Abstract
Adverse effects caused by the overuse of chemical fertilizers have led to the development of natural biostimulants like seaweed and microalga extracts being used as an alternative, environmentally-friendly approach to improve crop growth and increase agricultural yields. The current research aimed to study the interactions between seaweed and microalga extracts priming on the seed germination parameters, and the growth of Calotropis procera. A petri-dish trial was conducted under a controlled growth room, where the experimental seeds were treated individually with the two biostimulant primings, seaweed liquid extracts (SLEs) and microalga liquid extracts (MLEs), and combinations of SLEs + MLEs at different levels (0, 0.5, 1, and 1.5%). The results of the germination experiment indicated that most germination parameters except seed vigor were significantly increased at all levels of SLE, and 0.5% of MLE. Also, the primary growth indices such as shoot length, root length, shoot and root fresh and dry weight were remarkably enhanced in lower levels of their combined primings. Our data about combined biostimulant treatments showed that SLE 0.5 and SLE 1% + MLE 0.5% led to increasing the germination and growth of primed seeds compared to control. Ultimately, SLE 1% and MLE 0.5% priming were found to be more successful and better candidates for developing effective biostimulants for improving seed germination parameters of milkweed. Therefore, the results maintain the concept of marine algae extracts as an agricultural biocatalyst, which could be applied as alternative biostimulants in improving growth performance for some plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Milkweed (species: Calotropis procera Ait. family: Asclepiadaceae) is a desert shrub, which is widely distributed in arid and semiarid regions including Iran, Pakistan, India, neighboring countries of the Persian Gulf, etc. (Bahmani et al. 2018), which grows on alkaline and saline soils (Hindi 2013; Bahmani et al. 2018). This plant contains many bioactive compounds with medicinal properties such as anticancer, insecticidal, antimicrobial, etc. (Bahmani et al. 2018).
It is noteworthy that the habitat of the milkweed plant confronts some vital threats such as the change of land use and cut-off trees, and its overexploitation in local communities (Taghvaei et al. 2012; Bahmani et al. 2018). During recent decades, although milkweed has extensively been used to afforest, this plant faces germination and establishment problems in its habitats (Bahmani et al. 2018).
To overcome the aforementioned problems, seed priming can be assumed as an efficient approach to improve physicochemical processes in the seeds before germination (Ibrahim 2016). To date, the various techniques of seed priming have been suggested to accelerate germination and promote growth of plants, including biostimulants priming (Bahmani Jafarlou et al. 2021).
Bio-stimulant priming is a technique by which the seeds absorb algae base bioactive compounds for the bootstrap of some essential metabolic processes (e.g., protein synthesis and, or repair of the damaged proteins using RNA and DNA), that allows early germination, but does not complete the process (Jisha et al. 2012; Ibrahim 2016).
The polysaccharides obtained from algae constitute the subject of interest in agriculture, with emphasis on its application in sustainable agriculture (Khan et al. 2009). Another important commercial application of algae is the production of healthcare products and cosmetics, as well as the biochemical industry.
Over the centuries, various types of seaweeds like Sargassum sp. were grown and harvested in coastal areas. Successful reports have been presented concerning algae extracts as a biostimulant in the growth regulation, crop protection, yield enhancers, and supporters to plant tolerance towards environmentally stress conditions (Bahmani Jafarlou et al. 2021).
Sargassum angustifolium (C. Agardh) is a valuable brown seaweed, from which a considerable number of the species have bloomed in the Persian Gulf and Oman Sea. This seaweed provides a desirable opportunity to assay the efficacy of extractions of S.angustifolium on terrestrial plants, concerning its numerous bioactive compounds, affordability, easy access, and environment-friendliness (Sohrabipour and Rabiei 1996; Salehpour et al. 2021).
Seaweed liquid extracts (SLEs) have a wide range of applications in the world. With the emphasis on the bio-stimulating activity of the seaweed, it can be used as Bio-stimulant priming (Raghunandan et al. 2019). SLEs contain macroelements and microelements, amino acids, vitamins, and phytohormones (Khan et al. 2009; Carrasco-Gil et al. 2019).
The results of many studies illustrated that SLEs could improve growth, enhance seed germination, and efficiency of horticultural crops such as Vitis vinifera L. (Norrie et al. 2002), Solanum lycopersicum L. (Hernandez-Herrera et al. 2013), and Capsicum annuum L. (Yildiztekin et al. 2018).
