Abstract
Introduction
Fatigue is a frequent problem during and after cancer treatment. We investigated different courses of fatigue from pre-diagnosis, through therapy, to long-term survivorship and evaluated potential implications on long-term quality of life (QoL).
Methods
Breast cancer patients diagnosed in 2001–2005 were recruited in a case–control study in Germany (MARIE). At follow-up in 2009 (median 5.8 years, MARIEplus), patients self-reported current fatigue and QoL status using validated questionnaires (FAQ, EORTC QLQ-C30). In addition, survivors retrospectively rated fatigue levels pre-diagnosis, during different treatment phases, and 1 year post-surgery. Our analyses included 1,928 disease-free cancer survivors and comparisons with fatigue and QoL scores from the general population.
Results
Fatigue levels were substantially increased during chemotherapy and radiotherapy. Among patients who received both therapies, 61.4% reported higher, 30.0% same, and 8.6% lower fatigue levels during chemotherapy compared to radiotherapy. Courses of fatigue varied widely between individuals. Survivors with persisting long-term fatigue had significantly and markedly worse scores for all QoL functions and symptoms about 6 years post-diagnosis than other survivors and compared to the general population. Survivors without substantial fatigue post-treatment had QoL scores largely comparable to the general population.
Discussions/conclusion
Chemotherapy appears to have a stronger impact on fatigue than radiotherapy. Breast cancer survivors may experience long-term QoL comparable to the general population, even when suffering from substantial fatigue during treatment. Yet, persistent fatigue post-treatment may lead to extensive long-term loss in QoL concerning physical, social, cognitive, and financial aspects.
Implications for cancer survivors
Fatigue management should be obligatory during and post cancer treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The population of breast cancer survivors is already large and still growing. About 242,000 women who are living in Germany have had a breast cancer diagnosis within the last 5 years [1]. In the United States, approximately 2.5 million women are breast cancer survivors [2]. Thus, quality of life (QoL) after cancer diagnosis has become increasingly important. One of the most disturbing factors that has a severe impact on QoL is cancer-related fatigue (CRF) [3].
CRF is a common multidimensional syndrome during or after cancer treatment, which includes physical, emotional, and cognitive exhaustion, often described by patients as “a tiredness they never felt before” or a “total lack of energy” [3–5]. In various studies, between 80% and 96% of breast cancer patients undergoing chemotherapy and between 60% and 93% receiving adjuvant radiotherapy have reported fatigue, and some observed elevated fatigue levels already after surgery in about 10% of patients [6]. While several studies reported that CRF resolves after the end of treatment for most patients, there are observations that fatigue may persist for up to 10 years in about one third of women treated for breast cancer [3, 7]. A systematic review of 18 studies on fatigue after treatment in breast cancer survivors with a follow-up period of between 4 months and 10 years concluded that there is good evidence of fatigue persisting up to 5 years after completion of adjuvant therapy [8]. However, most studies assessed fatigue cross-sectionally at a single time point. To our knowledge, so far no study investigated the course of fatigue during the different therapy phases up to long-term survivorship. Moreover, it is unclear how QoL in long-term survivors may be related to different fatigue courses.
Interpreting the current body of acquired knowledge is further hindered by several methodological issues. There is a wide variation in definition, assessment, and cutoff points for fatigue. Most studies used a dichotomized fatigue variable, categorizing patients or survivors in “fatigued” or “not fatigued.” Yet, fatigue occurs with a wide continuous intensity spectrum. Thus, studies using a single cutoff point for fatigue may provide only limited information on the degree of fatigue.
In addition, in the general population, fatigue levels appear to increase with age or other comorbidities. Thus, a certain baseline level of fatigue might be quite normal for some women, hence relevant for evaluations of CRF. However, we are not aware of any study accounting for pre-diagnosis fatigue levels.
