Abstract
Introduction
The subjective experience of cancer survivorship can be assessed by various patient-reported outcome (PRO) methods, including measures of symptom burden and health-related quality of life (HRQOL). Symptom burden includes the presence and severity of multiple symptoms and the level of distress caused by symptoms that go untreated or unrelieved. The concept of symptom burden is more limited in scope than HRQOL but may provide information that better describes the status of various stages of survivorship. This paper contrasts symptom burden with general HRQOL and addresses the importance of including symptom burden as research tool throughout the trajectory of cancer survivorship.
Methods
We summarized studies that illustrate both HRQOL and symptoms as outcomes of treatment and of descriptive studies of cancer survivorship. Survivorship was operationally defined as beginning at the completion of primary anticancer treatment.
Results
HRQOL and symptom burden measures both provide meaningful but conceptually different data. Both types of measures are important in portraying aspects of cancer survivorship over time, although symptom burden may provide sufficient information to inform treatment decisions and identify long-term effects of cancer therapies.
Conclusions
Cancer survivors are at risk for multiple severe and persistent symptoms, and assessing and monitoring the severity and impact of these multiple symptoms is critical to understanding the survivorship experience. The inclusion of multiple symptom measures along with the development of new and better methods of long-term symptom tracking in survivors is a critical step in improving the heath status of survivors.
Implications for cancer survivors
Late and long-term effects seen in cancer survivors have historically been understudied. Symptom burden is an important area of assessment that can be used to specifically describe the symptoms that distress survivors. More descriptive data in this growing population may help identify biological processes in symptom production and maintenance, and facilitate in the development of better treatment and prevention to enhance cancer survivorship.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number of people with cancer who are living longer is increasing dramatically each year. As a result, there is an emerging emphasis on providing better long-term health care to these individuals [39]. It is well known that patients undergoing active treatment for cancer experience multiple symptoms, including fatigue, pain, lack of appetite, shortness of breath, constipation, numbness and tingling, and cognitive and sexual dysfunction, that cause significant distress and impair post-treatment function and rehabilitation [39]. Patients who have completed primary anti-cancer treatment, whom we define herein as “cancer survivors,” sometimes experience similar symptoms [4, 27, 28, 39], many of which persist indefinitely [4]. However, there is a paucity of longitudinal research within the population of cancer survivors compared with the vast research on patients still undergoing treatment.
Clinicians and researchers are being challenged not only to seek more effective methods of cancer prevention and cure, but also to address the prevalence and severity of the symptoms experienced by cancer survivors and to develop the most effective means to monitor, treat, and prevent them. Long-term and late effects, which negatively affect a person’s ability to function and enjoy life, are beginning to be appropriately recognized as significant factors in the cancer survivorship experience.
Cancer survivors can report the effects of the symptoms they experience in a variety of ways. One method of patient report includes measures of differences in symptom occurrence, severity, and impact (symptom burden), and another includes the widely used generic measures of health-related quality of life (HRQOL) [19] (Table 1). The appropriateness of using one of these methods over the other (or both used in conjunction) is determined by the intended purpose of the research.
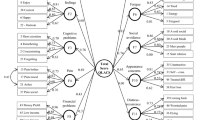
HRQOL is a measure of an individual’s perception of health-related well-being, in contrast to overall well-being, or quality of life (QOL). HRQOL is generally defined as a multidimensional construct comprising at least four dimensions: physical function (daily activities, self care), psychological function (mental state, mood), social role function (interpersonal dynamics), and disease-related or treatment-related symptoms (fatigue, pain) [21]. Because HRQOL relies on the underlying assumption that a patient’s perception of the impact of symptoms influences the more abstract concepts included in the meaning of HRQOL, the most widely used HRQOL measures include domains that evaluate the severity of at least some symptoms. However, as shown in Fig. 1, HRQOL assessment does not stop at the perception of symptom severity and the impact of interference on the patient’s life, but includes constructs more distal from actual disease-related or treatment-related effects (e.g., social and role functions and concerns about social support).
Symptoms are not synonymous with HRQOL. Symptoms and symptom interference (circled) constitute symptom burden and are closest to the disease and treatment processes. HRQOL includes multiple domains, such as social relations and general health perception, more distal from those that are most likely to be affected by disease and treatment. (© Charles S. Cleeland. Used by permission).
In contrast to measures of HRQOL, the measure of symptom burden is “a summative indicator of the severity of the symptoms that are most associated with a disease or treatment and a summary of the patient’s perception of the impact of these symptoms on daily living” [19]. Symptom burden can be defined as the combined impact of disease-related and treatment-related symptoms on the ability of persons to function as they did before onset of their disease or therapy [19], and includes the presence, frequency, and severity of multiple symptoms and the level of distress caused by symptoms that go untreated or unrelieved [45, 59, 66]. This combination of symptom severity and interference with functioning provides a more comprehensive snapshot of the specific impact of the illness and its treatment than do measures of individual symptoms.
