Abstract
Cell cultures of Commiphora wightii (Arnott.) Bhandari were grown in shake flasks and a bioreactor and an increase in guggulsterone accumulation up to 18 μg l−1 was recorded in cells grown in the production medium containing a combination of sucrose:glucose (4% total), precursors (phenylalanine, pyruvic acid, xylose, and sodium acetate), morphactin, and 2iP. A yield of 10 g l−1 biomass and ∼200 μg l−1 guggulsterone was recorded in a 3-l flask and in a 2-l stirred tank bioreactor compared with 6.6 g biomass and 67 μg l−1 guggulsterone in 250-ml flasks. Increased vessel size was correlated with increased biomass and guggulsterone accumulation. 2iP alone was not effective for biomass and guggulsterone accumulation in cell cultures of C. wightii.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Guggulsterone (two isomers, E and Z) is an effective anti-hyperlipidemic agent obtained from gum resin of the tree Commiphora wightii (Arnott.) Bhandari (Wang et al. 2004). It is the only natural hypolipidemic agent. Purified gum resin is used in traditional medicine in India for obesity and arthritis (Dev 2006). Its approval by the FDA as a food supplement in 1994 resulted in intensive work describing guggulsterone as a farnesoid X receptor (FXR) antagonist (Urizar et al. 2002) and for FXR regulation of several cholesterol-related genes (Burris et al. 2005).
Plant cell cultures offer an alternative to natural biomass for production of useful metabolites. However, the productivity obtained by in-vitro techniques remains low (Merillon and Ramawat 2007). Optimum growth is negatively correlated with production of secondary metabolites. Consequently strategies have been proposed to increase productivity. Manipulation of the culture medium (e.g. carbon source, nitrogen, and phosphate) and culture conditions (e.g. inoculum density, growth period) has been successfully used to increase the production of secondary metabolites (Ramawat and Mathur 2007).
Earlier we reported guggulsterone accumulation by manipulating medium constituents (Mathur et al. 2007), the marked influence of morphactin on guggulsterone accumulation in callus cultures (Tanwar et al. 2007), and guggulsterone accumulation in cell cultures grown in shake flasks and a bioreactor (Mathur and Ramawat 2007). In this communication, we report improvement of guggulsterone accumulation as a result of formulating a production medium by combining optimal conditions of medium salts, precursors, and morphactin. These cultures were grown in shake flasks and a bioreactor.
Materials and methods
Cell-suspension cultures were maintained in modified Murashige and Skoog (1962) medium containing 2,4-dichlorophenoxyacetic acid (0.5 mg l−1), kinetin (0.25 mg l−1), and 3% w/v sucrose, referred to as CN4 medium (NH4NO3 825 mg l−1, KNO3 475 mg l−1, and CaCl2.6H2O 220 mg l−1). These salts in different concentrations were used for production medium referred to as CN7 medium (NH4NO3 1650 mg l−1, KNO3 475 mg l−1, and CaCl2.6H2O 220 mg l−1) containing 0.1 mg l−1 kinetin and 0.1 mg l−1 2,4-dichlorophenoxyacetic acid. The cultures were grown for 12 days in 250-ml Erlenmeyer flasks containing 100 ml CN4 medium and then the cells (∼500 mg dry weight) were transferred to 100 ml CN7 medium. The culture conditions and medium preparation were same as described earlier (Mathur et al. 2007; Mathur and Ramawat 2007). Six replicate flasks were used in each treatment and experiments were conducted twice. All results are averaged over two separate analyses for guggulsterone estimation and expressed as μg guggulsterone g−1 cell dry weight. The data were analyzed by one-way ANOVA followed by mean separation using a post-hoc least-significant difference (LSD) test at P ≤ 0.05.
Modification of CN7 medium
Sugars (sucrose, glucose, maltose) at different concentrations (4–6% w/v) and in different combinations (sucrose:glucose, 1:1 ratio) were incorporated in CN7 medium. Six combinations in CN7 medium were used: PM1 (CN7 + 50 g l−1sucrose), PM2 (CN7 − 2,4-D), PM3 (CN7 + pyruvic acid, xylose 250 mg l−1), PM4 (CN7 + phenylalanine 250 mg l−1), PM5 (CN7 − 110 mg l−1CaCl2), and PM6 (NH4NO3 1650 mg l−1, KNO3 475 mg l−1, CaCl2.6H2O 110 mg l−1, KH2PO4 85 mg l−1, with 2,4-D 0.1 mg l−1 and kinetin 0.1 mg l−1, and 40 g l−1 sucrose:glucose, pyruvic acid, sodium acetate, xylose, phenylalanine each 250 mg l−1, thiourea 100 mg l−1).
