h, k) 1.5 mm, (i, l) 12 mm, (j, m) 7 mm
Callus induction frequencies (expressed as number of segments with calli and number of calli per segment) showed differences between Agrobacterium strains and controls (Table 2; Fig. 3d–g). Untransformed leaf segments cultured on medium without antibiotic formed callus as described earlier. However, those cultured on selection medium did not produce any callus (Fig. 3e), confirming our previous findings. Comparatively, callus formation was around 10-fold higher on leaf segments inoculated with strain C58C1RifR (pMP90) than on those inoculated with EHA105 (Table 2). This result contradicts the transient expression analysis where no differences were observed between the strains. It should be noted that blue spots assessed histochemically after co-cultivation represent zones of transient expression (mainly from non-integrated DNA copies), whereas calli formed after selection most probably derives from cells where DNA integration has occurred (Gelvin and Kim 2007). GUS assay of transformed calli showed stable uidA expression (Fig. 3h) in 100 % of the tested calli irrespective of the Agrobacterium strain used. Untransformed calli did not show any GUS activity (Fig. 3k).
As expected from the number of calli obtained after selection, there were differences in the number of plantlets regenerated between strains. A total of 518 putative transgenic lines (each regenerated plantlet was considered as a line) were regenerated out of 75 inoculated segments with C58C1RifR (pMP90) strain, while only 24 lines were regenerated from 83 segments inoculated with EHA105 strain (Table 2).
The selection of an efficient Agrobacterium strain plays an important role in improving transformation efficiency (Rajesh et al. 2013). Because identical bacterial densities were used to inoculate the segments with each Agrobacterium strain, differences in callus induction and plant regeneration must be related to the strain virulence and the specific interaction with D. purpurea host plant. Indeed, these effects have been reported by Maheshwari et al. (2011) in canola and by Chetty et al. (2012) in tomato. An A. tumefaciens-mediated transformation procedure was developed for the first time in the genus Digitalis by Sales et al. (2003). This protocol includes inoculation of leaf segments with EHA105 Agrobacterium strain obtaining 8.4 % of transformation frequency and a high incidence of escapes. The strains tested in this investigation have the same chromosomal background (C58), but different vir genes on the disarmed Ti-plasmid. The vir genes in strain EHA105, derived from Ti-plasmid pTIBo542 (Lee and Gelvin 2008), often confer supervirulence and increase transformation efficiency, for example in pea (Nadolska-Orczyk and Orczyk 2000), apple (De Bondt et al. 1994), and tomato (Chetty et al. 2012). However, this is not always the case; for example, in Camelina sativa cultivars, the lowest transformation efficiency was obtained with EHA105 (Liu et al. 2012).
Stable expression of β-glucuronidase was determined in regenerated plantlets (Fig. 3i) and roots (Fig. 3j), but was not present in untransformed segments (Fig. 3l, m). The percentage of GUS-positive lines was similar between regenerated lines from segments transformed with EHA105 and C58C1RifR (pMP90) (58.5 and 62.3 %, respectively). Regarding GUS-negative plants, there are three possible explanations: firstly, plants are not transgenic; secondly, the inserted T-DNA was truncated; or thirdly, integrated T-DNA became silenced (Gelvin 2003; Li et al. 2012).
PCR and Southern analysis
One hundred putative transgenic lines were selected for PCR analysis, 86 lines derived from leaf segments inoculated with strain C58C1RifR (pMP90) (65 GUS-positive and 21 GUS-negative) and 14 lines derived from EHA105 (10 GUS-positive and four GUS-negative). The nptII and uidA genes were present in all GUS-positive lines, as determined by PCR amplification of 488-bp and 1031-bp fragments of these genes- respectively (Fig. 4a). Only four GUS-negative lines obtained with strain C58C1RifR (pMP90) showed neither uidA nor nptII genes (4.6 % of escapes for this strain), and no escapes were detected among lines obtained with strain EHA105. These results suggest that, probably, few non-transgenic cells were protected from the selection agent by the high number of calli obtained per leaf segment with strain C58C1RifR (pMP90). Perhaps the increase of selection pressure (e.g., by applying an antibiotic gradient) could prevent this problem. As observed in Fig. 4a, one GUS-negative line (lane 5) did not contain the uidA gene but the nptII gene was present. These results indicate the integration of a truncated T-DNA. All transgenic plants analyzed that were positive for the presence of the uidA gene in the PCR assay also showed constitutive GUS expression in leaves, indicating the presence of a full functional transgene.