Historically, cancer researchers have relied upon HRQOL measures to assess daily functioning and well-being after cancer and treatment [3]. These measures have also been used to gather information for physician decision-making, including treatment planning. We propose that, although HRQOL provides meaningful information about the impact of symptoms on various aspects of a cancer survivor’s life, the disease-specific measurement of symptom burden is invaluable and, in some cases, more sensitive to the changes in late and long-term symptoms experienced by cancer survivors. On the basis of the above research and our clinical and research experience, it seems important to discuss the differences between these two areas of patient-reported measure. In this article we will explore in greater detail the contrasts between HRQOL and symptom burden and present results from a number of cancer survivorship studies that illustrate these differences.
What is a symptom?
The word “symptom” is derived from the Greek root “symptoma,” which can be literally translated to “anything that has befallen one.” Webster’s Third New International Dictionary defines a symptom as “the subjective evidence of disease or physical disturbance observed by a patient” [65]. In medical populations, symptoms are subjective reports by patients that indicate a change in normal functioning or sensation due to disease or treatment. Therefore, by definition, symptoms can only be known through direct patient report, known as patient-reported outcomes [16].
It is frequently difficult for patients or their clinicians to accurately ascertain the underlying causes of symptoms. They can be produced by the disease process itself, treatment of the disease, comorbid medical conditions, or even other symptoms. To date there is very little research on the risk factors related to the development of these symptoms. However, it is increasingly recognized that pain, fatigue, sleep disturbance, cognitive dysfunction, and affective symptoms can be caused by anti-cancer treatments (chemotherapy, surgery, radiotherapy, and biological agents) [39]. The extent to which these enduring and distressing symptoms are directly related to the toxicities of cancer therapy is beginning to be better understood. Irrespective of the cause, symptoms collectively impose a significant burden upon the patient.
What is a symptom cluster?
Historically, symptoms have been studied individually, but patients, their clinicians, and their families are distinctly aware that symptoms rarely present by themselves. Recent research has supported this observation. For instance, pain, fatigue, and sleep disturbance often co-occur in the symptom profiles of patients with cancer [18, 49]. Other studies have shown that pain, fatigue, sleep disturbance, emotional distress, and poor appetite are found to occur together in patients with cancer [24, 33]. This co-occurrence of multiple cancer-related symptoms has evolved into the relatively new concept of “symptom clustering.” Most definitions propose that a symptom cluster constitutes two [43] or three [23] symptoms that are interrelated. These symptoms can be caused by the disease process, disease treatment, or a combination [6]. Although the complex interdependence and relationship of symptoms in clusters is not yet clear, researchers have proposed that the symptoms in a cluster may share a common etiology [48] and have a cumulative impact on the patient’s functioning [23, 32].
The clinical importance of measuring symptom burden
HRQOL and symptom burden are influenced by different variables and outcomes
Factors other than symptoms influence HRQOL during cancer survivorship. Hewitt et al. note that “factors that have been associated with poorer ratings of QOL among breast cancer survivors are impaired physical function, poor body image, a lack of social support, coping strategies, and aspects of care such as poor communication with physicians” (pp. 77) [39], evidencing that general HRQOL measures are affected by many factors far removed from side effects or symptoms of cancer and its treatments. Some psychological and social domains measured in generic HRQOL measures are predicted more by non-disease factors, such as socioeconomic status, personality, coping skills, social support, and cultural variables [8], than by symptoms, which are more proximal to disease and treatment processes [18]. Whereas these domains probably influence both HRQOL and symptom burden, the extent of their relative influence on each type of assessment remains unclear.
Major factors influencing symptom burden during survivorship include disease status and residuals or toxicities of anti-cancer treatments that persist as patients shift to disease-free or chronic-disease status. Both biological and behavioral host factors affect disease status before, during, and after treatment. These factors, in combination with disease, yield a biological response that produces the symptom burden. During treatment, symptom management affects both the biological response and symptom burden, and host factors (genetic, behavioral) may modify the effectiveness of symptom management.
HRQOL can improve while symptoms persist
Studies have shown that cancer survivors report high HRQOL ratings, even while they are still highly symptomatic. According to a study by Ganz et al., more than half of the women at the end of primary treatment for breast cancer were experiencing significant symptoms, including hot flashes (60%), cognitive dysfunction (56%), and aches and pains (60%) [31]. However, even given the presence of these symptoms, the women reported high levels of functioning and HRQOL as measured by the Medical Outcomes Study Short Form-36 (SF-36), an extensively used measure of HRQOL. In a review of ten studies of HRQOL and generic QOL among long-term breast cancer survivors, most of the studies supported the fact that long-term (more than 5 years) survivors experienced good overall HRQOL and that non-symptom domains such as social support and income were important predictors of overall QOL and HRQOL [50]. Even so, these studies also reported that survivors continued to experience long-term effects such as pain, lymphedema, and sexual problems.