Therefore, we investigated in 1,928 disease-free survivors the course of fatigue during and after breast cancer treatment under consideration of the pre-diagnosis fatigue level. We further studied the associations between course of fatigue and long-term QoL including comparisons with women from the general German population of similar age.
Material and methods
Study setting
Incident breast cancer patients aged 50–75 years were recruited in a case–control study conducted in 2002–2005 in Germany (MARIE). In 2009, a follow-up of the cases was performed (MARIEplus study). Out of 3,813 patients who had completed the MARIE assessment, 453 were deceased and 16 were lost to follow-up. Of the remaining 3,344 cases, 2,327 (70%) returned the fatigue and QoL questionnaire. Out of these, 209 had a relapse, metastases, or new tumors and were excluded. We further excluded 32 disease-free survivors with missing pre-diagnosis fatigue data and 158 survivors who reported high pre-diagnosis fatigue levels (values ≥7 on a 0–10 scale) because these women either suffered from fatigue already before cancer treatment or might have misinterpreted the 0–10 scale. Thus, this analysis included 1,928 disease-free survivors without substantial fatigue before diagnosis.
The MARIE/MARIEplus study has been approved by the ethics committees of the University of Heidelberg and Hamburg. All subjects gave informed consent prior to participation in the study. Baseline data were assessed in standardized personal interviews; follow-up assessment was done with computer-assisted telephone interviews and validated questionnaires mailed by post.
Fatigue assessment
Fatigue at the time of follow-up was assessed with the Fatigue Assessment Questionnaire (FAQ) [9], which is a 20-item, multidimensional self-assessment questionnaire that has been validated for a German-speaking population. It covers the physical, affective, and cognitive fatigue dimensions and includes one item on sleep disorders. Scores are derived by summing the answers (0 = not at all, 1 = a little, 2 = quite a bit, 3 = very much) of the appropriate items. All scores were linearly transformed to a 0–100 points scale. If more than half of the items from one scale were missing, the score was set to missing. In addition, the questionnaire includes a rating scale ranging from 0 (not tired at all) to 10 (totally exhausted). Using this rating scale, we retrospectively assessed the fatigue levels 1 year after breast surgery, during radiotherapy and during chemotherapy (if applicable), some days after breast surgery, and in the year before cancer diagnosis, i.e., obtaining a value between 0 and 10 for each single time point. Reference values of the FAQ scores are available from a representative sample of the German population including 1,340 women stratified by age [10].
Quality of life assessment
QoL was assessed with the EORTC QLQ-C30, version 3.0, a validated 28-item self-assessment questionnaire that includes 5 multi-item functional scales (physical, role, emotional, cognitive, and social function), 3 multi-item symptom scales (fatigue, pain, and nausea/vomiting), and 6 single items assessing further symptoms (dyspnea, insomnia, appetite loss, constipation, and diarrhea) and financial difficulties. Further, seven items of the breast cancer-specific module (EORTC QLQ-BR23) were applied, assessing problems with the affected breast and arm. Scores were derived according to the EORTC scoring manual [11] and linearly transformed to a 0–100 points scale. In addition, the EORTC QLQ-C30 includes a scale rating overall QoL from 1 (very bad) to 7 (excellent). Using this 1–7 scale, we retrospectively assessed QoL levels for the same time periods as used for the fatigue ratings, except the time directly after surgery. As reference values, we used the QLQ-C30 scores derived from 197 women aged 60–69 years randomly selected from the German population [12]. Evidence-based guidelines for the interpretation of the clinical relevance of changes in the different EORTC QLQ-C30 subscales were recently published [13], categorizing difference between scores (on the 0–100 points scale) in trivial, small, medium, or large effect sizes. For example, effects are considered as medium size for differences of 19–29 in role function, differences of 14–22 in physical function, 11–15 in social function, 9–14 in cognitive function, and 13–19 for fatigue.
Statistical analyses
The course of fatigue on the 0–10 scale was descriptively investigated using box–whisker plots. It is likely that there are individual differences in how patients rate a certain fatigue severity on a 0–10 scale. Thus, for each assessed time period, we anchored the responses at the baseline fatigue rating by calculating the difference to the pre-diagnosis value.
