Abstract
Purpose
To examine the prevalence of comorbidities and the association of these comorbidities with demographics, tumor characteristics, treatments received, overall survival, and causes of death in a population-based cohort of colorectal cancer (CRC) patients.
Methods
Adult patients with stage I–III CRC diagnosed between 2004 and 2015 were included. Comorbidities were captured using Charlson comorbidity index. Causes of death were categorized using International Classification of Diseases, tenth revision codes. Patients were categorized into five mutually exclusive comorbid groups (cardiovascular disease alone, diabetes alone, cardiovascular disease plus diabetes, other comorbidities, or no comorbidities). Data were analyzed using descriptive statistics, Kaplan-Meier survival analyses, and Cox proportional hazards models.
Results
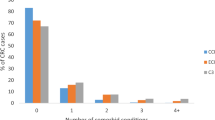
There were 12,265 patients. Mean follow-up was 3.8 years. Approximately one third of patients had a least one comorbidity, with cardiovascular disease and diabetes being most common. There were statistically significant differences across comorbid groups on treatments received and overall survival. Those with comorbidity had lower odds of treatment and greater risk of death than those with no comorbidity. Those with cardiovascular disease plus diabetes fared the worst for prognosis (median overall survival 3.3 [2.8–3.7] years; adjusted HR for death, 2.27, 95% CI 2.0–2.6, p < .001). Cardiovascular disease was the most common cause of non-CRC death.
Conclusions
CRC patients with comorbidity received curative intent treatment less frequently and experienced worse outcomes than patients with no comorbidity. Cardiovascular disease was the most common cause of non-cancer death.
Implications for Cancer Survivors
Management of comorbidities, including healthy lifestyle coaching, at diagnosis and into survivorship is an important component of cancer care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Colorectal cancer (CRC) is the second most commonly diagnosed cancer in the developed world [1]. The number of CRC survivors is expected to grow due to an increased incidence of CRC and longer term survival in some patients [2]. To plan for and meet the comprehensive health needs of this growing number of cancer survivors, the influence of comorbidities on outcomes in survivors is increasingly relevant [2, 3]. Although the comorbidities that can affect cancer patients are highly variable depending on cancer type, age, and how comorbidities are measured, there is general agreement that comorbid conditions likely play an important role in overall outcomes [2].
CRC is frequently diagnosed after the age of 50 years, with 54% of those diagnosed being over the age of 70 years [1]. The prevalence of comorbid medical conditions, most notably cardiovascular disease and diabetes, also increases with age [4]. Thus, as our population grows older, it is expected that the number of CRC patients with comorbidities at diagnosis will also increase [5]. In addition, causes of death in an aging population need to be considered in the context of cancer follow-up care. Cardiovascular and diabetes are among the leading causes of death, other than cancer [6]. For these reasons, there is an impetus to understand how this changing health profile will impact patients with CRC during and after treatment, what changes may need to occur to manage their care, and what the long-term health burden will be for CRC survivors.
While some literature exists on the prevalence of comorbidities and implications for long-term survivorship in select tumor types [1,2,3, 7,8,9], there is a paucity of research that is specific to the CRC population. Most studies have evaluated the impact of overall comorbidity burden using the Charlson comorbidity index [3, 9, 10] and have not evaluated specific comorbid conditions that may be more clinically relevant, such as cardiovascular disease and diabetes. Many studies are dated from over 10 years ago when treatment for CRC was different from current guidelines and when comorbidity profiles may have also been different [9, 11, 12]. Considering these limitations, the specific objectives of this research were:
-
1.
To determine the prevalence of comorbidities by clinically relevant groups for patients with CRC
-
2.
To describe the association of different comorbidities with demographics, tumor characteristics, treatments received, and overall survival
-
3.
To examine non-cancer causes of death
We hypothesized that CRC patients with comorbid medical conditions would have significantly worse outcomes than patients with CRC and no comorbid medical conditions.
