Abstract
Isoflavonoid production in cell cultures of Pueraria tuberosa as influenced by an angiospermic parasite, Cuscuta reflexa, was studied. During the time course, maximum isoflavonoid content was recorded when Cuscuta elicitor was added on day 15 of culture. Among various concentrations of elicitor tried, 1 g l−1 of Cuscuta elicitor was found to be the most effective. The optimized elicitation conditions were used in vessels of varying capacity where maximum yield of ~91 mg l−1 of isoflavonoid was recorded in a 2-l bioreactor which was about 19% higher than the control cultures. In this case, puerarin content increased up to 11 mg l−1 which was 580% higher that the value recorded in the control cultures. In the bioreactor, 8 days of elicitation was optimal for the high accumulation of isoflavonoid, giving productivity of ~4 mg l−1 day−1. The study showed persistent high isoflavonoid yield even during scale-up. Use of a preparation of Cuscuta reflexa as an elicitor is reported for the first time. The increase in isoflavonoid content was elicitor dose-dependent and can be explored to trigger high yields of isoflavonoid/secondary metabolites in production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tubers of Pueraria tuberosa (Roxb. ex. Willd.) DC are widely used in various Ayurvedic (Indian system of medicine) formulations and contain isoflavonoids, viz., puerarin, daidzein, genistein and genistin (Dev 2006). These isoflavonoids, mostly limited to the family Fabaceae, are effective antioxidants and are used in the treatment of cardiovascular diseases (Mizushige et al. 2007), osteoporosis and postmenopausal symptoms (Dai et al. 2008), and are also under phase I and II clinical trials for kidney failure and prostate cancer (Bingham et al. 1998). These utilities urge in vitro research to develop the technology for their competent controlled production (Ren et al. 2001).
Plants are a potential source of a large number of valuable metabolic products (Ramawat and Goyal 2008; Ramawat et al. 2009). Biotic and abiotic elicitors are frequently used to stimulate the biosynthetic activity to optimize the secondary metabolites accumulation in plant cell cultures (Zhao et al. 2005), which is finding commercial applications (Savitha et al. 2006). The cell cultures of Glycyrrhiza echinata, Cicer arietinum, Pueraria lobata and P. thomsonii have been studied for elicitor-induced manipulation of isoflavonoid production (Luczkiewicz 2008; Maojun et al. 2006). Production of secondary metabolites through in vitro culture is promising, but a breakthrough is required to produce a metabolite at a commercially viable level. In search of effective triggering agents for production of secondary metabolites, we demonstrated a new role of plant growth regulators (Tanwar et al. 2007; Goyal and Ramawat 2008a) and a new elicitation agent such as plant gums (Dass and Ramawat 2009).
In the present communication, we report an elicitation effect of a preparation from Cuscuta reflexa, an angiospermic parasite, on isoflavonoid accumulation in cell cultures of P. tuberosa. As far as we know, there is no report of the use of an angiosperm plant parasite as a source of elicitor. Cuscuta represent a unique group of holoparasitic dicotyledonous plants which can infect nearly all dicotyledonous species (Albert et al. 2008). This parasitic property of Cuscuta was used for creating the elicitation effect in P. tuberosa. Earlier, we reported a marked effect of plant growth regulators, biotic and abiotic elicitors on the production of isoflavonoids in cell cultures of P. tuberosa and a maximum yield of ~12 mg l−1 was obtained with elicitation (Goyal and Ramawat 2008a, b, c). Thus, in order to explore new efficient, biotic, and economically feasible sources of elicitor, Cuscuta was selected. In this study, the effects of elicitation duration, growth period and vessel size were studied in relation to stable isoflavonoid production.
Materials and methods
Cultures and experimental setup
Cell suspension cultures were grown in modified MS medium (Murashige and Skoog 1962) containing morphactin 0.1 mg l−1 and 2iP 5 mg l−1, which yielded the highest isoflavonoid production as described earlier (Goyal and Ramawat 2008a). The pH of the medium was adjusted to 5.8 and autoclaved at 121°C for 15 min. These cultures were incubated on a rotary shaker at 100g and 25 ± 0.2°C.