Spirulina platensis is one of the photosynthetic green microalgae. Microalgae perform nitrification under anaerobic conditions in heterocysts, include 5–10% of cells in a filament (Fleming and Haselkorn 1973).
Nowadays, microalga is produced commercially as a food source, animal feed, and bio-fertilizer with high nutritional value (Sànchez et al. 2003). S. platensis comprises high amino acids and the spectrum of mixed natural carotene and, xanthophyll phytopigments, which are considered a rich natural source of vitamin B12 and antioxidants (Kemka et al. 2007).
Microalgae biofertilizers have been applied for various purposes, including soil enrichment and the growth and yield of crop plants. Besides, algae produce secondary metabolites that inhibit the growth of plant pathogenic microbes (Falch et al. 1995; Bahmani Jafarlou et al. 2021).
Spirulina microalga is considered an organic nitrogenous source, for example, amino acids, which have a critical function in some plants like chlorophyll synthesis (Amin et al. 2011), the optimization of the nutrient uptake, translocation and metabolism, vitamin biosynthesis, the production of dry matter of plant (Khalilzadeh et al. 2012), preserving the protein structure required for cell division, helping cells divide, enlarge, differentiation, and growth-efficient polyamines (Kakkar et al. 2000), growth promotion and the reduction of biotic and abiotic stress conditions (Souri and Hatamian 2019). Several studies have demonstrated that biological seed priming with microalgae extracts has positive effects on seed germination and plant growth of cowpea and mung bean seeds (Dmytryk et al. 2015), rice plant (Dineshkumar et al. 2018), Zea mays L. (Seifikalhor et al. 2020), Allium cepa L. (Geries and Elsadany 2021), and Gossypium barbadense L. (Yanni et al. 2020).
According to the aforementioned beneficial effects, microalgae species are recommended to be used as biofertilizers instead of chemical fertilizers because of affordability and environment—friendliness.
The objectives of this study were (1) to investigate the role of SLEs priming on germination rate and seed vigor of milkweed, (2) to study the primary growth parameters of milkweed seedling after biostimulant priming with MLEs, and (3) to evaluate the effects of dual algae priming (SLEs + MLEs) at various doses on milkweed performance.
Materials and methods
Seaweed
Seaweed Sargassum angustifolium C. Agardh was collected at the intertidal zone of the rishehr coastal area of Bushehr, Iran, in the Persian Gulf in November 2019. Seaweed species were identified using the taxonomic key reference followed by Sohrabipour and Rabiei (1996). Collected samples were washed with seawater to remove redundant materials such as debris, shells, and sand. Samples were transported to the laboratory, washed with water, dried under shade conditions, and then ground in an electric mill (Parskhzar, CP802P, Iran). Next, 100 g of seaweed powder and 1000 ml of distilled water (DW) were mixed with constant stirring for 15 min and then boiled for 15 min. The resulting extract was passed through a filter paper and designated as a stock SLE, and then maintained at 4 ºC until further use (Salehpour et al. 2021).
Microalga
Microalga Spirulina platensis was acquired from the marine biotechnology department of the Persian Gulf research institute, Bushehr, Iran. This microalga was cultured on Zarrouk special medium (Zarrouk 1966). Each Erlenmeyer flask was inoculated with 10 ml stock, incubated for seven days in a growth chamber with the appropriate conditions (continuous illumination 2000 lx and the temperature 35 ± 2 °C), and then considered an inoculum for 1000 ml of the medium and transferred into 20 l white fiberglass tank, incubated for 14 days under controlled greenhouse conditions of 35 ± 2 °C and continuous illumination from 5500–6500 lx. The tank received sterile air through a pump to contentiously mix/circulate/distribute the culture during the incubation period. To prepare microalga liquid extract (MLE), S. platensis (100 g) was suspended in 1000 ml sterilized distilled water using a blender. Finally, the obtained solution was filtered using a filter paper (Whatman No. 42 mm) to remove debris and design. The final filtrate is considered a 100% stock (Dineshkumar et al. 2018).