Similarly, in a small but well-controlled study by Huguenin et al., disease-free head and neck cancer patients treated with radiation therapy were assessed with the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30-HN (head and neck module), a disease-specific HRQOL instrument [40]. Five to six years after treatment, survivors of nasopharyngeal cancer reported high rates of long-term treatment side effects (e.g., dry mouth, mucus overproduction, sticky saliva, dental problems, and difficulty eating), yet these long-term symptoms did not have a high impact on global HRQOL or functional scores on the QLQ-C30-HN. Hammerlid et al. reported longitudinal HRQOL results of 232 patients with head and neck cancer [36]. Although significant dry mouth, impaired senses of smell and taste, and dental problems persisted even 3 years after treatment, these symptoms were not evidenced in HRQOL scores, which returned slowly to pretreatment values during those 3 years. These findings suggest that general HRQOL tends to return to levels similar to baseline despite the presence of serious and persistent symptoms.
If cancer survivors are experiencing significant tumor-related and treatment-related symptoms years after treatment, why are their general HRQOL ratings not reflecting these difficulties? High HRQOL scores in the presence of persistent or deteriorating physical symptoms may reflect psychosocial adaptation, improved coping, or response shift [60]. Response shift has been defined as the phenomenon of individuals changing their perceptions of HRQOL because of changes in internal standards or values that may be a normal process of adaptation over months and years [58]. For example, Ganz reported that survivors of breast cancer identified several positive aspects of their cancer experience 12 months after surgery, such as a more positive view of life and changes in life values [29].
Other studies in cancer populations report similar positive changes in life domains that affect HRQOL as a result of “benefit finding,” changes in life priorities, and richer interpersonal relationships [2]. A 2005 review of the National Cancer Institute Community Clinical Oncology Program (CCOP) reported that a conceptual and evidence-based connection between symptom reduction and actual changes in HRQOL is often not delineated [11], possibly because of the distal nature of HRQOL relative to actual symptoms that patients experience. Although HRQOL provides important information about the survivorship experience, HRQOL tools used alone may not adequately assess the persistent symptoms that need to be included as variables in treatment outcome measures. Consequently, given the proximity of symptom burden to biological and physiological factors, symptom assessment could be an important tool in tracking the late and long-term effects experienced by cancer survivors.
Enhancing survivorship research using symptom burden
There are specific areas in which cancer survivorship research could be enhanced through the use of symptom burden measures. For instance, these measures would help patients and clinicians address specific symptoms and symptom-burden issues related to treatment options. The relevance of symptom-based measures is supported by a review of 24 clinical trials by Bottomley et al., in which 13 outcome trials exhibited differences in patient-reported symptoms between treatment arms [9]. Nine of these trials showed arm differences in symptom reports such as fatigue, pain, and peripheral neuropathy, yet global HRQOL differences were found in only four of the studies. Fallowfield et al. studied multiple treatment arms in adjuvant therapy for early-stage breast cancer [25] and found that after 2 years of treatment with anastrozole, tamoxifen, or a combination (ATAC trial), all treatment arms had a similar global impact on HRQOL. Not only were the groups comparable in HRQOL, they improved over time. However, symptom profiles after 2 and 5 years of treatment revealed differences between treatment groups in levels of diarrhea, vaginal dryness, dizziness, and libido. The authors concluded that researchers should identify the symptoms associated with different trial arms to assist in treatment decision-making. Symptom burden as a decision-making endpoint can be informative when choosing between treatments that have similar trial outcomes as to tumor status but less symptom burden during survivorship.
Another area of research that could be enhanced by the addition of symptom burden measures is describing age-related versus cancer-related symptomology in cancer and non-cancer cohorts. It is known that cancer survivors exhibit more health-related symptoms than matched non-cancer populations [55]. However, studies have shown that, although this discrepancy in symptom presence and severity exists, cancer populations have similar measures of HRQOL compared with non-cancer cohorts.
In a descriptive study of HRQOL, psychological distress, and health perceptions of survivors of bone marrow transplantation, Bush et al. found no differences in HRQOL measures in individuals sampled from the general population compared with survivors 6 to 18 years after treatment [12]. Importantly, even 10 or more years after transplantation, survivors experienced persistent symptoms such as fatigue, sleep disturbance, general pain, and cognitive dysfunction. A second study by Hammerlid and Taft followed 135 head and neck cancer survivors 3 years after diagnosis [37]. In this study, the general health status of the survivors and overall QOL measured by the generic SF-36 was comparable to age and sex-matched non-cancer groups, even though patients exhibited significantly more disease-related and treatment-related symptoms such as localized pain, difficulty swallowing, and dry mouth. The same trend can be seen in studies that conclude that there are no overall HRQOL differences between survivors of testicular cancer and age-matched non-cancer populations [26, 57]. This has also been shown to be true in survivors of breast [30], rectal [54], and esophageal [20] cancer. Nonetheless, research has shown that cancer survivors continue to experience late and long-term effects, including fatigue [13, 55], pain [55], and depression [5, 55], 1 to 10 years after treatment.