Preparation of elicitor and elicitor treatment
The plant material of C. reflexa was collected from plants growing near Udaipur, dried at 60°C for 48 h and ground. Fatty acids and pigments were removed by overnight extraction with chloroform. The chloroform was removed by filtration. Polyphenol and low molecular weight compounds were extracted from the residue with methanol several times until a colorless extract was obtained. After filtration, the residue was washed in ice-cold water (thrice), dried and strained through sieves (0.4-mm mesh; Sigma, USA). The Cuscuta powder thus obtained was analyzed by HPLC for isoflavonoids and incorporated (0.2 and 2.0 g l−1) on three different days of culture, either at the day of inoculation (0 day) or on day 10 or 15 after inoculation and harvested at day 20 of culture.
Cell cultures were also treated with different concentrations of Cuscuta elicitor (see “Results and discussion”). These concentrations of Cuscuta elicitor were incorporated in the medium on day 15 of culture and harvested after 5 days of incorporation. The optimal concentration and treatment duration were combined with vessels of different sizes (see “Results and discussion”) and a 2-l bioreactor to determine optimal conditions for stable isoflavonoid accumulation. The bioreactor was constructed from a Borosil three-necked flask (No. 4384; Borosil, India) fitted with air sponge type sparger, sample tube, air inlet and outlet through PTFE filter (Sartorius® Midisart® 2000, 0.2 μm). The bioreactor flask was fixed in a stand and autoclaved. It contained 1.5 l medium with 30% v/v aeration and was placed at the controlled temperature of 25 ± 0.2°C. To evaluate the efficacy of the plant-based biotic elicitor, a time course study was carried out using the bioreactor.
Sample preparation and HPLC analysis
The cultures were washed with distilled water and filtered under mild vacuum. The dry mass (DM) was determined by drying the cells at 60°C in an oven to a constant weight. Dried homogenized cells (100–150 mg) were extracted in 5 ml methanol for 12 h and analyzed by HPLC, as described earlier (Vaishnav et al. 2006).
The HPLC system used for the separation of compounds was equipped with a pump (model L2130; Merck-Hitachi), autosampler (model L-2200; Merck-Hitachi) and a UV detector (L-2400; Merck-Hitachi) controlled with “Lachrome Elite” software. Separation was accomplished on a (LichroCART)® 250 × 4 mm LiChrospher® (5 μm) RP-18 column protected by a guard column of the same material. The auto sampler was programmed to inject 20 μl sample per injection. During HPLC analysis, the solvent system used was: solvent A, 0.0025% trifluoroacetic acid in water and solvent B, 80% acetonitrile (Merck, Mumbai, India) in solvent A. The mobile phase consisted of solvent (A) and (B). The step-gradient programme of solvent A was as follows: 0–2 min: 85%; 2–5 min: 85–80%; 5–15 min: 80–50%; 15–20 min: 50–40%; 20–30 min: 40–30%; 30–35 min: 30–20%; 35–45 min: 20–0%; 45–48 min: 0%; 48–50 min: 0–85%; 50–55 min: 85%. The separation was performed at a flow rate of 1.0 ml min−1, and chromatographic peaks were monitored at 254 nm.
Statistical analysis
All results were averaged over three separate analyses from three flasks and each experiment was done in duplicate. For individual isoflavonoid content, the data were analyzed by ANOVA followed by mean separation using a post hoc least significant difference (LSD) test at P ≤ 0.05 using Prism statistical software.