Mineral assay
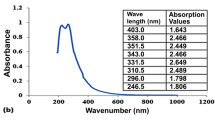
For the mineral analysis of the algae, samples were placed in an oven at 80 °C for two days and grinding the dried algae by a mixer, the obtained fine powder was transferred into a furnace (at 460 °C for 6 h). Then, a weight of 0.5 g of the attained ash was added by nitric acid (HNO3, 2%) and passed through a 0.45 μm filter (glass fiber) (AOAC 1990). The absorption of minerals (P, K, Fe, Mn, and Zn) was conducted using atomic absorption spectroscopy (240FS AA, Agilent, America).
Nitrogen (total N) content in algae sample was determined after mineralization with sulfuric acid (H2SO4, 96%) in the presence of potassium sulfate (K2SO4) and a low concentration of copper (Cu) according to the Kjeldahl method (Bremner 1965). Moreover, the pH and electrical conductivity (EC) of the seaweed liquid extract and microalga liquid extract were measured by pH meter (PHS, China) and EC meter (DDS-22C, China).
Seed collection
The ripe seeds of the milkweed shrubs were randomly collected from Abad village (3,213,206 m N and 523,703 m E; 59 m a.s.l) of the Tangestan region, South of Iran, in fall 2019.
To assay purity percentage, the immature and malformed seeds were manually separated and placed in distilled water. Tetrazolium chloride was used to evaluate seed viability. The moisture percentage of seeds was calculated as follows (AOSA 1981):
The results of seed physiological characteristics indicated 100% the purity percentage, 95% viability, and 6% moisture percentage.
Experimental Setup
Sodium hypochlorite (5%) was used to sterilize the milkweed seeds for 10 min. Next, they were rinsed three times with DW, transported to Petri dishes, and soaked in 20 ml of the algae biostimulant solutions at concentrations 0.5, 1, and 1.5% (v/v) to perform the seed priming method. After the soaking period, the seeds between two filter paper (Whatman No. 42 mm) were placed in the Petri dish (darkness at 25 ºC) and allowed to dry for 24 h to reduce moisture percentage (approximately 6%). Control seeds were also soaked in a Petri dish containing distilled water.
The treatments were prepared in a completely randomized design with three replication per treatment, amounting to a total of 16 experimental units (n = 48 petri dishes). All experimental seeds were transferred to sterile Petri dishes (total seed = 960) containing a moist filter paper, which was soaked with 5 ml of distilled water and then transferred to a growth room (temperature 25 °C and relative humidity 65%, photoperiod 16 h light/8 h dark) (AOSA 1981). Filter papers were replaced every day to inhibit microbial infections, including fungi and bacteria, etc., in Petri dishes containing seeds.
For studying the effect of seed priming with biostimulants SLEs and MLEs, The experiment carried out by the following treatments:
-
1. Control, 0% extract (10 ml of DW), pH 7.0; EC 0.0 dS.m−1
-
2. T1, 0.5% concentration (0.05 ml SLE in 9.95 ml DW), pH 7.2; EC 0.54 dS.m−1
-
3. T2, 1%concentration (0.1 ml SLE in 9.9 ml DW), pH 7.4; EC 0.80 dS.m−1
-
4. T3, 1.5% concentration (0.15 ml SLE in 9.85 ml DW), pH 7.6; EC 1.23 dS.m−1
-
5. S1, 0.5%concentration (0.05 ml MLE in 9.95 ml DW), pH 7.6; EC 2.5 dS.m−1
-
6. S2, 1% concentration (0.1 ml MLE in 9.9 ml DW), pH 8.3; EC 3.79 dS.m−1
-
7. S3, 1.5% concentration (0.15 ml MLE in 9.85 ml DW), pH 8.6; EC 5.51 dS.m−1
-
8. T1 + S1, 0.5% + 0.5% concentration (0.05 ml SLE in 9.95 ml DW + 0.05 ml MLE in 9.95 ml DW), pH 7.6; EC 1.36 dS.m−1
-
9. T1 + S2, 0.5% + 1% concentration (0.05 ml SLE in 9.95 ml DW + 0.1 ml MLE in 9.9 ml DW), pH 8.1; EC 1.65 dS.m−1
-
10. T1 + S3, 0.5% + 1.5% concentration (0.05 ml SLE in 9.95 ml DW + 0.15 ml MLE in 9.85 ml DW), pH 8.2; EC 1.97 dS.m−1
-
11. T2 + S1, 1% + 0.5% concentration (0.1 ml SLE in 9.9 ml DW + 0.05 ml MLE in 9.95 ml DW), pH 7.9; EC 1.34 dS.m−1
-
12. T2 + S2, 1% + 1% concentration (0.1 ml SLE in 9.9 ml DW + 0.1 ml MLE in 9.9 ml mL DW), pH 8.1; EC 2.0 dS.m−1