Symptom-directed research can provide a more specific platform for comparing cancer and non-cancer populations to delineate late and long-term effects of cancer and cancer treatment while controlling for symptoms related to normal aging processes.
Future directions
This paper is not intended to be a systematic review or meta-analysis, but we do suggest that HRQOL and symptom burden are conceptually different types of assessments, each of which represent an essential aspect of the cancer survivorship experience. Although the information gleaned from HRQOL is important in certain aspects of survivorship research, adequately telling the whole story of the survivorship experience will require change in our conceptualization of symptom assessment so that the residuals of disease and treatment can be adequately assessed and tracked. We propose symptom burden to be a sensitive and clinically useful construct in outcomes where symptom profiles are important. Clear directions for the future of research in cancer survivors are outlined in Table 2 and described in detail below.
Understanding the survivor’s experience
Collecting longitudinal descriptive symptom data
First and foremost, the experiences and needs of cancers survivors must be understood. This will require more descriptive epidemiological studies in which survivors are followed for years to longitudinally assess the prevalence, severity, and interference caused by persistent multiple symptoms. The importance of this approach was affirmed by Alfano et al., who suggested that future quality-of-life and symptom-management intervention studies would benefit from more accurate assessment of disease-related and treatment-related symptoms [1].
Identifying persistent effects related to cancer and treatment types
To provide an opportunity for patients and clinicians to make empirically educated decisions about treatment plans, different anti-cancer therapies need to be contrasted in light of their persistent symptom effects. Steineck et al. summarized this issue well, stating that “evidence for how ...treatment modifications change the excess risk of long-term distressful symptoms varies considerably depending on the validity of the comparisons made and the means for assessing nature, occurrence, intensity, and duration of the appropriate symptoms” [61].
Comparing cancer survivors to non-cancer populations
Also, cancer survivor populations need symptom studies comparing them to age-matched and sex-matched non-cancer populations. This would help differentiate disease-related from treatment-related persistent effects while controlling for historical and aging factors that can influence symptom presentation. And, as previously mentioned, given the existence of symptom clusters in cancer patients and survivors, researchers will need to track clusters to understand trajectory profiles of late and long-term effects in survivors.
Identifying symptom clusters in cancer survivors
The 2002 State of the Science Conference on Symptom Management in Cancer addressed the co-occurrence, assessment, and management of pain, depression, and fatigue [52]. As a result of this effort, symptom-management researchers have initiated approaches for studying these cancer-related symptoms, but there has been little research addressing what symptom patterns and clusters look like in survivors of cancer. In a sample of patients with non-small cell lung cancer, Wang et al. identified four unique clusters of symptoms that exhibited distinct temporal relationships to chemoradiation treatment even after the therapy was completed [64]. The study showed that specific symptom clusters were positively correlated with increased symptom interference experienced by the patients. Another study by Bender et al. looked at symptom clusters before treatment (disease-related), during treatment (disease-related and treatment-related), and after treatment during survivorship (late and long-term effects) [7]. The symptom cluster consisting of fatigue, cognitive impairment, and mood problems existed throughout the continuum of breast cancer, from diagnosis to survivorship. This is an important study, given that the authors identified the existence of clusters during all three phases of breast cancer treatment.
Symptom control is dependent upon understanding the trajectory of physical and psychological symptoms that endure after treatment or have their onset after treatment, as with late effects. The temporal patterns of symptom presentation need to be identified more clearly in the survivorship period, and detailed longitudinal studies are imperative given the persistence of these symptoms. Such investigations could determine which symptom clusters cause the greatest interference in a patient’s daily functioning and life. Furthermore, symptoms should be studied by disease type and treatment so that possible causal and contributory factors of biological mechanisms can be assessed.
Assessing symptoms with multi-symptom tools
Identifying the phenomenon of late and long-term symptoms in cancer survivors will require simple, validated scales that can assess multiple symptoms. Researchers have confirmed that the subjective nature of symptoms make symptom tools more practical to use in patient-reported health care research [51]. An ideal symptom-assessment tool should be brief and easy to complete, and it should include the symptoms that occur most frequently in response to specific diseases and their treatments and that are most distressing to patients (i.e., that contribute the most interference from the patient's perspective). As new therapies arise, symptom-tracking tools must also be flexible, given that new therapies used in cancer treatment may exhibit new treatment-related symptoms, such as rash, that have not traditionally been assessed [16].
Kirkova et al. identified 21 tools deemed as appropriate for clinical use, of which 14 assayed more than five common cancer symptoms [44]. Thirteen of the instruments, including the M. D. Anderson Symptom Inventory [18] , the Rotterdam Symptom Checklist [22], and the Memorial Symptom Assessment Scale [53], assessed the patient’s perception of the impact of the symptoms, or interference with normal activities and life. In 2004, Paice identified several valid and reliable tools for assessing symptom clusters [51], including the Edmonton Symptom Assessment Scale [10], the M. D. Anderson Symptom Inventory [18], and the Condensed Memorial Symptom Assessment Scale [14].