Result and discussion
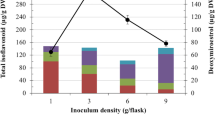
This paper describes for the first time an elicitation effect of C. reflexa on the increased production of isoflavonoid. Cuscuta elicitor was added to P. tuberosa cell cultures at different days of the culture period to identify the optimal incorporation day for the induction of isoflavonoid. Figure 1 shows the effect of time of Cuscuta incorporation on all the isoflavonoid production. It was observed that Cuscuta elicitor was most effective in increasing only the puerarin content in comparison to other isoflavonoids analyzed. It enhanced the puerarin production on all the concentrations used, irrespective of time of addition. Puerarin content increased up to ~392% when the cells were treated with 2 g l−1 of Cuscuta elicitor added at day 15 of culture. However, Cuscuta elicitor preparation did not contain any isoflavonoid as evident by HPLC analysis. Therefore, this increase in puerarin content was because of stimulation of the plant cells. Maximum total isoflavonoid content (~6.5 mg g−1 DM) was recorded when 0.2 and 2.0 g l−1 of Cuscuta elicitor was added on day 15 of the culture period (Fig. 2). Growth of the cells was also maximal on these treatments. When elicitor was added on the day of inoculation (day 0), it decreased the cell growth by 33% as well as isoflavonoid accumulation by 60%. It is evident that incorporation of Cuscuta elicitor at a later growth phase was beneficial for the isoflavonoid accumulation while a decrease in isoflavonoid content was recorded if added at the early growth phase. Thus, Cuscuta was acting as a triggering agent at the end of the growth phase of the cultures.
Results obtained with the cultures treated with various concentrations of Cuscuta elicitor on day 15 of the culture period are presented in Table 1. The cells grew equally well with increasing concentrations of Cuscuta elicitor without significant differences. Maximum isoflavonoid yield (~85 mg l−1) was observed in the cultures treated with 1.0 g l−1 Cuscuta elicitor which was ~12% higher than that recorded in the control cultures. Puerarin yield increased up to 8.4 mg l−1 which was 434% higher than the values recorded in control. On higher concentrations of Cuscuta elicitor (above 2.0 g l−1), the cultures turned dark brown.
The optimal conditions observed in the above experiments were used with vessels of different sizes (Table 2). Both the growth of the cells and isoflavonoid yield (~85 mg l−1) were almost stable with increases in vessel size and bioreactor culture. Table 3 shows the effect of elicitation period in the bioreactor. Cells were elicited on day 15 of culture and harvested at different time intervals (days 17, 20, 23 and 25). When cultures were harvested after 8 days of elicitation (on day 23), the isoflavonoid yield increased up to ~19% (~91 mg l−1) in comparison to control cultures maintained in flasks and the bioreactor, and puerarin yield increased up to 11 mg l−1 which was 580% of that recorded in the control. However, when the cultures were harvested after 10 days of elicitation (on day 25), the isoflavonoid yield increased up to ~20% (~92 mg l−1) in comparison to control cultures maintained in flasks and the bioreactor. Thus, there was no significant difference when the elicitation period was prolonged from 8 to 10 days. In scale-up cultures, the time period of culture is an important factor determining the cost of product. Thus, it was inferred that 8 days of elicitation was optimal for the high accumulation of isoflavonoid giving the productivity of ~4 and 0.54 mg l−1 day−1 for total isoflavonoids and puerarin, respectively. During the increased yield of total isoflavonoid by elicitation, puerarin content played a significant contribution. Since puerarin is a known antimicrobial compound (Jardin 2002), the isoflavone synthase gene might have activated to enhance puerarin synthesis when challenged by the elicitor. The pH of the medium decreased with the increase in the period of culture. The spent medium did not contain isoflavonoid and was comparable to the spent medium of control cultures. It was observed that a prolonged time of elicitation was beneficial for isoflavonoid production provided that the elicitation was done after the growth phase (day 15). Generally, addition of elicitor at a late exponential phase is beneficial for increasing the production of secondary metabolite, e.g., as observed in C. wightii cells treated with plant gums (Dass and Ramawat 2009).