-
13. T2 + S3, 1% + 1.5% concentration (0.1 ml SLE in 9.9 ml DW + 0.15 ml MLE in 9.85 ml DW), pH 8.2; EC 2.01 dS.m−1
-
14. T3 + S1, 1.5% + 0.5% concentration (0.15 ml SLE in 9.85 ml DW + 0.05 ml MLE in 9.95 ml DW), pH 7.9; EC 2.36 dS.m−1
-
15. T3 + S2, 1.5% + 1% concentration (0.15 ml SLE in 9.85 ml DW + 0.1 ml MLE in 9.9 ml DW), pH 8.1; EC 2.46 dS.m−1
-
16. T3 + S3, 1.5% + 1.5% concentration (0.15 ml SLE in 9.85 ml DW + 0.15 ml MLE in 9.85 ml DW), pH 8.2; EC 2.54 dS.m−1
Biometry for germination experiment
The germinated seeds were noted daily for 19 days (assessed as the length of root emergence reached 2 mm). After no seed germination was observed in three successive days, the seed trial was completed. At the end of the experiment, shoots were removed from roots, and their fresh weight was measured through digital beam balance (scale 0.01 mg), and afterward, they dried in an oven (70 ºC) for 48 h to assay their dry weight. Also, their length was carefully measured by a digital ruler (scale 0.01 mm). In addition, the seed germination percentage (Panwar and Bhardwaj 2005) and mean germination time (Kulkarni et al. 2007), and Seed vigor index (Marcos Filho 2015) were determined as follows:
where n is the number of germination per day, N is the total number of seeds, ti is the number of days that seeds germinated, ni is the number of germinated seeds among periods, SL is the shoot length, and RL is the root length.
Statistical analysis
We used a completely randomized design (three replications per treatment) through a factorial experiment (seaweed and microalga). A Two-way ANOVA test was used to analyze all data. Data were checked for normality by the Kolmogorov-Smirnov test and homogeneity of variances by the Levene test. Tukey HSD test (p ≤ 0.05) was used to compare means among treatments (SPSS software, version 21).
Results
Multi elemental composition of seaweed and microalga:
Mineral content revealed the presence of total N (1.34 and 6.64%), P (70.3 and 26.5 ppm), K (7580.4 and 142.8 ppm), Ca (4801.6 and 5083.3 ppm), Fe (46.5 and 60.2 ppm), Mn (21.2 and 18.5 ppm) and, Zn (16.3 and 8.7 ppm) in seaweed S. angustifolium and microalga S. platensis, respectively. Among the elements, total N, K, and Ca were abundant in the samples (Table 1).
Germination percentage and germination speed
In our experiments, the use of both algae extracts priming significantly promoted the rate of seed germination and primary growth of milkweed. There was a noticeable increase in all parameters, when 0.5% of SLE and MLE were applied to the milkweed plant, as presented in Table 2.
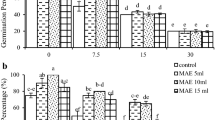
The results of two-way ANOVA indicated that the response of the seedlings was different in SLE, and MLE primings and no significant difference (p ≥ 0.05) was observed by increasing the concentration of SLE in GP and GS parameters. The germination percentage was (100%) in all concentrations of SLEs and exhibited an increase in GP (50%) in comparison with the control. Similarly, almost all concentrations of SLEs showed significant positive effects on the GS (11 seed.day−1), and an increase of 348% over to the control. Correspondingly, the different concentrations of MLEs priming produced a significant stimulatory effect (p ≤ 0.05) on milkweed GP and GS. The highest values of GP and GS were found in the seeds treated with 1% MLE from S. platensis (with an increase of 50 and 240%, respectively, compared to control). However, MLE had a negative effect on both parameters at higher concentrations (Fig. 1a, b). The combined priming of SLE and MLE showed an inhibitory effect on GP and GS parameters at higher concentrations. The highest values of GP, i.e., 100 and, 90 (%), and GS i.e. 11, 10 and 9 (seed.day−1) were found in seeds that received combined primings T1 + S1, T2 + S1, and T3 + S1, respectively (Fig. 1a, b).