Molecular analysis of putative transformed Digitalis purpurea L. plantlets. a PCR carried out with specific primers for nptII (upper panel) and uidA (lower panel) primers; lanes 1–3 genomic DNA from putative transgenic lines obtained with EHA105 (pTJK136); lanes 4–16 genomic DNA from putative transgenic lines obtained with C58C1RifR (pMP90) (pTJK136); C− untransformed plant; H 2 O water; C+ pTJK136 plasmid control; MW molecular weight marker Gene Ruler™ DNA Ladder Mix (Fermentas). b Southern hybridization of putative transformed D. purpurea L. PCR-positive plantlets; lanes 1–3 genomic DNA from putative transgenic lines obtained with EHA105 (pTJK136), lanes 4–9 genomic DNA from putative transgenic lines obtained with C58C1RifR (pMP90) (pTJK136); (−) untransformed plant; (+) pTJK136 plasmid control; MW digoxigenin-labeled DNA molecular weight marker VII (Roche). Twenty micrograms total DNA were digested with SacII, and separated fragments were hybridized with a digoxigenin-labeled nptII probe (488 bp)
Southern hybridization confirmed stable integration of the target gene in the genome of nine PCR-positive D. purpurea lines. Genomic DNA and pTJK136 were digested with SacII, which recognizes a single site within the T-DNA. Therefore, digestion of genomic DNA with SacII generates a unique fragment for each integrated copy. The results indicated that all the selected plants integrated one or two T-DNAs in their genome (Fig. 4b). The size of each band differed from line to line, indicating independent transformation events and random integration. No hybridization was detected in the non-transformed plants (controls).
When this paper was in preparation, Li et al. (2014) published a protocol for Agrobacterium-mediated transformation in D. purpurea by inoculating mature leaf segments. The main differences from our protocol are the A. tumefaciens strains (GV2260 and GV3101) used for T-DNA transfer, the plant regeneration mode (direct organogenesis), and the use of kanamycin as selective agent. Additionally, the authors reported an efficiency of 0.62 kanamycin-resistant shoots regenerated per explant, which is lower than the transformation efficiency obtained in our work (6.92 lines/leaf segment). Also, the authors detected the presence of GUS and nptII genes by PCR analysis in 9 out of 27 putative transgenic lines (GUS-positive plants), whereas GUS-negative plants were not analyzed by PCR (Li et al. 2014).
A. tumefaciens-mediated transformation has been used to engineer some medicinal plant species such as Catharanthus roseus L. (Van der Frits and Memelink 2000), Artemisia annua L. (Han et al. 2006), Humulus lupulus L. (Gatica-Arias et al. 2012), Podophyllum hexandrum Royle (syn. P. emodi Wall. ex Hook.f. & Thomas) (Rajesh et al. 2013), and Atropa belladonna L. (Song and Walworth 2013). This biological system exhibits several advantages for plant genetic transformation, since Agrobacterium efficiently integrates low copy numbers of relatively large segments of DNA with defined ends into plant chromosomes without much rearrangement (Komari et al. 2004).
To our knowledge, this is the most efficient report on A. tumefaciens-mediated transformation in D. purpurea. The genetic transformation method developed in our study provides an efficient tool to study metabolic engineering of cardenolide production and consequently genetic improvement of Digitalis species. New sequences related to cardenolide biosynthesis were recently described by Wu et al. (2012), which could be considered as possible targets to engineer D. purpurea plants.