Methods
We undertook a population-based study using provincial cancer registry and administrative data to define a cohort of adults diagnosed with CRC between January 1, 2004 and December 31, 2015, with follow-up to May 31, 2017. The cohort included adults aged 18 years or older from the province of Alberta, Canada. The population-based provincial cancer system (with tertiary and regional centers) is a part of Canada’s publicly funded healthcare system, and thus, it represents the sole provider of cancer care in the province. Patients were included if they had stage I-III CRC and had complete comorbidity information. This study received approval from the Health Research Ethics Board of Alberta (HREBA.CC-17-0034-REN1). Study findings are reported according to Strengthening of the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [13] (see Appendix 4).
Data sources and variables
Data from Alberta Health Services and Cancer Control Alberta repositories were used to define demographic and clinical variables. Demographic data included age at diagnosis and sex. Racial data and income status were not included in this dataset; however, more than 85% of the Alberta population is White [14] and the median income range between 2004 and 2015 was $87,000–$115,000 [15]. Cancer stage was coded using the Collaborative Staging System to derive American Joint Commission on Cancer (AJCC) Tumor Node Metastasis (TNM) stage [16]. Initial cancer treatment was obtained from Alberta Health Services pharmacy records. We divided type of treatment into three distinct categories: those who received surgery alone, those who received surgery plus some adjuvant treatment (either chemotherapy, radiation therapy, or both), and those who received non-curative treatment. Vital statistics at last contact with the healthcare system was coded as alive, confirmed deceased, or non-confirmed deceased. Patients were followed until death or end of study.
Comorbidities
Comorbidities were identified from inpatient hospital data and physician billing claims by using the Charlson comorbidity index (CCI) and included conditions diagnosed within 6 months prior to or after cancer diagnosis. This 1-year window for capturing comorbidities has been demonstrated to be a valid time frame in prior studies and represents the most commonly used definition [17]. The CCI is a widely used comorbidity classification system and has been broadly applied to cancer populations [3, 11, 12]. We used the Deyo adaptation of the CCI [18].
Patients were subsequently assigned to one of five mutually exclusive comorbid groups. These comorbid groups were determined based on the frequency of comorbidities in the present cohort and their clinical relevance to inform comprehensive survivorship care. Current standard chemotherapeutic treatments (fluorouracil and oxaliplatin) and medications to manage side effects from these treatments (steroids) have cardiotoxic, hematological, and endocrine side effects [19,20,21] that are particularly relevant to patients with pre-existing cardiovascular disease and diabetes. Thus, we opted to focus on cardiovascular disease and diabetes.
Patients were grouped into the following mutually exclusive groups: (1) those with cardiovascular disease including myocardial infarction, congestive heart failure, peripheral vascular disease, or cerebrovascular disease; (2) those with diabetes including diabetes with no complications and diabetes with complications; (3) those with both cardiovascular disease and diabetes; (4) those with non-cardiovascular and non-diabetes comorbidities including dementia, chronic obstructive pulmonary disease, connective tissue disorders, peptic ulcer disease, paraplegia, renal disease, mild or severe liver disease, and HIV/AIDS; and (5) those with no comorbidities.
Cause of death
Cause of death was captured using the International Classification of Diseases, tenth revision (ICD-10) codes [22]. Patients were categorized into death due to colorectal cancer (ICD-10 codes C18-C20), non-colorectal cancer cause of death (ICD-10 codes A00-Y98, excluding C18-C20), or unknown cause of death. See supplementary Table 1 for complete ICD-10 coding categories.
Statistical analysis
Descriptive statistics (means, standard deviations, or frequencies) were used to characterize demographic, clinical, and comorbidity data and to examine for differences between the five comorbid groups. The t test, chi-squared test, one-way ANOVA, or Fisher’s exact test were used to evaluate for group differences.
To compare survival across comorbidity groups, we used Kaplan-Meier method and Cox Proportional hazards regression models. Statistical significance for all Kaplan-Meier plots was determined used the log rank test.