Germination percentage (GP) – (a) and germination speed (GS) – (b) of milkweed seeds treated with biostimulant extracts priming at concentrations of T1 0.5%, T2 1%, T3 1.5%, S1 0.5%, S2 1% and S3 1.5% from S.angustifolium (T) and S.platensis (S). The different letters indicate significant differences among treatments based on the Tukey HSD test. The values are mean ± SE of three replicates (p value ≤ 0.05)
Meantime of germination and seed vigor index
The results of ANOVA showed that the response of the mean time germination and seed vigor was significantly different in seaweed and microalga primings. Application of SLEs priming showed constant changes with increasing of concentrations in MGT and SVI. The minimum value MGT was 5 seeds.day−1 at the concentration of SLE 0.5%, and this decrease was 97.2% compared to the control. The maximum value of SVI was (41.75) at the concentration of SLE 0.5%, and this increase was 111.92% compared to control. The 0.5% MLE from S. platensis showed a significant decrease of 34.4% (2 seed.day−1) in MGT and an increase of 72.57% (36.05) in SVI compared to control, respectively.
Application of MLE priming in these parameters, showed a decreasing trend with the increase of concentrations. Tukey comparisons showed a decreasing trend with increasing concentration of combined treatment. The minimum value of MGT, 1, 1, and 2 seed.day−1 was observed in combined primings, including T1 + S1, T2 + S1, and T3 + S1, respectively. The maximum value of SVI was observed 41.75 at T2 + S1 treatment (Fig. 2a and b).
Mean germination time (MGT) – (a) and seed vigor index (SVI) – (b) of milkweed seeds treated with biostimulant extracts primings at concentrations of T1 0.5%, T2 1%, T3 1.5%, S1 0.5%, S2 1% and S3 1.5% from S.angustifolium (T) and S.platensis (S). The different letters indicate significant differences among treatments based on the Tukey HSD test. The values are mean ± SE of three replicates (p value ≤ 0.05)
Shoot and root length
The shoot length did not show any significant change by increasing concentrations of SLEs primings. The highest average shoot length was 43 mm at all concentrations of SLE showed an increase of 23.7% compared to the control. The highest amount of SL (39.5 mm) was observed at 0.5% SLE and increased by 19.7% compared to the control. There was a noticeable decrease in shoot length parameter, when 1.5% of MLE of S. platensis was applied to the milkweed plant. The seaweed and microalga combined primings showed a reduction in SL with increasing liquid extraction concentration. The highest values of SL (40, 45, and 45.5 mm) were seen in T1 + S1, T2 + S1, and T3 + S1 combined treatments, respectively (Fig. 3a). The root length was decreased, when concentrations of SLE were increased in all treatments. The longest root length (39.5 mm) was observed at 0.5% concentration (64.5% increase compared to the control). Concurrently, a sharp decrease was seen in root length with increasing MLE concentrations. Application of 0.5% of MLE priming resulted in the highest root length (65.41 mm) with a 73.5% increment compared to the control. The longest root length enhancement was found in plants that received the blend of SLE 1% and MLE 0.5% with an increase of up to 60% (38.5 mm) compared to the control (Fig. 3a, b).
Shoot length (SL) – (a) and Root length (RL) – (b) of milkweed seeds treated with biostimulant extracts primings at concentrations of T1 0.5%, T2 1%, T3 1.5%, S1 0.5%, S2 1% and S3 1.5% from S.angustifolium (T) and S.platensis (S). The different letters indicate significant differences among treatments based on the Tukey HSD test. The values are mean ± SE of three replicates (p value ≤ 0.05)
Fresh and dry weight of shoot and root
The highest value of the fresh weight of shoot and root was observed in treatment with 0.5% SLE from S. angustifolium, with an increase of 55.4% (442.5 mg), and 107% (220.5 mg), respectively, compared to the control. There was a gradual decrease in the fresh weight of shoot and root parameters, when 1.5% of seaweed extract priming was applied to milkweed seeds. Increasing the concentration of MLE priming led to a sharp decrease in these parameters. In addition, SFW and RFW parameters were significantly decreased, when 1.5% concentration of MLE extract was applied.