As women are affected by fatigue with varying patterns, we classified the participants into five fatigue categories based on these calculated differences. Hereby, an increase in the fatigue level of more than one third of the scale range, i.e., at least four points, was considered as “substantial fatigue.” FCAT = 1: no substantial fatigue at any assessed time period; FCAT = 2: substantial fatigue only during cancer treatment (post-surgery, chemotherapy, radiotherapy); FCAT = 3: substantial fatigue during cancer treatment sustained until 1 year post-diagnosis but improvement thereafter, i.e., prolonged fatigue; FCAT = 4: substantial fatigue throughout all periods, i.e., persistent long-term fatigue; and FCAT = 5: others with fluctuating fatigue course; n = 52 had incomplete ratings.
The FAQ dimensions and EORTC scores were investigated stratified by the above-described categories. Here, FCAT = 5 is not presented because it includes different fluctuating fatigue courses such as (re)increase of fatigue level several years post-treatment. Since for most scales the normality assumption did not hold, differences between the fatigue categories were tested using Wilcoxon’s rank sum test. Reported p values are two-sided. SAS statistical software version 9.2 (SAS Institute, Cary, NC, USA) was used.
Results
The 1,928 disease-free breast cancer survivors had a median age of 69 years (range, 54–83 years) at a median follow-up time of 5.7 years (range, 3.6–8.6 years). The majority of women (54.8%) had basic education and 16.2% had higher education. Most survivors had received radiotherapy, either with (35.1%) or without (44.5%) chemotherapy, while 11.9% had neither chemotherapy nor radiotherapy. About two thirds of survivors reported treatment with tamoxifen, and nearly half with aromatase inhibitors (Table 1).
Considering the course of fatigue, rated on the scale from 0 (not tired at all) to 10 (totally exhausted), fatigue levels increased from a median pre-diagnosis level of 1 to a level of 3 after breast surgery, reached a very high level of 8 during chemotherapy, a lower level of 5 during radiotherapy, and decreased further until the time of follow-up about 6 years post-diagnosis, yet remaining at a value of 3 still above the pre-diagnosis fatigue level. This overall fatigue course did not differ substantially between treatment groups. Only 1 year post-surgery, fatigue levels differed statistically significantly between the treatment groups with slightly higher fatigue levels among patients who had received chemotherapy (with or without radiotherapy). At all other time points, fatigue levels were similarly distributed between treatment groups (Table 2). For each treatment group and each time point, the fatigue increase from pre-diagnosis was statistically significant (all p < 0.0001, Wilcoxon signed-rank test, data not shown).
Among patients who received both therapies, 61.4% reported higher fatigue levels during chemotherapy compared to radiotherapy, 30.0% reported the same fatigue level during both treatment periods, and the remaining 8.6% reported higher fatigue during radiotherapy.
There was large interindividual variation in this overall fatigue course. We, therefore, classified survivors into five fatigue categories as described in the “Material and methods” section. Of all survivors, 619 were free of substantial fatigue at all recorded time periods (FCAT = 1), 452 reported substantial fatigue during the treatment period only (FCAT = 2), 320 had prolonged substantial fatigue up to 1 year post-surgery (FCAT = 3), and 242 suffered from substantial fatigue persisting until follow-up about 6 years post-diagnosis (FCAT = 4). The remaining 243 survivors were categorized as FCAT = 5 (fluctuating fatigue pattern).
The fatigue course stratified by this categorization is presented in Fig. 1a. Participants in FCAT = 4 had moderately higher fatigue levels during chemotherapy (p = 0.0033) and radiotherapy (p = 0.028) than those in FCAT = 3 and markedly higher levels than those in FCAT = 2. Figure 1b shows that the QoL courses by FCAT are approximately inverse to the fatigue courses. For participants in FCAT = 1, QoL reached pre-diagnosis levels about 6 years post-diagnosis.