Multivariate Cox proportional hazard regression was performed to examine the effect of comorbidity on survival (unadjusted) and then adjusted for age at diagnosis, sex, year of diagnosis, type of initial treatment received, type of cancer (colon, rectum or rectosigmoid), and cancer stage. The proportional hazards assumption was checked by examining log-minus-log survival plots and plots of partial Schoenfeld residuals [23]. One covariate, year of diagnosis, violated the proportional hazards assumption and was removed from our final model. Statistical analyses of data were performed using IBM Statistical Package for Social Sciences software (IBM SPSS) version 23.0.
Results
We identified 12,265 participants for our analysis. Descriptive statistics are detailed in Table 1. The majority had colon cancer (61.81%), and the median age at diagnosis was 68 years. There were more men (56.17%) than women (43.83%). For those with stage I disease (33.63%), surgery alone was the most common treatment (94.34%), whereas 70.25% of those with stage II disease received surgery alone and 25.30% received surgery plus adjuvant treatment, 27.35% with stage III disease received surgery alone, and 69.33% received surgery plus adjuvant treatment. Approximately one third of patients had at least one comorbidity, with 16.40% having a weighted CCI score of 2 or greater. The most common comorbidities were cardiovascular disease and diabetes.
Comorbidity subgroups
Of the 12,265 patients, 1153 (9.40%) were classified into cardiovascular disease alone, 1711 (13.95%) diabetes, 515 (4.20%) diabetes and cardiovascular disease, 1141 (9.30%) non-cardiovascular and non-diabetes comorbidities, and 7745 (63.15%) no comorbidities. Patients in the cardiovascular disease alone as well as the cardiovascular disease plus diabetes groups were older (median age 77 and 76 years, respectively) compared to those with no comorbidities (median age 65 years). There were also statistically significant differences across the comorbid groups in terms of stage of disease at initial diagnosis and the types of treatments received. For example, 31.37% of stage II patients in the no comorbidities group were treated with surgery plus adjuvant treatment, while less than 20% of patients in all of the other groups received the same treatment. Likewise, surgery and adjuvant treatment were given to 37.56 and 31.61% of stage III patients in the cardiovascular disease and cardiovascular disease plus diabetes groups, respectively, compared to 78.26% in the no comorbidities group (Table 2).
Survival analysis by subgroups
Unadjusted overall survival for the cohort was 8.58 years (95% CI, 8.28–8.88) with a mean follow-up of 45.80 months. Median survival between the five groups was statistically significantly different (p < .001) (Fig. 1). The cardiovascular disease plus diabetes group had the worst median overall survival (3.25 years, 95% CI, 2.76–3.73), followed by the cardiovascular disease group (4.16 years, 95% CI 3.63–4.70), the other comorbidities group (6.00 years, 95% CI, 5.33–6.67), the diabetes group (7.25 years, 95% CI, 6.7–7.8), and the no comorbidities group (10.75 years, 95% CI 10.36–11.14). We further divided the diabetes group into those with complications (n = 1195) and those without complications (n = 516) (as classified by Charlson). The Kaplan-Meier survival curves followed a similar pattern and statistically significant differences were found between the groups (p < .001). Patients who had diabetes and complications had worse median overall survival (4.80 years, 95% CI 4.11–5.55) compared to those with uncomplicated diabetes (8.33 years, 95% CI 7.47–9.19) (see Fig. 1, supplementary material). The Kaplan-Meier survival plot in the subset of patients with a minimum of 2 years of follow-up showed similar overall survival patterns to those with any length of follow-up, where the cardiovascular disease plus diabetes group continued to fare the worst while the no comorbidities group continued to experience the longest median overall survival (data not shown). We also generated Kaplan-Meier survival curves to examine differences in survival in stage III disease between those treated with surgery plus adjuvant treatment compared to those not treated with surgery plus adjuvant treatment for each of the five comorbid subgroups. We found statistically significant differences in overall survival, with surgery plus adjuvant treatment patients having better survival, regardless of the comorbidity group (see Figs. 2–6, supplementary material).