The 0.5% MLE solution of S. platensis showed the highest increase in the SFW and RFW of the milkweed plant with an increase of 21.4% (318 mg), and 53% (163 mg) as compared to the control. In addition, the values of these parameters were demonstrated 318 and 163 mg, respectively.
Both concentrations 1.5 and 1% blended from SLE and MLE primings (T3 + S2, T2 + S1) showed the highest increase in fresh weight of shoot, i.e., 66.4% (436 mg), and root, i.e., 45.5% (155 mg) over the control (Fig. 4a, b). Tukey test showed a gradual decrease in dry weight of shoot and root parameters, when the milkweed plant received seaweed priming at 1.5% concentration. The highest rate of SDW and RDW parameters was recorded 27.16 and 3.95 mg in SLE, respectively. These parameters were enhanced 196% and 197% compared to the control, when 0.5% concentration of SLE was applied. These parameters in the milkweed plant significantly decreased, when MLE priming at 1.5% concentration was applied. Also, The 0.5% MLE priming of S. platensis showed the highest increase in the SDW and RDW of the milkweed plants with an increase of 32.6% (12.15 mg), and 114.2% (2.85 mg) as compared to the control.
Shoot fresh weight (SFW) – (a) and root fresh weight (RFW) – (b) of milkweed seeds treated with biostimulant extracts primings at concentrations of T1 0.5%, T2 1%, T3 1.5%, S1 0.5%, S2 1% and S3 1.5% from S.angustifolium (T) and S.platensis (S). The different letters indicate significant differences among treatments based on the Tukey HSD test. The values are mean ± SE of three replicates (p value ≤ 0.05)
The combined treatments T1 + S1 and T2 + S1 showed the highest increase in dry weight of shoot, i.e., 22.6% (11.23 mg), and 26% (11.55 mg) over the control (Fig. 4a, b). Also, an increase of root dry weight (134.5%) was observed in plants treated with the 0.5% blend from SLE and MLE priming (T1 + S1) compared to the control (Fig. 5a, b).
Shoot dry weight (SDW) – (a) and root dry weight (RDW) – (b) of milkweed seeds treated with biostimulant extracts primings at concentrations of T1 0.5%, T2 1%, T3 1.5%, S1 0.5%, S2 1% and S3 1.5% from S.angustifolium (T) and S.platensis (S). The different letters indicate significant differences among treatments based on the Tukey HSD test. The values are mean ± SE of three replicates (p value ≤ 0.05)
Discussion
The present study indicates that the priming of S. angustifolium, S. platensis extracts and S. angustifolium + S. platensis have significant potential as biostimulants to enhance the growth and yield of milkweed plants. These results support earlier works (Hernandez-Herrera et al. 2013; Dineshkumar et al. 2018).
This positive effect can be attributed to some growth regulators present in marine algal extracts like ethylene, kinetin, and gibberellic acid, which play an essential role in accelerating germination time, boosting growth, and development (Raghunandan et al. 2019). The amplifying effect of algae extracts may be due to phytohormones, which cause the synthesis of hydrolytic enzymes (Bahmani Jafarlou et al. 2021). The researchers have demonstrated that seaweed extracts may reduce the likely inhibitors of abscisic acid in the seeds, which improves the germination indices (Battacharyya et al. 2015; Hussein et al. 2021).
Furthermore, there are other possible mechanisms, which can demonstrate the valuable performance of algae priming on the early emergence of milkweed seeds, including the activation of metabolic functions of seeds before germination and the seeds preparation for more rapid germination.
In addition, the brown algal extract contains compatible betaines and betaine-like compounds (Yildiztekin et al. 2018) in plants to reduce the osmotic stress caused by salt stress (Yousefi et al. 2017; Liu et al. 2018). Khan et al (2009) illustrated that the bioactive compounds had a compelling performance in the successfully forming of somatic embryos seeds.
In contrast, the extracts priming of seaweed and microalga and combined of them (EC from 1.23 to 5.51 dS.m−1) showed a significant negative effect on the germination of milkweed seeds at high concentration (1.5%), by inhibiting the ability of seeds to absorb water. Basher et al (2012) studied the effect of seaweed, and saline water on the germination rate, and growth of tomato seedlings, that their results were similar to our data.