Harnessing technology to track symptoms over time
Innovative methods for repeated and more efficient symptom assessment would allow clinicians and researchers to monitor patients throughout treatment and survivorship. Repeated assessment could be done in various ways, including automated phone systems or computerized or Web-based questionnaires. Researchers at The University of Texas M. D. Anderson Cancer Center are using a computerized telephone-response system to contact patients about their symptoms weekly for an extended time after treatment. In some of these projects, an alerts system sends e-mail notifications to health personnel when certain symptoms exceed acceptable, predetermined thresholds.
Managing the late and long-term effects of cancer
Managing symptoms through monitoring
The residual symptoms of therapy and disease are targets for intervention and treatment in cancer survivors. Management of the late and long-term effects of cancer could be improved by the symptom assessment and longitudinal symptom monitoring described above. By understanding the presentation of a specific symptom or group of symptoms, clinicians would be provided specific opportunities for intervention. Further, funding should be directed towards clinical trials that examine various biological and pharmaceutical interventions that may treat persistent symptoms in cancer survivors. For instance, certain anti-convulsants have been shown to be effective treatment for neuropathic pain [56], and studies of multi-dimensional oncology rehabilitation programs show beneficial effects on psychological and physical symptom improvement in breast cancer patients [63].
Identification of possible mechanisms of symptoms and symptom clusters
Although the mechanisms underlying the development of treatment-related symptom clusters in general are not well understood, there is a growing awareness that common biological mechanisms may cause or contribute to some of these symptoms at the same time [17, 47]. Identification of the underlying causes of symptom evolution would be beneficial for controlling disease-related and treatment-related late and long-term effects. This includes identification of possible biological mechanisms, such as inflammation, that may account for the generation and sustenance of symptoms, and development of biological interventions that ameliorate those processes.
Current research on cytokines has shown that biologic mechanisms (such as an inflammatory response produced by disease or treatment) appear to contribute to expressions of animal sickness behavior that are similar to the symptoms seen in cancer patients [15, 17, 35]. “Sickness behavior” refers to a constellation of behavioral and physiological responses (decreased locomotor activity, decreased feeding, reduced sexual activity, and increased sleep) observed in animals after administration of inflammatory agents or specific proinflammatory cytokines [38, 42]. Laboratory studies have identified cytokines as central mediators of sickness behavior resulting from infections [41]. In humans, both cognitive impairment and fatigue have been related to cytokines during cancer treatment [46, 62]. The longitudinal tracking of clusters utilizing symptom-based research may help to clarify the processes involved in symptom formation and should lead to the development of mechanism-based treatments.
Preventing the late and long-term effects of cancer
Identification of host risk factors for symptom development
The ultimate goal of symptom-based research in survivors is to prevent debilitating long-term and late effects from ever developing. To further this goal, researchers must study factors, which could include genetic markers, comorbidity, and age, that could put an individual at high risk for certain symptoms. Health care providers must be knowledgeable about risks related to specific treatments to assist in the selection of interventions on a case-by-case basis. Identification of patient risk factors and variables affecting those risk factors that lead to susceptibility to late or long-term effects related to specific treatments would be an important area enhanced by symptom-based research.
Development of methods to prevent biological mechanisms of treatment-related symptoms
Another area of prophylaxis that is gaining more research attention is geared towards preventing the mechanisms underlying the development of symptoms from ever occurring. Identification of biological mechanisms, for example inflammatory processes, would be essential not only in treating symptoms as previously mentioned, but also in preventing them with novel interventions. Drugs such as carbamazepine, gabapentin, and amifostine have demonstrated some prophylaxis of chemotherapy-induced neuropathy, in addition to treating it [34]. Clinical trials testing the protective capability of biological substrates are imperative and should be incorporated into preventative trials that adequately measure symptom development.
Conclusions
The number of people surviving cancer is dramatically increasing each year and will continue to increase as more effective methods of cancer prevention, screening, and treatment emerge. Multidisciplinary collaboration among institutions, departments, clinicians and researchers can lead to a systematic, effective approach to symptom treatment that will improve our understanding of the survivorship experience. Symptom-directed research is a means to improve the current care provided to cancer survivors and to open new avenues for symptom prevention, an area that has been neglected far too long. A better understanding of symptom mechanisms may lead to new treatments that reduce symptom burden. By developing new treatments for symptoms produced by cancer and its treatment and finding ways to prevent the emergence of symptoms, we should be able to improve function and decrease the distress experienced by cancer survivors.
References
Alfano, C. M., McGregor, B. A., Kuniyuki, A., Reeve, B. B., Bowen, D. J., Baumgartner, K. B., et al. (2006). Psychometric properties of a tool for measuring hormone-related symptoms in breast cancer survivors. Psychooncology, 15(11), 985–1000.