We also used unadjusted and adjusted Cox proportional hazard ratio models to compare risk of death between the comorbid groups (Table 3). We found an almost 4-fold higher risk of death for patients with cardiovascular disease plus diabetes, compared to those with no comorbidities (unadjusted hazard ratio [HR] 3.63, 95% CI 3.23–4.10). Similarly, all comorbid groups had a statistically significant increased risk of death compared to the no comorbidities group. After adjusting for age at diagnosis, sex, cancer site (colon, rectum, or rectosigmoid), treatment type, and stage, those in any of the comorbid groups had a statistically significant increased risk of death compared to those with no comorbidities.
Causes of death by subgroups
There were also differences across the comorbidity groups with respect to vital status and causes of death (Table 4). More patients in the no comorbidities group were alive (76%) at the end of the study compared to any other group. Those who died more often from CRC compared to non-CRC causes were in the no comorbidities group and diabetes group. For all the other groups, a larger percentage died from non-CRC causes than they did from their CRC. The most common causes of non-CRC death included diseases of the cardiovascular system (ischemic heart disease, hypertensive diseases, and cerebrovascular disease), other solid and hematological malignancies, respiratory system disorders, and diseases of the digestive system. A detailed list of causes of death is found in supplementary Table 2.
Discussion
We conducted a population-based cohort study of CRC patients to examine the prevalence and effect of comorbidities on outcomes and to characterize the causes of death in this population. Our study adds novel findings to the current literature for many reasons, including its large cohort size, its inclusion of patients treated according to most recent guidelines, and its focus on specific and clinically relevant comorbidities.
We observed that over one third of patients had at least a comorbidity, with cardiovascular disease and diabetes being the most prevalent. These results are consistent with recent Canadian population statistics that cite 29.2% of those aged 20 years or older have at least one chronic disease [24], with the most common in the general population being cardiovascular diseases, cancer, chronic respiratory diseases, and diabetes. Our findings are also similar when compared to prior studies on the prevalence of comorbidities in CRC [9, 11, 25,26,27]. Prevalence rates have been documented as low as 23.3% to as high as 43% in the CRC population depending on country and age of the cohort being studied. The most common comorbidities cited in these studies include cardiovascular disease, cerebrovascular disease, chronic pulmonary disease, and diabetes [11, 26].
The presence of comorbidities and the burden of comorbid diseases in relation to treatment and outcomes have been infrequently described in the setting of CRC. In this study, we noted differences in the type and amount of treatment received based on the presence of comorbidities. Across all stages, for example, those with cardiovascular disease or cardiovascular disease plus diabetes were less likely to receive guideline-concordant treatment. In our cohort, only 37.6% of stage III patients with cardiovascular disease and 31.7% of patients with cardiovascular disease plus diabetes received the recommended treatment. Similar patterns of undertreatment among patients with comorbidities have been reported [28,29,30,31,32]. While some studies have attributed undertreatment to the combined effects of comorbidities and advanced age [32], it has also been demonstrated that comorbidities alone are associated with undertreatment [28, 31].
The undertreatment of CRC patients with comorbidities is complex and driven by multiple potential factors including physician discretion [33, 34], patient preferences [2, 35], and lack of information from randomized controlled trials about the effectiveness of treatments in diverse populations (older, racial minorities, or those with comorbidities) [2, 3]. Although there may be concerns that comorbid patients could suffer more toxicities and experience worse quality of life if treated aggressively, little data exist to substantiate this claim [2, 32]. In addition, some research has shown that patients with high comorbidity burden can still tolerate treatment well [30,31,32, 36] and stand to gain survival benefits from receiving guideline concordant treatment [32]. While it can be challenging to understand the nuances underlying the treatment versus comorbidity relationship, it is important to recognize that comorbidities alone may not always be a clinically appropriate reason for undertreatment. As shown in our study, the type of comorbidity rather than comorbidity burden may be important to consider. However, further research comparing the relative importance of overall comorbidity burden versus specific comorbid conditions is required to substantiate this finding.