References
Abou-Alaiwi WA, Potlakayala SD, Goldman SL, Josekutty PC, Karelia DN, Rudrabhatla SV (2012) Agrobacterium-mediated transformation of the medicinal plant Centaurea montana. Plant Cell Tissue Organ Cult 109:1–8
Andrade GM, Nairn CJ, Le HT, Merkle SA (2009) Sexually mature transgenic American chestnut trees via embryogenic suspension-based transformation. Plant Cell Rep 28:1385–1397
Boszoradova E, Libantova J, Matusikova I, Poloniova Z, Jopcik M, Berenyi M, Moravcikova J (2011) Agrobacterium-mediated genetic transformation of economically important oilseed rape cultivars. Plant Cell Tissue Organ Cult 107:317–323
Canter PH, Thomas H, Ernst E (2005) Bringing medicinal plants into cultivation: opportunities and challenges for biotechnology. Trends Biotechnol 23:180–185
Chetty VJ, Ceballos N, García D, Narváez-Vásquez J, López W, Orozco-Cárdenas ML (2012) Evaluation of four Agrobacterium tumefaciens strains for the genetic transformation of tomato (Solanum lycopersicum L.) cultivar Micro-Tom. Plant Cell Rep 34:747–754
Chong-Pérez B, Reyes M, Rojas L, Ocaña B, Pérez B, Kosky RG, Angenon G (2012) Establishment of embryogenic cell suspension cultures and Agrobacterium-mediated transformation in banana cv. ‘‘Dwarf Cavendish’’ (Musa Cavendish, AAA): effect of spermidine on transformation efficiency. Plant Cell Tissue Organ Cult 111:79–90
De Bondt A, Eggermont K, Druart P, De Vil M, Goderis I, Vanderleyden J, Broekaert WF (1994) Agrobacterium-mediated transformation of apple (Malus × domestica Borkh.): an assessment of factors affecting gene transfer efficiency during early transformation steps. Plant Cell Rep 13:587–593
Elliott AR, Campbell JA, Brettell RIS, Grof CPL (1998) Agrobacterium-mediated transformation of sugarcane using GFP as a screenable marker. Aust J Plant Physiol 25:739–743
Fahim M, Ayala-Navarrete L, Millar AA, Larkin PJ (2010) Hairpin RNA derived from viral NIa gene confers immunity to wheat streak mosaic virus infection in transgenic wheat plants. Plant Biotechnol J 8:821–834
Fatima Z, Mujib A, Fatima S, Arshi A, Umar S (2009) Callus induction, biomass growth, and plant regeneration in Digitalis lanata Ehrh.: influence of plant growth regulators and carbohydrates. Turk J Bot 33:393–405
Gatica-Arias A, Farag MA, Stanke M, Matoušek J, Wessjohann L, Weber G (2012) Flavonoid production in transgenic hop (Humulus lupulus L.) altered by PAP1/MYB75 from Arabidopsis thaliana L. Plant Cell Rep 31:111–119
Gavidia I, Tarrio R, Rodríguez-Trelles F, Pérez-Bermúdez P, Seitz HU (2007) Plant progesterone 5β-reductase is not homologous to the animal enzyme. Molecular evolutionary characterization of P5βR from Digitalis purpurea. Phytochemistry 68:853–864
Gelvin SB (2003) Improving plant genetic engineering by manipulating the host. Trends Biotechnol 21:95–98
Gelvin SB, Kim S-I (2007) Effect of chromatin upon Agrobacterium T-DNA integration and transgene expression. Biochim Biophys Acta 1769:410–421
Gómez-Galera S, Pelacho AM, Gene A, Capell T, Christou P (2007) The genetic manipulation of medicinal and aromatic plants. Plant Cell Rep 26:1689–1715
Gurel E, Yücesan B, Aglic E, Gurel S, Verma SK, Sokmen M, Sokmen A (2011) Regeneration and cardiotonic glycoside production in Digitalis davisiana Heywood (Alanya Foxglove). Plant Cell Tissue Organ Cult 104:217–225
Hagimori M, Matsumoto T, Obi Y (1983) Effects of mineral salts, initial pH and precursors on digitoxin formation by shoot-forming cultures of Digitalis purpurea L. grown in liquid media. Agricult Biol Chem 47:565–571
Han JL, Liu BY, Ye HC, Wang H, Li ZQ, Li GF (2006) Effects of overexpression of the endogenous farnesyl diphosphate synthase on the artemisin content in Artemisia annua L. J Integr Plant Biol 48:482–487
Herl V, Frankenstein J, Meitinger N, Muller-Uri F, Kreis W (2007) Δ5-3β-Hydroxysteroid dehydrogenase (3βHSD) from Digitalis lanata. Heterologous expression and characterization of the recombinant enzyme. Planta Med 73:704–710
Herrera MT, Cacho M, Corchete MP, Fernández-Tarrago J (1990) One step shoot tip multiplication and rooting of Digitalis thapsi L. Plant Cell Tissue Org Cult 22:179–182
Hood EE, Gelvin SB, Melehers LS, Hoekema A (1993) New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res 2:208–218