Antoni, M. H., Lehman, J. M., Kilbourn, K. M., Boyers, A. E., Culver, J. L., Alferi, S. M., et al. (2001). Cognitive-behavioral stress management intervention decreases the prevalence of depression and enhances benefit finding among women under treatment for early-stage breast cancer. Health Psychology, 20(1), 20–32.
Ashing-Giwa, K. T. (2005). The contextual model of HRQoL: A paradigm for expanding the HRQoL framework. Quality of Life Research, 14(2), 297–307.
Aziz, N. M. (2002). Cancer survivorship research: Challenge and opportunity. Journal of Nutrition, 132(11 Suppl), 3494S–3503S.
Bailey, R. K., Geyen, D. J., Scott-Gurnell, K., Hipolito, M. M., Bailey, T. A., & Beal, J. M. (2005). Understanding and treating depression among cancer patients. International Journal of Gynecological Cancer, 15(2), 203–208.
Barsevick, A. M., Whitmer, K., Nail, L. M., Beck, S. L., & Dudley, W. N. (2006). Symptom cluster research: Conceptual, design, measurement, and analysis issues. Journal of Pain and Symptom Management, 31(1), 85–95.
Bender, C. M., Ergyn, F. S., Rosenzweig, M. Q., Cohen, S. M., & Sereika, S. M. (2005). Symptom clusters in breast cancer across 3 phases of the disease. Cancer Nursing, 28(3), 219–225.
Borgaonkar, M. R., & Irvine, E. J. (2000). Quality of life measurement in gastrointestinal and liver disorders. Gut, 47(3), 444–454.
Bottomley, A., Flechtner, H., Efficace, F., Vanvoorden, V., Coens, C., Therasse, P., et al. (2005). Health related quality of life outcomes in cancer clinical trials. European Journal of Cancer, 41(12), 1697–1709.
Bruera, E., Kuehn, N., Miller, M. J., Selmser, P., & Macmillan, K. (1991). The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. Journal of Palliative Care, 7(2), 6–9.
Buchanan, D. R., O’Mara, A. M., Kelaghan, J. W., & Minasian, L. M. (2005). Quality-of-life assessment in the symptom management trials of the National Cancer Institute-supported Community Clinical Oncology Program. Journal of Clinical Oncology, 23(3), 591–598.
Bush, N. E., Haberman, M., Donaldson, G., & Sullivan, K. M. (1995). Quality of life of 125 adults surviving 6–18 years after bone marrow transplantation. Social Science & Medicine, 40(4), 479–490.
Cella, D., Davis, K., Breitbart, W., & Curt, G. (2001). Cancer-related fatigue: Prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. Journal of Clinical Oncology, 19(14), 3385–3391.
Chang, V. T., Hwang, S. S., Kasimis, B., & Thaler, H. T. (2004). Shorter symptom assessment instruments: The Condensed Memorial Symptom Assessment Scale (CMSAS). Cancer Invest, 22(4), 526–536.
Cleeland, C. S. (2001). Cancer-related fatigue: New directions for research. Introduction. Cancer, 92(6 Suppl), 1657–1661.
Cleeland, C. S. (2007). Symptom burden: Multiple symptoms and their impact as patient-reported outcomes. Journal of the National Cancer Institute, (in press).
Cleeland, C. S., Bennett, G. J., Dantzer, R., Dougherty, P. M., Dunn, A. J., Meyers, C. A., et al. (2003). Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer, 97(11), 2919–2925.
Cleeland, C. S., Mendoza, T. R., Wang, X. S., Chou, C., Harle, M. T., Morrissey, M., et al. (2000). Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer, 89(7), 1634–1646.
Cleeland C. S., & Reyes-Gibby, C. C. (2002). When is it justified to treat symptoms? Measuring symptom burden. Oncology (Williston.Park), 16(9 Suppl 10), 64–70.
De Boer, A. G., Genovesi, P. I., Sprangers, M. A., Van Sandick, J. W., Obertop, H., & Van Lanschot, J. J. (2000). Quality of life in long-term survivors after curative transhiatal oesophagectomy for oesophageal carcinoma. British Journal of Surgery, 87(12), 1716–1721.
De Haes, J. C. (1988). Quality of life: Conceptual and theoretical considerations. In M. Watson, S. Greer, & C. Thomas (Eds.), Psychosocial oncology (pp. 61–70). Oxford: Pergamon.
De Haes, J. C., Van Knippenberg, F. C., & Neijt, J. P. (1990). Measuring psychological and physical distress in cancer patients: Structure and application of the Rotterdam Symptom Checklist. British Journal of Cancer, 62(6), 1034–1038.
Dodd, M. J., Miaskowski, C., & Paul, S. M. (2001). Symptom clusters and their effect on the functional status of patients with cancer. Oncology Nursing Forum, 28(3), 465–470.
Donnelly, S., Walsh, D., & Rybicki, L. (1995). The symptoms of advanced cancer: Identification of clinical and research priorities by assessment of prevalence and severity. Journal Of Palliative Care, 11(1), 27–32.