We also observed that patients with any type of comorbidity had decreased overall survival when compared to those with no comorbidities. In addition, those patients with cardiovascular disease or cardiovascular disease plus diabetes had the worst survival. While our findings are consistent with previous research on the general association between comorbidities and worse survival in CRC patients, some important distinctions should be noted [9, 11, 32, 37]. Iversen et al. [9] demonstrated that CCI scores of 1 or greater were associated with higher overall mortality rates in Danish CRC patients, but they did not examine specific comorbid conditions. Sarfati et al. [31] and Erichsen et al. [11] demonstrated that those with specific comorbid conditions (cardiovascular, cerebrovascular, renal, diabetes, and neurological diseases) had the highest adjusted hazard ratios for all cause and cancer specific mortality, but these studies included populations dating back to 1995. Thus, our findings continue to demonstrate the added risk of death in CRC patients with comorbidity and highlight the specific comorbidities that are important to consider for clinical practice and future research.
The mechanisms for increased mortality in cancer patients with comorbid diseases are likely multifactorial. As suggested by Sogaard [3] and Sarfati [2], factors such as undertreatment of cancer, the deleterious effects of multiple concurrent diseases, the direct and indirect effects of cancer on the chronic diseases, and the effects of the chronic diseases on the cancer biology could explain the relationship. While our findings largely corroborate previous research on undertreatment in CRC patients with comorbid diseases, this represents one of the few studies to specifically highlight that patients with cardiovascular disease plus diabetes were most likely to be undertreated when compared to other comorbidities. Our findings also demonstrate worse overall survival in this subgroup of patients.
Further, we found that cardiovascular disease was the most common non-CRC cause of death, even in those patients without cardiovascular disease at diagnosis. Cardiovascular disease has been noted as the leading cause of non-cancer death in CRC patients in other studies [38,39,40]. The percentage of CRC patients dying from cardiovascular disease in our study was higher compared to the general Canadian population. Recent population statistics show that 21.1% of Canadians aged 65–75 years died from heart and cerebrovascular disease in 2015 [6]. With increasing age, between 75 and 84 years, the percentage of Canadians dying from these same diseases increased slightly to 26% [6], but this is still lower than our CRC cohort.
The reasons for this high rate of cardiovascular disease related deaths in CRC patients are unclear and our findings may not directly address this issue, but there is emerging evidence of shared biological (inflammation and oxidative stress) and lifestyle factors (obesity, tobacco, diet, alcohol consumption, and inactivity) between cardiovascular disease and cancer [41]. There is also a growing body of data of the synergistic effects of comorbidity and cancer, which can worsen mortality in CRC patients [11]. In addition, it is well known that cancer treatment can have cardiotoxic effects, but long-term studies on the incidence of cardiovascular disease among CRC survivors and the impacts of cancer treatments on pre-existing cardiac comorbidity are lacking [2, 42]. Finally, there is very limited evidence for the optimal management of comorbid cardiac disease while CRC patients are undergoing treatment as well as during survivorship [42].
Strengths and limitations
Our findings should be considered in light of several limitations. This is an observational study, and thus, it does not provide evidence of causation. We did not evaluate treatment completion rates due to lack of data availability, and thus, we were unable to adjust for this in our survival analyses. In addition, we used administrative data to capture comorbidity status which may lead to misclassification. Moreover, we only searched for comorbidities coded within 6 months (before or after) of diagnosis and therefore we did not have data about changes to existing comorbidities (e.g. worsening of diabetes or cardiovascular disease). While this time frame has been previously demonstrated to be appropriate for modeling mortality and is most commonly used [17], it is important to note that there remains a potential risk of bias since some patients may have had comorbid conditions beyond this time window. While some limitations of the CCI have been noted in other studies [43, 44], this index is the most widely used and thus comparable across studies. It is important to note that this index may not capture other clinically relevant diseases in the CRC population including mental illnesses.
The above limitations should be weighed against the study’s many strengths, including its large sample size and population-based context. The time frame of the study (2004–2015) also meant that we captured recent systemic therapeutic agents as well as a long follow-up period with minimal missing data elements.