Course of fatigue and of QoL stratified by fatigue category. For each time period, the four box–whisker plots refer to fatigue categories FCAT = 1–4 as defined in the “Material and methods” section
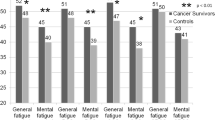
Figure 2 presents different aspects of fatigue and QoL about 6 years post-diagnosis, stratified by FCAT. Survivors in FCAT = 4 had significantly and markedly worse scores compared to those in FCAT = 1 in all FAQ dimensions (score differences: physical, 37; cognitive, 30; affective, 29) and all EORTC functions (role, 31; social, 27; emotional, 26; cognitive, 23; physical, 20) and symptoms (fatigue, 35; insomnia, 30; arm, 26; pain, 24; dyspnea, 24; financial, 16; breast, 16; appetite loss, 9; constipation, 9; nausea, 7; diarrhea, 6). In contrast, survivors without substantial fatigue post-treatment, irrespective of whether they had developed substantial fatigue during treatment (FCAT = 2) or not (FCAT = 1), showed levels comparable to the healthy reference group with regard to most scores (differences between 0 and 5 at the 0–100 EORTC scales, judged as clinically irrelevant [13]). Yet, cancer survivors reported more difficulties with sleeping/insomnia and dyspnea (differences ≥8) than the German reference population. Interestingly, about 6 years post-diagnosis survivors in FCAT = 3 also had significantly worse functional scores (all p < 0.001, differences between 6 and 9), more problems with pain, the affected arm or breast, insomnia, dyspnea, and more frequent financial difficulties compared to those in FCAT = 1 (all p < 0.001, differences of 11, 10, 6, 9, 9, and 9, respectively).
Fatigue and QoL (mean scores) in breast cancer survivors about 6 years post-diagnosis stratified by course of fatigue. CRF cancer-related fatigue, FCAT fatigue categories as defined in the “Material and methods” section. * p < 0.001, significant difference to FCAT = 1 (t test); + p < 0.01, significant difference to FCAT = 1 (t test)
In addition to the self-administered questionnaires, participants were asked in a telephone interview, among others, about their occupational and financial situation. A declined financial situation due to the cancer was reported by 9%, 13%, 21%, and 27% of survivors in FCAT = 1, 2, 3, and 4, respectively. Among the survivors that were still employed after breast cancer treatment, 7%, 13%, 13%, and 27% reported to be in a lower occupational position than before cancer treatment, respectively.
With increasing FCAT, i.e., increasing fatigue burden, participants made increasing use of supportive offers (Table 3). Nearly all survivors reported participation in the recommended breast cancer follow-up care, and women most frequently participated in rehabilitation programs.
Discussion
Our population included 1,928 breast cancer survivors who were free of relapse, metastases, or new tumors at the time of follow-up about 6 years post-diagnosis and who had not suffered from fatigue pre-diagnosis. Overall, these participants reported slightly increased fatigue levels already after breast surgery, highest fatigue levels during chemotherapy, and somewhat lower levels during radiotherapy, which decreased further post-treatment, approaching pre-diagnosis levels about 6 years post-diagnosis.
