Abstract
Simmondsia chinensis (Link) Schneider, a multipurpose dioecious shrub of arid zones, has emerged as a cash crop. It is being cultivated for its seeds which store liquid wax whose properties are similar to spermaceti (Sperm whale oil), a substitute for petro products and precious high-priced lubricants. Jojoba is a slow-growing desert shrub having a male biased (5:1; male:female ratio) population. Since there is no method available to determine the sex at the seedling stage, current investigations have been carried out to generate a sex-specific random amplified polymorphic DNA (RAPD) marker in jojoba which is based on the PCR amplification of random locations in the genome of plant. Of the 72 primers tested, only one random decamer primer, OPG-5, produced a unique ∼1,400 base pairs fragment in male DNA. To validate this observation, this primer was re-tested with the individuals of male and female samples of four cultivars. The unique ∼1,400 bp fragment was present in male individuals of all the four cultivars and completely absent in respective female individuals tested. To the best of our knowledge, this is the first report to ascertain the sex of jojoba plants at an early stage of development of the taxon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In most of the typically dioecious plants, pistil and stamen develop on separate individuals, which are distinguished as “pistillate plants” (female plants) and “staminate plants” (male plants), respectively. Such sex separation (dioecy) is found in some 15,000 species, in 1,300 genera and 60 families (Parker 1990).
In such plants, gender influences economic values, breeding schemes and opportunities for commercial harvest. The development of molecular strategies for early sex identification of dioecious taxa has been a priority in breeding programs for their greater economic potentials. Moreover, studies on marker technology regarding dioecy in general would render a better understanding of the developmental as well as the evolutionary pathways of dimorphism.
Simmondsia chinensis (jojoba), a dioecious shrub of arid zones, has emerged as a cash crop in India and abroad. It is a native of Sonoran deserts of south-western United States of America, north-western Mexico and Baja California (Gentry 1958; Benzioni 1992). The plant is important to commerce as its seeds store liquid wax (40–60% by dry weight). The jojoba oil is non-toxic, biodegradable and quite stable. It has promising physical properties, such as high viscosity index, high flash and fire points, high dielectric constant, and high stability and freezing point, and can be used in various industries. It does not get damaged by repeated heating to temperatures above 285°C. The viscosity index of jojoba oil is much higher than that of petroleum oil, and it is therefore being used as a high temperature and high pressure lubricant in heavy machinery. In addition to this, it is also being used in transformer oil, detergents, the leather and plastic industries, and in pharmaceutical as well as cosmetic industries all over the globe. Jojoba was introduced in 1978 from Israel and is being cultivated throughout India to provide a renewable source of its high quality oil. However, several constraints have been faced with jojoba cultivation by its growers: (1) it is a slow growing and has male biased (5:1; male:female ratio) population, (2) the waiting time from planting to harvesting is very long, and (3) it does not flower or produce seeds until 3–4 years after transplantation.
Realising these inherent problems, it is imperative for the sex of this plant to be identified at the seedling stage, prior to its transplantation to the field, so that a desired ratio of male and female plants can be achieved, and resources like planting space, fertilizers, water and the labor costs can be devoted to the cultivation of the desired sex (female plants and male plants). Thus, an increase in the number of fruit-bearing plants per hectare of land would directly increase the total yield in the field making its cultivation more profitable. In the Indian climate, one male plant is enough to pollinate five female plants (Harsh et al. 1987; Agrawal et al. 1999, 2000, 2002; Prakash et al. 2003). Therefore, it is of immense agricultural importance to identify the sex of this species at the juvenile stage.
In our earlier publications (Agrawal et al. 1999, 2002; Prakash et al. 2003), we have developed in vitro methods for characterization of male and female plants using growth regulators and growth adjuvants. We now, present PCR based markers, i.e. random amplified polymorphic DNA (RAPD) to ascertain the sex of S. chinensis at the seedling stage before the expression of sexual phenotypes which usually occurs 3–4 years after transplanting.
Materials and methods
Plant materials
Four cultivars of jojoba (Q-104, Clone-64, 17-22, Local) procured from the Association of the Rajasthan jojoba plantation and research project (AJORP) Jaipur, were used for the screening of sex-associated DNA markers by RAPD analysis. Leaf material was picked from fully developed, field grown plants after the complete expression of the sexual phenotypes, and the individual samples were stored at −80°C prior to use.