Conclusions, clinical implications, and future directions
To effectively manage complex cancer patients, such as those with comorbidities, we must have the ability to identify those at higher risk for short and long-term adverse outcomes, those who may need multidisciplinary care, and those who require targeted approaches to optimize their overall health and wellbeing [45]. Our findings indicate that those diagnosed with CRC and concomitant cardiovascular disease or diabetes, or both, have inferior cancer treatments and worse outcomes. Given that the management of future CRC patients will most likely occur in healthcare systems that are already facing constrained resources, we believe that better preparation to accommodate the increasing number of comorbid cancer survivors is essential. In addition, reasons for undertreatment in the comorbid population should be more thoroughly investigated. The management of comorbidities in CRC should start at diagnosis and be ongoing into survivorship. The early engagement of multidisciplinary and/or non-cancer specialist providers to address cardiovascular disease and diabetes could lead to more optimal coordination and delivery of appropriate cancer and non-cancer care. As has been noted in previous studies [2, 40, 42], there should be targeted interventions or programs to support lifestyle management and healthy behaviors in the CRC population, even in those without comorbidities at baseline, given their increased risk of dying from non-cancer causes. An appropriate model of cancer survivorship care that considers and incorporates comprehensive prevention and management of comorbidities is strongly warranted.
References
Canadian Cancer Society/Statistics Canada. Canadian Cancer Statistics 2016. 2016.www.cancer.ca. Accessed 21 Mar 2018.
Sarfati D, Koczwara B, Jackson C. The impact of comorbidity on cancer and its treatment. CA Cancer J Clin. 2016;66(4):338–50. https://doi.org/10.3322/caac.21342.
Sogaard M, Thomsen R, Bossen K, Sorensen HT, Horgaard M. The impact of comorbidity on cancer survival: a review. Clin Epidemiol. 2013;5:3–29.
Canadian Institute for Health Information. Seniors and the health care system: what is the impact of multiple chronic conditions. 2011 www.cihi.ca. Accessed 4 Feb 2018.
Ostenfeld EB, Norgaard M, Thomsen RW, Iversen LH, Jacobsen JB, Sogaard M. Comorbidity and survival of Danish patients with colon and rectal cancer from 2000–2011: a population-based cohort study. Clin Epidemiol. 2013;5(Suppl I):65–74. https://doi.org/10.2147/CLEP.S47154.
Statistics Canada. Table 102–0561. Leading causes of death, total population, by age group and sex, Canada. www5.statcan.gc.ca/cansim/a47. Accessed 29 Mar 2018.
Sarfati D, Tan L, Blakely T, Pearce N. Comorbidity among patients with colon cancer in New Zealand. N Z Med J. 2011;142:76–88.
Zafar SY, Abernethy AP, Abbott DH, Grambow SC, Marcello JE, Herndon JE, et al. Comorbidity, age, race and stage at diagnosis in colorectal cancer: a retrospective parallel analysis of two health systems. BMC Cancer. 2008;8:345.
Iversen LH, Norgaard M, Jacobsen J, Laruberg S, Sorensen HT. The impact of comorbidity on survival of Danish colorectal cancer patients from 1995-2006—a population-based cohort study. Dis Colon Rectum. 2009;52(1):71–8. https://doi.org/10.1007/DCR.0b013e3181974384.
Hahn EE, Gould MK, Munoz-Plaza CE, Lee JS, Parry C, Shen E. Understanding comorbidity profiles and their effect on treatment and survival in patients with colorectal cancer. J Natl Compr Cancer Netw. 2018;16(1):23–34. https://doi.org/10.6004/jnccn.2017.7026.
Erichsen R, Horvath-Puho E, Iversen LH, et al. Does comorbidity interact with colorectal cancer to increase mortality? A nationwide population-based cohort study. Br J Cancer. 2013;109:2005–13. https://doi.org/10.1038/bjc.2013.541.
Dasgupta P, Youlden DR, Baade PD. An analysis of competing mortality risks among colorectal cancer survivors in Queensland, 1996-2009. Cancer Causes Control. 2013;24:897–909. https://doi.org/10.1007/s10552-013-0166-4.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening of the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–0. https://doi.org/10.1016/j.jclinepi.2001.22.008.
Statistics Canada. Ethnocultural portrait of Canada highlight tables, 2016 census. http://www12.statcan.ca/english/census16/data/highlights/ethnic/index.cfm?Lang. Accessed 31 Mar 2011.