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Jiménez V (2005) Involvement of plant hormones and plant growth regulators on in vitro somatic embryogenesis. Plant Growth Regul 47:91–110
Kapila J, De Rycke R, Van Montagu M, Angenon G (1997) An Agrobacterium-mediated transient gene expression system for intact leaves. Plant Sci 122:101–108
Khayat E, Duvdevani A, Lehav E, Ballesteros BA (2004) Somaclonal variation in banana (Musa acuminata cv. Grande Naine). Genetic mechanism, frequency, and application as a tool for clonal selection. In: Jain SM, Swennen R (eds) Banana improvement: cellular, molecular biology, and induced mutation. Science, Plymouth, pp 99–109
Koga M, Hirashima K, Nakahara T (2000) The transformation system in Foxglove (Digitalis purpurea L.) using Agrobacterium rhizogenes and traits of the regenerants. Plant Biotechnol 17:99–104
Komari T, Ishida Y, Hiei Y (2004) Plant transformation technology: Agrobacterium-mediated transformation. In: Christou P, Klee H (eds) Handbook of plant biotechnology 1. Wiley, London, pp 233–261
Koncz C, Schell J (1986) The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet 204:383–396
Kreis W, Müller-Uri F (2013) Cardenolide aglycone formation in Digitalis. In: Bach TJ, Rohmer M (eds) Isoprenoid synthesis in plants and microorganisms: new concepts and experimental approaches. Springer, New York, pp 425–438
Kuate SP, Padua RM, Eisenbeiss WF, Kreis W (2008) Purification and characterization of malonyl-coenzyme A: 21-hydroxypregnane 21-O-malonyltransferase (Dp21MaT) from leaves of Digitalis purpurea L. Phytochemistry 69:619–626
Lee L-Y, Gelvin SB (2008) T-DNA binary vectors and systems. Plant Physiol 146:325–332
Lehmann U, Moldenhauer D, Thomar S, Diettrich B, Luckner M (1995) Regeneration of plants from Digitalis lanata cells transformed with Agrobacterium tumefaciens carrying bacterial genes encoding neomycin phosphotransferase II and β-glucuronidase. J Plant Physiol 147:53–57
Li WJ, Dai LL, Chai ZJ, Yin ZJ, Qu LQ (2012) Evaluation of seed storage protein gene 3´-untranslated regions in enhancing gene expression in transgenic rice seed. Transgenic Res 21:545–553
Li Y, Gao Z, Piao C, Lu K, Wang Z, Cui M-L (2014) A stable and efficient Agrobacterium tumefaciens-mediated genetic transformation of the medicinal plant Digitalis purpurea L. Appl Biochem Biotechnol 172:1807–1817
Linsmaier E, Skoog F (1965) Organic growth factor requirements of tobacco tissue cultures. Physiol Plant 18:100–127
Liu X, Brost J, Hutcheon C, Guilfoil R, Wilson AK, Leung S, Shewmaker CK, Rooke S, Nguyen T, Kiser J, De Rocher J (2012) Transformation of the oilseed crop Camelina sativa by Agrobacterium-mediated floral dip and simple large-scale screening of transformants. In Vitro Cell Dev Biol-Plant 48:462–468
Maheshwari P, Selvaraj G, Kovalchuk I (2011) Optimization of Brassica napus (canola) segment regeneration for genetic transformation. New Biotechnol 29:144–155
Menger L, Vacchelli E, Adjemian S, Martins I, Ma Y, Shen S, Yamazaki T, Sukkurwala AQ, Michaud M, Mignot G, Schlemmer F, Sulpice E, Locher C, Gidrol X, Ghiringhelli F, Modjtahedi N, Galluzzi L, André F, Zitvogel L, Kepp O, Kroemer G (2012) Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci Transl Med 4:143ra99. doi:10.1126/scitranslmed.3003807
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nadolska-Orczyk A, Orczyk W (2000) Study of the factors influencing Agrobacterium-mediated transformation of pea (Pisum sativum L.). Mol Breed 6:185–194
Nandy S, Srivastava V (2012) Marker-free site-specific gene integration in rice based on the use of two recombination systems. Plant Biotechnol J 10:904–912
Padilla IMG, Burgos L (2010) Aminoglycoside antibiotics: structure, functions and effects on in vitro plant culture and genetic transformation protocols. Plant Cell Rep 29:1203–1213
Patil JG, Ahire ML, Nitnaware KM, Panda S, Bhatt VP, Kavi Kishor PB, Nikam TD (2013) In vitro propagation and production of cardiotonic glycosides in shoot cultures of Digitalis purpurea L. by elicitation and precursor feeding. Appl Microbiol Biotechnol 97:2379–2393
Pérez-Alonso N, Wilken D, Gerth A, Jahn A, Nitzsche HM, Kerns G, Capote A, Jiménez E (2009) Cardiotonic glycosides from biomass of Digitalis purpurea L. cultured in temporary immersion systems. Plant Cell Tissue Org Cult 99:151–156