DNA isolation
Total genomic DNA was isolated from 5 g of leaf tissues of female and male individuals separately for the four cultivars with the modified CTAB method (Saghai-Maroof et al. 1984). The DNA was RNAse-treated and subsequently quantified on agarose gel by comparison with standard lambda DNA marker (Amersham Biosciences, USA).
Bulk segregant analysis (BSA)
Initially, two bulks of DNA samples were made by pooling an equal amount of DNA from individual plants of four cultivars, i.e. Q-104, Clone-64, 17-22 and Local, and amplified with the 100 decamer primers. A DNA marker present in the corresponding male and female bulk and absent in the alternate sex bulk was considered as a potential sex-linked marker. BSA (Michelmore et al. 1991) was carried out with each individual of known sex independently from all the four cultivars to ascertain the sex specificity of the marker. Only DNA fragments differentiating the male bulk and female bulk, and corresponding individuals of all the cultivars, were considered as putative sex-linked marker.
RAPD marker analysis
A total of 72 decamer RAPD primers (sets A, B, D, F and G; Operon Technologies, Alameda, CA, USA) were screened on the four cultivars of jojoba (Q-104, Clone-64, 17-22, Local) of known sex to determine their potential of clear polymorphisms and reproducibility. The PCR reactions were carried out in a 25-μl volume containing one unit of Taq polymerase (Invitrogen Corporation, USA), 50 ng of genomic DNA, 0.80 μM of RAPD primer, 0.1 mM of each dNTPs, 2.5 μl of 10 × PCR reaction buffer [500 mM KCl, 200 mM Tris-HCl (pH 8.4)] and 3 mM MgCl2. DNA amplifications were carried out in a myCycler thermal controller (Bio-Rad). The following steps were used for RAPD: 1 cycle consisting of 60 s at 94°C, 30 s at 36°C and 60 s at 72°C followed by 45 cycles of 5 s at 94°C, 15 s at 36°C, and 60 s at 72°C, and a final cycle of 7 min at 72°C. The amplification products were resolved on 1.2% agarose gels in Tris-borate EDTA buffer (45 mM Tris-borate and 1 mM EDTA) and stained with ethidium bromide.
Results and discussion
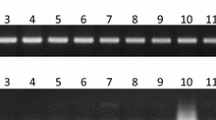
A total of 72 RAPD primers were used to amplify the bulk DNA of male and female individuals, and 38 primers gave a reproducible RAPD pattern. However, the number of amplification products varied from 2 to 9 and the fragment size ranged from 300 bp to 1,500 bp. Of all the 72 deacamer primers tested, only one primer, i.e. OPG-5, was found to have sex specificity in bulk analysis. Random decamer primer OPG-5 (5’ CTGAGACGGA 3’) produced a unique ∼1,400 base pairs fragment in male bulk DNA, and this band was absent in female bulk DNA (Fig. 1). In addition to this, many other bands were generated in both male and female samples. To confirm this observation, this primer was re-tested with the individuals of male and female samples of the four cultivars. The unique ∼1,400 bp fragment was present in male individuals of all the four cultivars and completely absent in respective female individuals tested. Thus, OPG-51,400 could be recognized as a putative sex-linked marker for four jojoba cultivars.
Sex-specific markers in dioecious taxa which could be generated through DNA analysis using PCR technology have been proved to be a reliable strategy, as such markers for sex prediction can be analyzed at any developmental stage of growth. The RAPD technique (Williams et al. 1990) is a simple identifier of polymorphism and has been used to screen markers of sex determination in several plants, i.e. Salix viminalis L. (Alstrom-Rapaport et al. 1998), Actinidia chinensis (Harvey et al. 1997; Gill et al. 1998), Asparagus (Jiang and Sink 1997), Cannabis sativa L. (Mandolino et al. 1999), Myristica fragrans Houtt. (Ganeshaiah et al. 2000), Eucommia ulmoides Oliv. (Xu et al. 2004), Encephalartos natalensis (Prakash and Van Staden 2006), Carica papaya (Urasaki et al. 2002; Chaves-Bedoya and Nunenz 2007).
To the best of our knowledge, this is the first report describing a method for sex identification of jojoba. Our findings would be helpful to identify the sex in S. chinensis at the early seedling stage by its commercial growers and for further breeding schemes. Further investigations are in progress to generate a SCAR marker (sequence characterized amplified regions), while the sex-specificity of the marker OPG51,400 with the other cultivars of S. chinensis is yet to be evaluated.