Statistics Canada. Table 206–0021. Income statistics by economic family type and income source, Canada, provinces and selected census metropolitan areas (CMAs). www5.statcan.gc.ca/cansim/a47. Accessed 17 Feb 2018.
Collaborative Stage Data Collection System. About collaborative stage. Retrieved from www.cancerstaging.org/cstage/about/Pages. Accessed 4 Feb 2018.
Preen DB, Holman CDJ, Spilsbury K, Semmens JB, Brameld KJ. Length of comorbidity lookback period affect regression model performance of administrative health data. J Clin Epidemiol. 2006;59:940–6. https://doi.org/10.1016/j.jclinepi.2005.12.013.
Deyo R, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9.
Alberta Health Services. Early stage colon cancer treatment. 2017. www.albertahealthservices.ca/cancerguidelines. Accessed 17 Mar 2018.
BC Cancer Agency. BC Cancer Agency drug manual: fluorouracil. 2015. www.bccanceragency.bc.ca/drug-database-site. Accessed 4 Feb 2018.
BC Cancer Agency. BC Cancer Agency drug manual: oxaliplatin. 2015. Retrieved from www.bccanceragency.bc.ca/drug-database-site. Accessed 4 Feb 2018.
WHO. Classification of Disease: ICD-10 online version. 2016. Accessed 10 Oct 2017.
Kleinbaum DG, Klein M. Survival analysis: a self learning text. Statistics for biology and health. 3rd ed. New York: Springer; 2013.
Public Health Agency of Canada. How healthy are Canadian? A trend analysis of the health of Canadians from a healthy living and chronic disease perspective. 2016. https://www.canada.ca/en/public-health/services/publications/healthy-living/how-healthy-canadians.html. Accessed 10 Mar 2018.
Cho H, Mariotto AB, Mann BS, Klabunde CN, Feuer EJ. Assessing non-cancer related health status of US cancer patients: other-cause survival and comorbidity prevalence. Am J Epidemiol. 2013;178(3):339–49. https://doi.org/10.1093/aje/kws580.
Sarfati D, Gurney J, Lim BT, et al. Identifying important comorbidity among cancer populations using administrative data: prevalence and impact on survival. Asia Pac J Clin Oncol. 2013;12(1) https://doi.org/10.1111/ajco.12130. published online ahead of print December 19, 2013.
Hahn EE, Gould MK, Munoz-Plaza CE, Lee JS, Parry C, Shen E. Understanding comorbidity profiles and their effect on treatment and survival in patients with colorectal cancer. J Natl Compr Cancer Netw. 2018;16(1):23–34. https://doi.org/10.6004/jccn.2017.7026.
Janssen-Heijnen MLG, Houterman S, Lemmens VEPP, Louwman MWJ, Maas HAAM, Coebergh JWW. Prognostic impact of increasing age and comorbidity in cancer patients: a population-based approach. Crit Rev Oncol Hematol. 2005;55:231–40. https://doi.org/10.1016/j.critrevonc.2005.04.008.
Rodrigues G, Sanatani M. Age and comorbidity considerations related to radiotherapy and chemotherapy administration. Semin Radiat Oncol. 2012;22:227–83. https://doi.org/10.1016/jsemradonc.2012.05.004.
Cronin DP, Harlan LC, Potosky AL, Clegg LX, Stevens JL, Mooney MM. Patterns of care for adjuvant therapy in a random population-based sample of patients diagnosed with colorectal cancer. Am J Gastroenterol. 2006;101:2308–18. https://doi.org/10.1111/j.1572-0241.2006.00775.x.
Sarfati D, Hill S, Blakely T, Robson B, Purdie G, Dennett E, et al. The effect of comorbidity on the use of adjuvant chemotherapy and survival from colon cancer: a retrospective cohort study. BMC Cancer. 2009;9:110. https://doi.org/10.1186/1471-2407-9-116.
Gross CP, McAvay GJ, Guo Z, Tinetti ME. The impact of chronic illnesses on the use and effectiveness of adjuvant chemotherapy for colon cancer. Cancer. 2007;109(12):2410–9. https://doi.org/10.1002/cncr.22726.