Scale-up of the production medium
Different sized vessels (250-ml to 3-l flasks and stirred tank bioreactor, 2 l; Inceltech, France) containing production medium (PM6) with two different combinations of plant-growth regulators, 2iP alone or morphactin with 2iP. Morphactin concentration and bioreactor settings, guggulsterone extraction, and HPLC analysis were same as described earlier (Mathur and Ramawat 2007; Tanwar et al. 2007).
Results and discussion
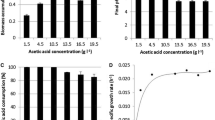
Transferring cells to the production medium resulted in an approximately two fold improvement in guggulsterone accumulation compared with growth medium. The cells harvested after different intervals of time grown in CN7 medium showed increased accumulation of guggulsterone for up to 15 days growth (Fig. 1). Growth and guggulsterone content declined after fifteen days of culture. Guggulsterone E content remained high during the entire growth period.
Increased sugars from 3% to 4–6% w/v in the medium significantly enhanced dry weight yield of C. wightii cell cultures compared with the 3% control level. Sucrose or maltose alone were not effective in enhancing guggulsterone accumulation as compared with combined sugars. A combination of sucrose:glucose (40 g l−1) in the medium enhanced guggulsterone accumulation (18 μg g−1) and yield (165 μg l−1) after 15 days growth (Table 1). This increase was about 2.5 times that of the control.
More than a threefold increase in guggulsterone accumulation over control was recorded in the cells grown in PM6 medium followed by PM3 medium whereas maximal growth was recorded in cells grown in PM2 medium (Fig. 2). Increased sugar concentration (PM1), withdrawal of 2,4-D (PM2), addition of phenylalanine (PM4), or reduction in calcium chloride (PM5) in CN7 medium did not enhance biomass and guggulsterone content. Therefore, PM6 was used for another set of experiments based on this and earlier results of Tanwar et al. (2007). Replacement of 2,4-D and kinetin by 1 mg l−1 2iP resulted in low yield of guggulsterone (67–78 μg l−1) (Table 2). Addition of morphactin (0.1 mg l−1) to PM6 medium containing 1 mg l−1 2iP and SG 40, not only increased growth (dry biomass from 6.6 to 8.5 g l−1) but also guggulsterone accumulation from 10.2 μg g−1 to 14.4 μg g−1 in cells grown in 250-ml flasks (Table 2). Increase in vessel size with PM6 medium containing 1 mg l−1 2iP and 0.1 mg l−1 morphactin was correlated with an increase in dry biomass and increased yield of guggulsterone. Maximum yield of biomass and guggulsterone was obtained in cells grown in a 2-l bioreactor, which was also highest among all the treatments used so far in cell cultures of C. wightii.
A marked increase in guggulsterone accumulation has been achieved by permutation and combination in a production medium. Earlier, we had recorded 40 g l−1 maltose as an effective sugar in enhancing guggulsterone accumulation in callus cultures of C. wightii (Mathur et al. 2007). But in this work with cell cultures, 40 g l−1 sucrose:glucose (1:1 ratio) was effective in enhancing the yield 2.5-fold over other treatments. A high concentration of cytokinin (Merillon et al. 1991; Tanwar et al. 2007) is known to enhance secondary metabolites as shown in earlier work on C. wightii callus cultures (Tanwar et al. 2007) but failed to enhance guggulsterone accumulation in cell cultures grown in the production medium containing 2iP alone. Biomass and guggulsterone accumulation were higher in production medium used with large vessels, whereas growth medium was negatively correlated with increased vessel size for yield of biomass and guggulsterone (Mathur and Ramawat 2007). This different behavior for guggulsterone accumulation in callus (4 weeks) and cell cultures (15 days) might be due to differences in growth pattern and availability of nutrients to the cells in the two systems. Morphactin had profound effect even with high concentrations of 2iP (Tanwar et al. 2007). Use of low concentration of morphactin as an auxin in cell suspension cultures of C. wightii for production of guggulsterone was unique. The effectiveness of morphactin in conjunction with 2iP has also been validated for the production of isoflavonoids in cell cultures of Pueraria tuberosa cell cultures (Goyal and Ramawat 2008).