Pérez-Bermúdez P, Moya García A, Tuñón I, Gavidia I (2010) Digitalis purpurea P5BR2, encoding steroid 5β-reductase, is a novel defense-related gene involved in cardenolide biosynthesis. New Phytol 185:687–700
Pradel H, Lehmann U, Diettrich B, Luckner M (1997) Hairy root cultures of Digitalis lanata: secondary metabolism and plant regeneration. J Plant Physiol 151:209–215
Prassas I, Diamandis EP (2008) Novel therapeutic applications of cardiac glycosides. Nat Rev Drug Discov 7:926–935
Rajesh M, Jeyaraj M, Sivanandhan G, Subramanyam K, Mariashibu TS, Mayavan S, Kapil Dev G, Anbazhagan VR, Manickavasagam M, Ganapathi A (2013) Agrobacterium-mediated transformation of the medicinal plant Podophyllum hexandrum Royle (syn. P. emodi Wall. ex Hook.f. & Thomas). Plant Cell Tissue Organ Cult 114:71–82
Roca-Pérez L, Boluda R, Gavidia I, Pérez-Bermúdez P (2004) Seasonal cardenolide production and Dop5βr gene expression in natural populations of Digitalis obscura. Phytochemistry 65:1869–1878
Rosellini D (2012) Selectable markers and reporter genes: a well furnished toolbox for plant science and genetic engineering. Crit Rev Plant Sci 31:401–453
Saito K, Shimomura MYK, Yoshimatsu K, Murakoshi I (1990) Genetic transformation of foxglove (Digitalis purpurea) by chimeric foreign genes and production of cardioactive glycosides. Plant Cell Rep 9:121–124
Sales E, Segura J, Arillaga I (2003) Agrobacterium-mediated genetic transformation of the cardenolide-producing plant Digitalis minor. Planta Med 69:143–147
Sales E, Muñoz-Bertomeu J, Arrillaga I, Segura J (2007) Enhancement of cardenolide and phytosterol levels by expression of an N-terminally truncated 3-hydroxy-3-methyglutaryl CoA reductase in transgenic Digitalis minor. Planta Med 73:605–610
Sales E, Müller-Uri F, Nebauer SG, Segura J, Kreis W, Arillaga I (2011) Digitalis. In: Kole C (ed) Wild crop relatives: genomic and breeding resources, plantation and ornamental crops. Springer, Berlin, pp 73–112
Song G, Walworth A (2013) Agrobacterium tumefaciens-mediated transformation of Atropa belladonna. Plant Cell Tissue Organ Cult 115:107–113
Tadesse Y, Sagi L, Swennen R, Jacobs M (2003) Optimisation of transformation conditions and production of transgenic sorghum (Sorghum bicolor) via microparticle bombardment. Plant Cell Tissue Organ Cult 75:1–18
Van der Frits L, Memelink J (2000) ORCA3, a jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science 289:295–297
Vancanneyt G, Schmidt R, O’Connor-Sanchez A, Willmitzer L, Rocha-Sosa M (1990) Construction of an intron-containing marker gene: splicing of the intron in transgenic plants and its use in monitoring early events in Agrobacterium-mediated plant transformation. Mol Gen Genet 220:245–250
Verma SK, Yücesan B, Gurel S, Gurel E (2011) Indirect somatic embryogenesis and shoot organogenesis from cotyledonary leaf segments of Digitalis lamarckii Ivan., an endemic medicinal species. Turk J Biol 35:743–750
Warren B (2005) Digitalis purpurea. Am J Cardiol 95:544
Wu B, Li Y, Yan H, Ma Y, Luo H, Yuan L, Chen S, Lu S (2012) Comprehensive transcriptome analysis reveals novel genes involved in cardiac glycoside biosynthesis and mlncRNAs associated with secondary metabolism and stress response in Digitalis purpurea. BMC Genom 13:15
Xue GP, Way HM, Richardson T, Joyce PA, Drenth J, McIntyre CL (2011) Overexpression of TaNAC69 leads to enhanced transcript levels of stress up-regulated genes and dehydration tolerance in bread wheat. Mol Plant 4:697–712
Acknowledgments
The authors thank the support of the EU through the ALFA Network CARIBIOTEC (project AML/B7-311/97/0666/II-0201) and the Institutional University Collaboration programme with Universidad Central “Marta Abreu” de Las Villas funded by the Flemish Interuniversity Council (VLIR-IUC UCLV).
Author information
Authors and Affiliations
Corresponding author
Additional information
N. Pérez-Alonso and B. Chong-Pérez contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Pérez-Alonso, N., Chong-Pérez, B., Capote, A. et al. Agrobacterium tumefaciens-mediated genetic transformation of Digitalis purpurea L.. Plant Biotechnol Rep 8, 387–397 (2014). https://doi.org/10.1007/s11816-014-0329-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-014-0329-0