Fallowfield, L., Cella, D., Cuzick, J., Francis, S., Locker, G., & Howell, A. (2004). Quality of life of postmenopausal women in the Arimidex, Tamoxifen, alone or in Combination (ATAC) Adjuvant Breast Cancer Trial. Journal of Clinical Oncology, 22(21), 4261–4271.
Fossa, S. D., Dahl, A. A., & Haaland, C. F. (1999). Health-related quality of life in patients treated for testicular cancer. British Journal of Cancer, 9(5), 425–429.
Ganz, P. A. (2001). Late effects of cancer and its treatment. Seminars in Oncology Nursing, 17(4), 241–248.
Ganz, P. A. (2006). Monitoring the physical health of cancer survivors: A survivorship-focused medical history. Journal of Clinical Oncology, 24(32), 5105–5111.
Ganz, P. A., Coscarelli, A., Fred, C., Kahn, B., Polinsky, M. L., & Petersen, L. (1996). Breast cancer survivors: Psychosocial concerns and quality of life. Breast Cancer Research and Treatment, 38(2), 183–199.
Ganz, P. A., Kwan, L., Stanton, A. L., Krupnick, J. L., Rowland, J. H., Meyerowitz, B. E., et al. (2004). Quality of life at the end of primary treatment of breast cancer: First results from the moving beyond cancer randomized trial. Journal of the National Cancer Institute, 96(5), 376–387.
Ganz, P. A., Rowland, J. H., Meyerowitz, B. E., & Desmond, K. A. (1998). Impact of different adjuvant therapy strategies on quality of life in breast cancer survivors. Recent Results in Cancer Research, 152, 396–411.
Gaston-Johansson, F., Fall-Dickson, J. M., Bakos, A. B., & Kennedy, M. J. (1999). Fatigue, pain, and depression in pre-autotransplant breast cancer patients. Cancer Practice, 7(5), 240–247.
Grond, S., Zech, D., Diefenbach, C., & Bischoff, A. (1994). Prevalence and pattern of symptoms in patients with cancer pain: a prospective evaluation of 1635 cancer patients referred to a pain clinic. Journal of Pain and Symptom Management, 9(6), 372–382.
Grothey, A. (2005). Clinical management of oxaliplatin-associated neurotoxicity. Clinical Colorectal Cancer, 5 Suppl 1, S38–S46.
Gutstein, H. B. (2001). The biologic basis of fatigue. Cancer, 92(6 Suppl), 1678–1683.
Hammerlid, E., Silander, E., Hornestam, L., & Sullivan, M. (2001). Health-related quality of life three years after diagnosis of head and neck cancer-a longitudinal study. Head and Neck, 23(2), 113–125.
Hammerlid, E., & Taft, C. (2001). Health-related quality of life in long-term head and neck cancer survivors: A comparison with general population norms. British Journal of Cancer, 84(2), 149–156.
Hart, B. L. (1988). Biological basis of the behavior of sick animals. Neuroscience and Biobehavioral Reviews, 12(2), 123–137.
Hewitt, M. E., Greenfield, S., Stovall, E. (Eds.) (2005). From cancer patient to cancer survivor: Lost in transition. Washington, DC: National Academies Press.
Huguenin, P. U., Taussky, D., Moe, K., Meister, A., Baumert, B., Lutolf, U. M., et al. (1999). Quality of life in patients cured from a carcinoma of the head and neck by radiotherapy: The importance of the target volume. International Journal of Radiation Oncology, Biology, Physics, 45(1), 47–52.
Kelley, K. W., Bluthe, R. M., Dantzer, R., Zhou, J. H., Shen, W. H., Johnson, R. W., et al. (2003). Cytokine-induced sickness behavior. Brain, Behavior, and Immunity, 17 Suppl 1, S112–S118.
Kent, S., Bluthe, R. M., Kelley, K. W., & Dantzer, R. (1992). Sickness behavior as a new target for drug development. Trends in Pharmacological Sciences, 13(1), 24–28.
Kim, H. J., McGuire, D. B., Tulman, L., & Barsevick, A. M. (2005). Symptom clusters: Concept analysis and clinical implications for cancer nursing. Cancer Nursing, 28(4), 270–282.
Kirkova, J., Davis, M. P., Walsh, D., Tiernan, E., O'Leary, N., LeGrand, S. B., et al. (2006). Cancer symptom assessment instruments: A systematic review. Journal of Clinical Oncology, 24(9), 1459–1473.
Klinkenberg, M., Willems, D. L., van der, W. G., & Deeg, D. J. (2004). Symptom burden in the last week of life. Journal of Pain and Symptom Management, 27(1), 5–13.
Kurzrock, R. (2001). The role of cytokines in cancer-related fatigue. Cancer, 92(6 Suppl), 1684–1688.