In existing literature, results are conflicting whether chemotherapy or radiotherapy has a higher impact on fatigue. Most studies were performed cross-sectionally, hence were unable to compare fatigue levels during both treatment phases within patients. In contrast, in our study, survivors rated fatigue severity for different treatment periods in relation to each other. Our data indicate that fatigue severity was perceived higher during chemotherapy than during radiotherapy, which is in accordance with another study that had investigated the course of fatigue during chemotherapy and/or radiotherapy [14]. Among our participants without substantial fatigue, 77% had received radiotherapy but only 24% had received chemotherapy, while among those with substantial increase in fatigue during therapy, more than half had been treated with chemotherapy. Hence, patients should be thoroughly informed about this potential severe side effect of chemotherapy before making a treatment decision. There was large variation in individual courses of fatigue. Survivors categorized according to their course of fatigue showed significant differences with respect to long-term QoL scores. Following the recently published evidence-based guidelines for the interpretation of differences in EORTC QLQ-C30 QoL scores [13] (see the “Material and methods” section), all decreases in QoL functional scores and increases in symptom scores observed in the patients reporting persistent fatigue can be considered as medium to large differences compared to scores from the general German female population of comparable age. The exceptions are the less frequent symptoms of appetite loss and nausea where the differences are considered to be small. Thus, survivors with persistent fatigue experienced serious, clinically relevant loss in QoL. These results substantiate previous findings from a study of Alexander et al. [15] comparing 200 disease-free breast cancer survivors with and without fatigue, showing significantly worse scores for all EORTC QLQ functional scales (except the cognitive scale, which did not reach statistical significance) among those with fatigue. Similarly, the HEAL study [16] found significant deficits especially in role functioning and social functioning in 324 fatigued disease-free survivors compared to 476 nonfatigued survivors assessed cross-sectionally 2–5 years post-diagnosis. The substantially increased symptom scores for problems with the affected breast and arm in the survivors with persistent fatigue are also in accordance with Alexander et al. [15], while the difference in pain scores did not reach statistical significance in their study. Yet, our findings of a significant, relevant increase in pain in survivors with persistent fatigue confirm results from other studies, which had observed co-occurrence of fatigue and pain [16–19]. The etiological relationships and mechanisms are still unclear. Stress hormones have been hypothesized as potential common etiological mechanisms [20]. Pain and arm/shoulder problems have been found to be associated with poorer long-term QoL in cancer survivors [21, 22].
Interestingly, several years post-treatment, also constipation, diarrhea, appetite loss, nausea, and dyspnea, which represent typical side effects during cancer treatment, were more frequent in survivors with persistent fatigue, similar to the study of Alexander et al. [15]. Regarding dyspnea in our study, among survivors with persistent fatigue, 41% reported “quite a bit” or “very much” and only 24% “not at all” compared to 15% and 59%, respectively, among never-fatigued survivors. Dyspnea has multiple causes, including cardiovascular diseases, anemia, or psychosocial factors, and is also a common side effect of chemotherapy and radiotherapy. With our analyses, we cannot draw conclusions whether dyspnea contributes to persistent fatigue or rather shares common etiological factors. However, these observed associations should provoke further investigations in potential causal links and pathways of dyspnea and fatigue.
Our results further indicate that fatigue does not only impact health but may also have negative impact on the financial situation, too. The EORTC score for financial difficulties (about 6 years post-diagnosis) rose with increasing fatigue category. This cross-sectional association does not allow causal interpretation. However, on additional questions specifically asking about financial and occupational changes caused by the cancer experience, 27% of women with persistent fatigue reported a declined financial situation due to the disease (compared to only 7% among nonfatigued women), and among those still employed post-treatment, similar proportions reported a disease-related decline in their occupational position.
But not only survivors with long-term persistent fatigue, also those survivors who have had prolonged substantial fatigue upon 1 year post-surgery and then recovered reported impaired QoL about 6 years post-diagnosis. Medium to large size differences to the scores of the German reference population were found for insomnia and dyspnea, and smaller but still clinically relevant differences in financial problems, fatigue, role, cognitive, and social function. In contrast, survivors who never experienced substantial fatigue did not differ in long-term QoL from the general population, with the exception of slightly higher scores for dyspnea and sleep problems. Survivors who have had substantial fatigue solely during treatment showed some statistically significant differences in long-term QoL compared to never-fatigued survivors. Yet, most differences were not clinically relevant according to the guidelines. Thus, a positive message from our results might be that, even when patients suffer from substantial fatigue during therapy, they may have long-term QoL comparable to the general population, if fatigue decreases after end of treatment.