Oligosaccharides of both fungal and plant origin have been reported as potent signaling molecules, that regulate growth, development and defense reaction in plants (Sudha and Ravishankar 2002). Suri and Ramawat (1996) demonstrated that latex of Calotrips procera, particularly its protein and polysaccharide fraction, markedly enhanced differentiation of laticifers, while phenolics, amino acids and terpene fraction did not cause a marked increase. Changes (morphological) recorded in the cytodifferentiation of cells of C. procera were comparable to that recorded in the cells of P. tuberosa (physiological changes) in the present investigation as influenced by cell wall polysaccharides of Cuscuta elicitor. Besides fungal elicitors which are used in many cultures for the increased accumulation of secondary metabolites (Zhao and Verpoorte 2007), a higher plants-based elicitor like cork showed a ~eightfold increase in daidzein and genistein production in P. montana (Kirakosyan et al. 2006). However, the levels of daidzin and genistin decreased up to five- and eightfold, respectively. Thus, in that system, net accumulation of total isoflavonoid decreased up to 50% as compared to that of control cultures. Such results were different from the positive results observed in cell suspension cultures of Sophora flovescens by the use of cork tissues by Yamamoto et al. (1996).
Puerarin production was enhanced by 580% by use of a biotic elicitor obtained from C. reflexa. Similar to this, use of plant gum as a biotic elicitor of higher plant origin was demonstrated for the first time from our laboratory, which increased guggulsterones accumulation up to twofold in the cell cultures of Commiphora wightii (Dass and Ramawat 2009). Cuscuta elicitor was also very effective in enhancing another class of polyphenolics, stilbenes, in Cayratia trifolia (Arora et al. 2010).
Stability of cultures with scale-up is important, and P. tuberosa cultures produced high yields of isoflavonoid from the past 2 years in the designed medium (Goyal and Ramawat 2008a; Sharma et al. 2009). In the present work, ~91 mg l−1 of total isoflavonoid yield and 11 mg l−1 of puerarin were recorded. It may be concluded from the present work that Cuscuta preparation can be used as an elicitor, provided cells are elicited at the end of the exponential phase. This work reports for the first time an angiosperm parasite as a new source of elicitor for the production of useful metabolites.
Abbreviations
- 2iP:
-
N6-(2-isopentenyl) adenine
- DM:
-
Dry mass
- Morphactin:
-
Chloroflurenol-butylester
- MS:
-
Murashige and Skoog’s medium
References
Albert M, Belastegui-Macadam X, Bleischwitz M, Kaldenhoff R (2008) Cuscuta spp.: parasitic plants in the spotlight of plant physiology, economy and ecology. In: Luttge U, Beyschlag W, Murata J (eds) Progess in botany, vol 69. Springer, Berlin, pp 267–281
Arora J, Goyal S, Ramawat KG (2010) Enhanced stilbene production in cell cultures of Cayratia trifolia through co-treatment with abiotic and biotic elicitors and sucrose. In Vitro Cell Dev Biol Plant 46:430–436
Bingham SA, Atkinson C, Liggins J, Bluck L, Coward A (1998) Phytoestrogens: where are we now? Br J Nutr 79:393–406
Dai R, Ma Y, Sheng Z, Jin Y, Zhang Y, Fang L, Fan H, Liao E (2008) Effects of Genistein on vertebral trabecular bone microstructure, bone mineral density, microcracks, osteocyte density, and bone strength in ovariectomized rats. J Bone Miner Metab 26:342–349
Dass S, Ramawat KG (2009) Elicitation of guggulsterone production in cell cultures of Commiphora wightii by plant gums. Plant Cell Tissue Org Cult 96:349–353
Dev S (2006) Selection of prime ayurvedic plant drugs ancient–modern concordance. Anamaya, New Delhi, India
Goyal S, Ramawat KG (2008a) Synergistic effect of morphactin on cytokinin-induced production of isoflavonoid in cell cultures of Pueraria tuberosa (Roxb. ex. Willd.) DC. Plant Growth Regul 55:175–181
Goyal S, Ramawat KG (2008b) Increased isoflavonoid accumulation in cell suspension cultures of P. tuberosa by elicitors. Indian J Biotech 7:378–382