Lee, B. N., Dantzer, R., Langley, K. E., Bennett, G. J., Dougherty, P. M., Dunn, A. J., et al. (2004). A cytokine-based neuroimmunologic mechanism of cancer-related symptoms. Neuroimmunomodulation, 11(5), 279–292.
Miaskowski, C., Dodd, M., & Lee, K. (2004). Symptom clusters: the new frontier in symptom management research. Journal of the National Cancer Institute. Monographs, 32, 17–21.
Miaskowski, C., & Lee, K. A. (1999). Pain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: A pilot study. Journal of Pain and Symptom Management, 17(5), 320–332.
Mols, F., Vingerhoets, A. J., Coebergh, J. W., & van de Poll-Franse L. V. (2005). Quality of life among long-term breast cancer survivors: A systematic review. European Journal of Cancer, 41(17), 2613–2619.
Paice, J. A. (2004). Assessment of symptom clusters in people with cancer. Journal of the National Cancer Institute. Monographs, 32, 98–102.
Patrick, D. L., Ferketich, S. L., Frame, P. S., Harris, J. J., Hendricks, C. B., Levin, B., et al. (2004). National Institutes of Health State-of-the-Science Conference Statement: Symptom management in cancer: Pain, depression, and fatigue, July 15–17, 2002. Journal of the National Cancer Institute. Monographs, 32, 9–16.
Portenoy, R. K., Thaler, H. T., Kornblith, A. B., Lepore, J. M., Friedlander-Klar, H., Kiyasu, E., et al. (1994). The Memorial Symptom Assessment Scale: An instrument for the evaluation of symptom prevalence, characteristics and distress. European Journal of Cancer, 30A(9), 1326–1336.
Rauch, P., Miny, J., Conroy, T., Neyton, L., & Guillemin, F. (2004). Quality of life among disease-free survivors of rectal cancer. Journal of Clinical Oncology, 22(2), 354–360.
Reyes-Gibby, C. C., Aday, L. A., Anderson, K. O., Mendoza, T. R., & Cleeland, C. S. (2006). Pain, depression, and fatigue in community-dwelling adults with and without a history of cancer. Journal of Pain and Symptom Management, 32(2), 118–128.
Ross, J. R., Goller, K., Hardy, J., Riley, J., Broadley, K., A’hern, R., et al. (2005). Gabapentin is effective in the treatment of cancer-related neuropathic pain: A prospective, open-label study. Journal of Palliative Medicine, 8(6), 1118–1126.
Rudberg, L., Carlsson, M., Nilsson, S., & Wikblad, K. (2002). Self-perceived physical, psychologic, and general symptoms in survivors of testicular cancer 3 to 13 years after treatment. Cancer Nursing, 25(3), 187–195.
Schwartz, C. E., & Sprangers, M. A. (1999). Methodological approaches for assessing response shift in longitudinal health-related quality-of-life research. Social Science & Medicine, 48(11), 1531–1548.
Silveira, M. J., Kabeto, M. U., & Langa, K. M. (2005). Net worth predicts symptom burden at the end of life. Journal of Palliative Medicine, 8(4), 827–837.
Sprangers, M. A. G., & Schwartz, C. E. (1999). Integrating response shift into health-related quality of life research: A theoretical model. Social Science & Medicine, 48(11), 1507–1515.
Steineck, G., Bergmark, K., Henningsohn, L., al-Abany, M., Dickman, P. W., & Helgason, A. (2002). Symptom documentation in cancer survivors as a basis for therapy modifications. Acta Oncologica, 41(3), 244–252.
Valentine, A. D., & Meyers, C. A. (2001). Cognitive and mood disturbance as causes and symptoms of fatigue in cancer patients. Cancer, 92(6 Suppl), 1694–1698.
Van Weert, E., Hoekstra-Weebers, J., Grol, B., Otter, R., Arendzen, H. J., Postema, K., et al. (2005). A multidimensional cancer rehabilitation program for cancer survivors: Effectiveness on health-related quality of life. Journal of Psychosomatic Research, 58(6), 485–496.
Wang, X. S., Fairclough, D. L., Liao, Z., Komaki, R., Chang, J. Y., Mobley, G. M., et al. (2006). Longitudinal study of the relationship between chemoradiation therapy for non-small-cell lung cancer and patient symptoms. Journal of Clinical Oncology, 24(27), 4485–4491.
Webster’s Third New International Dictionary of the English Language, U. (1966). (15th ed.) Springfield, MA: Merriam.
Zambroski, C. H., Moser, D. K., Bhat, G., & Ziegler, C. (2005). Impact of symptom prevalence and symptom burden on quality of life in patients with heart failure. European Journal of Cardiovascular Nursing, 4(3), 198–206.
Acknowledgement
The authors would like to thank Jeanie Woodruff, ELS, for assistance in organizing and editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burkett, V.S., Cleeland, C.S. Symptom burden in cancer survivorship. J Cancer Surviv 1, 167–175 (2007). https://doi.org/10.1007/s11764-007-0017-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-007-0017-y