A sharp increase of saponin content was also recorded around days 10–14 at an initial sucrose concentration of 60 g l−1 in Panax ginseng (Akalezi et al. 1999). A similar method has been used to produce high yields of stilbenes in Vitis vinifera cell cultures using two-stage culture medium (Vitrac et al. 2002). Incorporation of the precursor in a biosynthetic pathway depends upon several factors, for example culture conditions including growth stage, hormone composition (El-Sayed et al. 2004) and elicitation (Vitrac et al. 2002). These strategies are being applied for enhanced guggulsterone production.
References
Akalezi CO, Liu S, Li QS, Yu JT, Zhong JJ (1999) Combined effects of initial sucrose concentration and inoculum size on cell growth and ginseng saponin production by suspension cultures of Panax ginseng. Process Biochem 34:639–642
Burris TP, Montrose C, Houck KA, Osborne HE, Bocchinfuso WP, Yaden BC, Cheng CC, Zink RW, Barr RJ, Hepler CD, Krishan V, Bullock HA, Burris LL, Galvin RJ, Bramlett K, Stayrook KR (2005) The hypolipidemic natural product guggulsterone is a promiscuous steroid receptor ligand. Mol Pharmacol 67:948–954
Dev S (2006) A selection of prime ayurvedic plant drugs: ancient modern concordance. Anamaya Pub, New Delhi
El-Sayed M, Choi YH, Fredrich M, Roytrakul S, Verpoorte R (2004) Alkaloid accumulation in Cathranthus roseus cell suspension cultures fed with stemmadenine. Biotechnol Lett 26:793–798
Goyal S, Ramawat KG (2008) Synergistic effect of morphactin on cytokinin induced production of isoflavonoid in the cell cultures of Pueraria tuberosa. Plant Growth Reg. doi:10.1007/s10725-008-927/-x
Mathur M, Jain AK, Dass S, Ramawat KG (2007) Optimization of guggulsterone production in callus cultures of Commiphora wightii (Arnott.) Bhandari. Indian J Biotechnol 6:525–531
Mathur M, Ramawat KG (2007). Guggulsterone production in cell suspension cultures of Commiphora wightii grown in shake flasks and bioreactors. Biotechnol Lett 29:979–982
Merillon JM, Liu D, Huguet F, Chenieux JC, Rideau M (1991) Effect of calcium entry blockers and calmodulin inhibitors on cytokinin enhanced alkaloid accumulation in Catharanthus roseus cell cultures. Plant Physiol Biochem 29:289–296
Merillon JM, Ramawat KG (2007) Biotechnology for medicinal plants: research need. In: Ramawat KG, Merillon JM (eds) Biotechnology secondary metabolites. Scientific Publisher, Enfield, USA, pp 1–20
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physol Plantarum 15:473–497
Ramawat KG, Mathur M (2007) Factors affecting production of secondary metabolites. In: Ramawat KG, Merillon JM (eds) Biotechnology secondary metabolites. Scientific Publisher, Enfield, USA, pp 59–101
Tanwar YS, Mathur M, Ramawat KG (2007) Morphactin influences guggulsterone production in callus cultures of Commiphora wightii. Plant Growth Reg 51:93–98
Urizar NL, Liverman AB, Dodds DT (2002) A natural product that lowers cholesterol as an antagonist ligand for FXR. Science 296:1703–1706
Vitrac X, Krisa S, Decendit A, Vercauteren J, Nuhrich A, Monti JP, Deffieux G, Merillon JM (2002) Carbon 14 biolabelling of wine polyphenols in Vitis vinifera cell suspension cultures. J Biotechnol 95:49–56
Wang X, Greilberger J, Ledinski G, Kager G, Paigen B, Jurgens G (2004) The hypolipidemic natural product Commiphora mukul and its component guggulsterone inhibit oxidative modification of LDL. Atherosclerosis 172:239–246
Acknowledgments
This work was supported by financial assistance (grant No. BT/PR3214/PBD/17/210/2002) from the DBT, Government of India, New Delhi, and partially by DST-FIST program for infrastructure development and UGC-DRS under special assistance program for medicinal plant research to KGR.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mathur, M., Ramawat, K.G. Improved guggulsterone production from sugars, precursors, and morphactin in cell cultures of Commiphora wightii grown in shake flasks and a bioreactor. Plant Biotechnol Rep 2, 133–136 (2008). https://doi.org/10.1007/s11816-008-0051-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-008-0051-x