Goyal S, Ramawat KG (2008c) Improvement of isoflavonoid accumulation by ethrel in cell suspension cultures of Pueraria tuberosa, a woody legume. Acta Physiol Plant 30:849–853
Jardin A (2002) Synthesis of natural occurring isoflavones and their analogues. In: Keung WM (ed) Pueraria. Taylor & Francis, London, pp 225–242
Kirakosyan A, Kaufman PB, Chang SC, Warber S, Bolling S, Vardapetyan H (2006) Regulation of isoflavone in hydroponically grown Pueraria montana (kudzu) by cork pieces, XAD-4, and methyl jasmonate. Plant Cell Rep 25:1387–1391
Luczkiewicz M (2008) Research into isoflavonoid: phytoestrogens in plant cell cultures. In: Ramawat KG, Merillon JM (eds) Bioactive molecules and medicinal plants. Springer, Heidelberg, pp 54–84
Maojun XU, Jufang D, Muyuan Z (2006) Nitric oxide mediates the fungal elicitor-induced puerarin biosynthesis in Pueraria thomsonii Benth suspension cells through a salicylic acid (SA)-dependent and a jasmonic acid (JA)-dependent signal pathway. Sci China Ser C Life Sci 49:1–11
Mizushige T, Mizushige K, Miyatake A, Kishida T, Ebihara K (2007) Inhibitory effects of soy isoflavones on cardiovascular collagen accumulation in rats. J Nutr Sci Vitaminol 53:48–52
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Ramawat KG, Goyal S (2008) The Indian herbal drug scenario in global perspectives. In: Ramawat KG, Merillon JM (eds) Bioactive molecules and medicinal plants. Springer, Heidelberg, pp 325–347
Ramawat KG, Dass S, Mathur M (2009) The chemical diversity of bioactive molecules and therapeutic potential of medicinal plants. In: Ramawat KG (ed) Herbal drugs: ethnomedicine to modern medicine. Springer, Berlin, pp 7–32
Ren MQ, Kuhn G, Wegner J, Chen J (2001) Isoflavones, substances with multi-biological and clinical properties. Eur J Nutr 40:135–146
Savitha BC, Timmaraju R, Bhagyalaksami N, Ravishankar GA (2006) Different biotic and abiotic elicitors influence betalain production in hairy root cultures of Beta vulgaris in shake flask and bioreactor. Process Biochem 41:50–60
Sharma V, Goyal S, Ramawat KG (2009) Scale up production of isoflavonoid in cell suspension cultures of Pueraria tuberosa grown in shake flasks and bioreactor. Eng Lif Sci 9:267–271
Sudha G, Ravishankar GA (2002) Involvement and interaction of various signaling compounds on the plant metabolic events during defense response, resistance to stress factors, formation of secondary metabolites and their molecular aspects. Plant Cell Tissue Org Cult 71:181–212
Suri SS, Ramawat KG (1996) Effect of Calotropis latex on laticifers differentiation in callus cultures of Calotropis procera. Biol Planta 38:185–190
Tanwar YS, Mathur M, Ramawat KG (2007) Morphactin influences guggulsterone production in callus cultures of Commiphora wightii. Plant Growth Regul 51:93–98
Vaishnav K, Goyal S, Ramawat KG (2006) Isoflavonoid production in callus culture of Pueraria tuberosa, the India kudzu. Indian J Exp Biol 44:1012–1017
Yamamoto H, Yamaguchi M, Inoue K (1996) Absorption and increase in the production of prenylated flavanones in Sophora flavescens cell suspension cultures by cork pieces. Phytochemistry 43:603–608
Zhao J, Verpoorte R (2007) Manipulating indole alkaloid production by Catharanthus roseus cell cultures in bioreactors: from biochemical processing to metabolic engineering. Phytochem Rev 6:435–457
Zhao J, Lawrence CD, Verpoorte R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 23:283–333
Acknowledgments
This work was supported by financial assistance from UGC-DRS under the special assistance program for medicinal plant research to K.G.R., and S.G. and V.S. thank CSIR, and UGC New Delhi for granting of a RA and JRF fellowship, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goyal, S., Sharma, V. & Ramawat, K.G. Marked effect of Cuscuta on puerarin accumulation in cell cultures of Pueraria tuberosa grown in shake flasks and a bioreactor. Plant Biotechnol Rep 5, 121–126 (2011). https://doi.org/10.1007/s11816-011-0167-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-011-0167-2