References
Agrawal V, Prakash S, Gupta SC (1999) Differntial hormonal requirements for clonal propaation of male and female jojoba plants. In: Altman A, Ziv M, Izhar S (eds) Current science and biotechnology in agriculture: plant biotechnology and in vitro biology in the 21st century. Kluwer, Dordrecht, pp 23–26
Agrawal V, Prakash S, Gupta SC (2000) Somatic embryogenesis in jojoba (Simmondsia chinensis). Kluwer, Dordrecht, pp 587–604
Agrawal V, Prakash S, Gupta SC (2002) Effective protocol for in vitro shoot production through nodal explants of Simmondsia chinensis. Biol Plant 45:449–453
Alstrom-Rapaport C, Lascoux M, Wang YC, Roberts G, Tuskan GA (1998) Identification of a RAPD marker linked to sex determination in the basket willow (Salix viminalis L.). J Hered 89:44–49
Benzioni A (1992) Flower bud dormancy: ABA concentration and survival during frost of jojoba genotype under water stress. J Am Soc Hort Sci 117:976–980
Chaves-Bedoya G, Nunenz V (2007) A SCAR marker for the sex types determination in Colombian genotypes of Carica papaya. Euphytica 153:215–220
Ganeshaiah KN, Ravishankar KV, Anand L, Shibu MP, Shaanker U (2000) Identification of sex-specific DNA markers in the dioecious tree, nutmeg (Myristica fragrans Houtt.). PGR Newsletter 121:59–61
Gentry HS (1958) The natural history of Jojoba, Simmondsia chinensis and its culture aspects. Econ Bot 12:261–295
Gill GP, Harvey CF, Gardner RC (1998) Development of sex-linked PCR markers for gender identification in Actinidia. Theor Appl Genet 97:439–445
Harsh LN, Tiwari JC, Patwal DS, Meena GL (1987) Package and practice for cultivation of Jojoba Simmondsia chinensis in arid zone. Central Arid Zone Research Institute, Jodhpur, India
Harvey CF, Gill GP, Fraser LG (1997) Sex determination in Actinidia. 1. Sex-linked markers and progeny sex ration in diploid A. chinensis. Sex Plant Reprod 10:149–154
Jiang C, Sink KC (1997) RAPD and SCAR markers linked to the sex expression locus M in Asparagus. Euphytica 94:329–333
Mandolino G, Carboni A, Forapani S (1999) Identification of DNA markers linked to the male sex in dioecious hemp (Cannabis sativa L.). Theor Appl Genet 98:86–92
Michelmore R, Paran I, Keselli V (1991) Identification of markers linked to disease-resistance genes by bulk segregante analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc Natl Acad Sci USA 88:9828–9832
Parker JS (1990) Sex chromosomes and sexual differentiation in flowering plants. Chromosome Today 10:187–198
Prakash S, Van Staden J (2006) Sex identification in Encephalartos natalensis (Dyer and Verdoorn) using RAPD markers. Euphytica 152:197–200
Prakash S, Agrawal V, Gupta SC (2003) Influence of some adjuvants on in vitro clonal propagation of male and female jojoba plants. In Vitro Cell Dev Biol Plant 39:217–222
Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW (1984) Ribosomal DNA spacer-length polymorphism in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci USA 81:8014–8018
Urasaki N, Tokumoto M, Tarora K, Ban Y, Kayano T, Tanaka H, Oku H, Chinen I, Terauchi R (2002) A male and hermaphrodite specific RAPD marker for papaya (Carica papaya L.). Theor Appl Genet 104:281–285
Williams JGK, Rubelik AR, Livak KJ, Rafalski A, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:6531–6535
Xu WJ, Wang BW, Cui KM (2004) RAPD and SCAR markers linked to sex determination in Eucommia ulmoides Oliv. Euphytica 136:233–238
Acknowledgments
V.A. is grateful to the University Grants Commission and University of Delhi, New Delhi for financial assistance. Facilities provided by the Director, National Institute for Plant Genome Research, New Delhi are gratefully acknowledged. We are also thankful to the AJORP (Association of the Rajasthan Jojoba Plantation and Research Project) for research material. K.D. is indebted to CSIR, New Delhi for awarding SRF.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Agrawal, V., Sharma, K., Gupta, S. et al. Identification of sex in Simmondsia chinensis (Jojoba) using RAPD markers. Plant Biotechnol Rep 1, 207–210 (2007). https://doi.org/10.1007/s11816-007-0031-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-007-0031-6