Keating NL, Landrum MB, Klabunde CN, Fletcher RH, Rogers SO, Doucette WR, et al. Adjuvant chemotherapy for stage III colon cancer: do physicians agree about the importance of patient age and comorbidity. J Clin Oncol. 2008;26(15):2532–7. https://doi.org/10.1200/JCO.2007.15.9434.
Shayeb ME, Scarfe W, Yasui Y, Winget M. Reasons physicians do not recommend and patients refuse adjuvant chemotherapy for stage III colon cancer: a populations-based chart review. BMC Res Notes. 2012;5:269. https://doi.org/10.1186/1756-0500-5-2569.
Kutner JS, Vu KO, Prindiville SA, Byers TE. Patient age and cancer treatment decisions: patient and physician views. Cancer Pract. 2000;8(3):114–9.
Etzioni DA, El-Khoueiry AB, Beart RW. Rates and predictors of chemotherapy use of stage III colon cancer: a systematic review. Cancer. 2008;113(12):3289. https://doi.org/10.1002/cncr.23958.
Lemmens VEPP, Janssen-Heijnen MLG, Verheij CDGW, Houterman S, van Driel OJR, Coebergh JWW. Comorbidity leads to altered treatment and worse survival of elderly patients with colorectal cancer. Br J Surg. 2005;92:615–23. https://doi.org/10.1002/bjs.4913.
Sjodahl R, Rosell J, Starkhammar H. Causes of death after surgery for colon cancer—impact of other diseases, urgent admittance, and gender. Scand J Gastroenterol. 2013;48(10):1160–5. https://doi.org/10.3109/00365521.2013.828771.
van Erning RN, van Steenbergen LN, Lemmens VEPP, et al. Conditional survival for long-term colorectal cancer survivors in the Netherlands: who do best? Eur J Cancer. 2014;50:1731–9. https://doi.org/10.1016/j.ejca.2014.04.009.
Zaorsky NG, Churilla TM, Egleston BL, Fisher SG, Ridge JA, Horwitz EM, et al. Causes of death among cancer patients. Ann Oncol. 2017;28:400–7. https://doi.org/10.1093/annonc/mdw604.
Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133(11):1104–14. https://doi.org/10.1161/CIRCULATIONAHA.115.020406.
Weaver KE, Foraker RE, Alfano CM, Rowland JH, Arora NK, Bellizzi KM, et al. Cardiovascular risk factors among long-term survivors of breast, prostate, colorectal, and gynecological cancers: a gap in survivor care? J Cancer Surviv. 2013;7:253–61. https://doi.org/10.1007/s11764-013-0267-9.
Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2013;173(6):676–82. https://doi.org/10.1093/aje/kwq433.
Extermann M. Measuring comorbidity in older cancer patients. Eur J Cancer. 2000;36:453–71.
Institute of Medicine and National Research Council. From cancer patient to cancer survivor: lost in transition. 2006. http://nap.edu/11468. Accessed 21 May 2018.
Funding
Dr. Cuthbert has postdoctoral salary support through the University of Calgary, Cumming School of Medicine Arnie Charbonneau Cancer Institute and O’Brien Institute for Public Health and the Canadian Institutes of Health Research. No additional funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
Colleen Cuthbert: conceptualization, formal analysis, and writing, reviewing, and editing. Brenda Hemmelgarn: conceptualization, formal analysis, and writing, reviewing, and editing. Yuan Xu: data abstraction, reviewing, and editing. Winson Cheung: conceptualization, formal analysis, and writing, reviewing, and editing.
Corresponding author
Ethics declarations
This study received approval from the Health Research Ethics Board of Alberta (HREBA.CC-17-0034-REN1). Study findings are reported according to Strengthening of the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human subjects
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Cuthbert, C.A., Hemmelgarn, B.R., Xu, Y. et al. The effect of comorbidities on outcomes in colorectal cancer survivors: a population-based cohort study. J Cancer Surviv 12, 733–743 (2018). https://doi.org/10.1007/s11764-018-0710-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-018-0710-z