Supportive actions were more frequently sought for by patients with increasing fatigue burden. Rehabilitation programs (covered by health insurance in Germany) were attended by 78% of survivors with persistent fatigue and 70% with prolonged fatigue compared to only 48% of survivors who never had substantial fatigue. With our data, we cannot determine the effect of rehabilitation actions on fatigue, but it seems that the 3- to 4-week rehabilitation program usually offered in Germany after end of chemotherapy/radiotherapy may not be sufficient to prevent persistence of fatigue. Psychological consultation had been used at least once by 31% of survivors with persistent fatigue and 25% with prolonged fatigue. As psychosocial interventions are among the few promising treatment options [23], it appears as if the need for treatment of this devastating cancer side effect has not yet been fully recognized.
As a limitation of our study, it needs to be recognized that our survivor population cannot be considered as a fully representative sample. The participation rate of eligible cases in the MARIE study was 65.5%. Further, 453 participating cases had died upon the time of follow-up, and of the remaining survivors, 30% did not respond to the fatigue/QoL questionnaire. Hence, most severely diseased or fatigued patients might have been lost to our analyses, potentially leading to an underestimation of the true fatigue problem. However, our analyses did not aim to provide prevalences for fatigue, but rather to investigate different fatigue courses and their potential impact on long-term QoL.
To our knowledge, this is the first study evaluating fatigue courses in cancer patients and survivors, accounting for pre-diagnosis fatigue level. This seems important as women have different baseline fatigue levels [24], and moreover, rating on a 0–10 scale is individually different. Except for current fatigue at the time of follow-up, fatigue levels at the different time points were assessed retrospectively, which might be prone to recall error. Yet, this retrospective rating also provides some benefits over longitudinal fatigue assessment. In longitudinal studies, intraindividual fatigue estimation may vary from time point to time point because no objective definition exists as to which (subjective) fatigue condition leads to, e.g., a value of “4” or the answer “quite a bit.” In contrast, women may recall well whether fatigue worsened or improved from one period to another. Another strength of our study is the availability of fatigue ratings for relevant periods during the cancer experience. Thus, we were able to thoroughly investigate the different courses of fatigue from pre-diagnosis up to several years post-treatment.
In conclusion, we could distinguish between different fatigue courses in breast cancer survivors. Overall, chemotherapy appears to have a stronger negative impact on fatigue than radiotherapy. Yet, even when breast cancer patients suffer under substantial fatigue during cancer treatment, they may have long-term QoL comparable to the general population, if fatigue decreases after end of treatment. However, prolonged or persistent fatigue can lead to extensive continuing loss in QoL with respect to physical, social, cognitive, and financial aspects. This severe adverse effect of cancer and its treatment needs to be better recognized by health professionals. Fatigue management should be obligatory during and post cancer treatment.
References
Husmann B, Kaatsch P, Katalinic A, Bertz J, Kraywinkel K, Wolf U. Krebs in Deutschland 2005/2006. Häufigkeiten und Trends, Berlin: Robert Koch-Institut und die Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V.; 2010.
American Cancer Society. Breast cancer facts & figures 2009–2010. Atlanta: American Cancer Society, Inc.; 2010.
Arndt V, Stegmaier C, Ziegler H, Brenner H. A population-based study of the impact of specific symptoms on quality of life in women with breast cancer 1 year after diagnosis. Cancer. 2006;107:2496–503.
Prue G, Rankin J, Allen J, Gracey J, Cramp F. Cancer-related fatigue: a critical appraisal. Eur J Cancer. 2006;42:846–63.
Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5:353–60.
Bardwell WA, Ancoli-Israel S. Breast cancer and fatigue. Sleep Med Clin. 2008;3:61–71.
Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH, Meyerowitz BE, et al. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006;106:751–8.
Minton O, Stone P. How common is fatigue in disease-free breast cancer survivors? A systematic review of the literature. Breast Cancer Res Treat. 2008;112:5–13.
Glaus A, Muller S. Measuring fatigue of cancer patients in the German-speaking region: development of the Fatigue Assessment Questionnaire. Pflege. 2001;14:161–70.
Beutel ME, Hinz A, Albani C, Brahler E. Fatigue assessment questionnaire: standardization of a cancer-specific instrument based on the general population. Oncology. 2006;70:351–7.
Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A, on behalf of the EORTC Quality of Life Group. The EORTC QLQ-C30 scoring manual. 3rd ed. Brussels: European Organisation for Research and Treatment of Cancer; 2001.
Schwarz R, Hinz A. Reference data for the quality of life questionnaire EORTC QLQ-C30 in the general German population. Eur J Cancer. 2001;37:1345–51.
Cocks K, King MT, Velikova G, Martyn St-James M, Fayers PM, Brown JM. Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J Clin Oncol. 2011;29:89–96.
Donovan KA, Jacobsen PB, Andrykowski MA, Winters EM, Balducci L, Malik U, et al. Course of fatigue in women receiving chemotherapy and/or radiotherapy for early stage breast cancer. J Pain Symptom Manage. 2004;28:373–80.
Alexander S, Minton O, Andrews P, Stone P. A comparison of the characteristics of disease-free breast cancer survivors with or without cancer-related fatigue syndrome. Eur J Cancer. 2009;45:384–92.
Meeske K, Smith AW, Alfano CM, McGregor BA, McTiernan A, Baumgartner KB, et al. Fatigue in breast cancer survivors two to five years post diagnosis: a HEAL Study report. Qual Life Res. 2007;16:947–60.
Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–53.
Reinertsen KV, Cvancarova M, Loge JH, Edvardsen H, Wist E, Fossa SD. Predictors and course of chronic fatigue in long-term breast cancer survivors. J Cancer Surviv. 2010;4:405–14.
So WK, Marsh G, Ling WM, Leung FY, Lo JC, Yeung M, et al. The symptom cluster of fatigue, pain, anxiety, and depression and the effect on the quality of life of women receiving treatment for breast cancer: a multicenter study. Oncol Nurs Forum. 2009;36:E205–14.
Thornton LM, Andersen BL, Blakely WP. The pain, depression, and fatigue symptom cluster in advanced breast cancer: covariation with the hypothalamic–pituitary–adrenal axis and the sympathetic nervous system. Health Psychol. 2010;29:333–7.
Caffo O, Amichetti M, Ferro A, Lucenti A, Valduga F, Galligioni E. Pain and quality of life after surgery for breast cancer. Breast Cancer Res Treat. 2003;80:39–48.
Nesvold IL, Reinertsen KV, Fossa SD, Dahl AA. The relation between arm/shoulder problems and quality of life in breast cancer survivors: a cross-sectional and longitudinal study. J Cancer Surviv. 2011;5:62–72.
Goedendorp MM, Gielissen MF, Verhagen CA, Bleijenberg G. Psychosocial interventions for reducing fatigue during cancer treatment in adults. Cochrane Database Syst Rev 2009;(1):CD006953.
David A, Pelosi A, McDonald E, Stephens D, Ledger D, Rathbone R, et al. Tired, weak, or in need of rest: fatigue among general practice attenders. BMJ. 1990;301:1199–202.
Acknowledgements
Funding: The MARIE/MARIEplus study is funded by the Deutsche Krebshilfe e.V. [grant no. 70-2892-BR I and 108253/108419], the Hamburg Cancer Society, the German Cancer Research Center, and the German Federal Ministry for Education and Research [grant no. 01KH0402]. We thank U. Eilber, C. Krieg, S. Behrens, R. Birr, and T. Olchers for data collection and management.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmidt, M.E., Chang-Claude, J., Vrieling, A. et al. Fatigue and quality of life in breast cancer survivors: temporal courses and long-term pattern. J Cancer Surviv 6, 11–19 (2012). https://doi.org/10.1007/s11764-011-0197-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-011-0197-3