The low concentrations of SLEs and MLEs enhanced GP, GS, SL, and RL, whereas they decreased MGT led to greater seed vigor in treated seeds. The high concentration of both algae extracts priming as well as their combined extracts priming (especially, 1.5%) decreased the parameters as mentioned earlier in treated milkweed seeds. High concentrations of salt present in algal extracts significantly enhanced the germination time and reduced the germination indices in tomatoes (Hernandez-Herrera et al. 2013; Rehmat et al. 2021). In the present study, SLE of S. angustifolium and MLE of S. platensis, and their combined extracts (at higher concentrations), decreased germination and growth that this reduction could be caused by high salt in extracts.
Algal extracts contain the amount of salt, which causes to reduce the absorption of water by seeds of milkweed at high levels, thereby decreasing growth parameters, like shoot and root (Woodell 1985). Increasing germination indices at low concentrations of algal extracts can be due to ameliorating the metabolic activities during the priming stages (Bahmani Jafarlou et al. 2021). Pretreatment priming may regulate signaling pathways during early growth stages and cause a faster defense response by trees (Jisha et al. 2012). Therefore, biostimulants served in our experiment may be triggered essential phytochemical changes like hydrolysis, enzyme activation, and breaking physiological dormancy in milkweed seeds to germinate and grow under undesirable conditions (Jisha et al. 2012).
In our study, the high concentrations of algal extracts, i.e., 1.5% (individually and combined), had toxic and negative effects on roots and shoots of milkweed plants. In line with our findings, Hernandez-Herrera et al. (2013) also indicated the negative effects of algae extracts (at high levels) on the growth of tomato seedlings, which these effects may be caused by a high electrical conductivity in the growth medium. In agreement with our results, Kumari et al. (2011) observed the detrimental effects of marine algal extracts at high concentrations (from 2.0 to 10%) on Vigna mungo seedlings. Workers have previously reported with respect to algal extracts on seed germination of plant species such as pine tree (Atzmon and Van Staden 1994) and soybean (Rathore et al. 2009) and tomato (Hernandez-Herrera et al. 2013; Garcia-Gonzalez and Sommerfeld 2016; Carrasco-Gil et al. 2019).
There are several possible reasons for the adverse responses is associated with the effects of high dosages of algal extracts on the early growth of milkweed, such as shoot, root length, and their FW can be mentioned some factors like the existence of regulator hormones or high levels of minerals, including Na+ and Cl− (Garcia-Gonzalez and Sommerfeld 2016; Latique et al. 2017).
The enhancement of germination and early growth indices of milkweeds can be due to secondary metabolites present in seaweed like macro-and microelement nutrients, amino acid, vitamins, cytokinins, auxins, and abscisic acid, which effectively stimulate cellular metabolisms in plants and finally lead to raised growth parameters and yield (Raghunandan et al. 2019). The increment in shoots indices can be attributed to some phytohormones, including the auxins content in the algal extracts, which play an influential role in cell division and elongation of plants resulting in the betterment of the shoot and root growth and biomass (Rouphael et al. 2017; Yildiztekin et al. 2018).
Besides, the capability of microalgae to increase milkweed plant growth can be associated with their high nitrogen contents, amino acides, vitamins, and phytohormones (Priyadarshani and Rath 2012). There were similar reports about the beneficial efficiencies of microalgae extracts like Dunaliella salina, Phaeodactylum tricornutum, S. maxima, Chlorella ellipsoida, and S. platensis on seedlings of pepper, wheat, and rice, which improved early growth during the germination process (Abd El-Baky et al. 2010; Guzman-Murillo et al. 2013; Dineshkumar et al. 2018).
The high concentrations of the SLE and MLE primings increased some primary growth parameters (shoot and root weight and length) in treated milkweed caused by the mineral elements present in extracts. Another positive effect of algal extract use at different concentrations may be due to its various components working synergistically (Fornes et al. 2002). Similar studies performed about algae extractions lead to increase growth and improve phytochemical contents in cowpea, soybean, and tomato plants (Hernandez-Herrera et al. 2013; Garcia-Gonzalez and Sommerfeld 2016; Vasantharaja et al. 2019; Kocira et al. 2019; Rehmat et al. 2021).
Our data showed a high concentration of macro elements such as Ca, K, and total N in both seaweed and microalga. This finding has also been confirmed by Dineshkumar et al (2018) and Hussein et al (2021) on S. platensis and Cystoseira compressa for the growth and yield of Zea mays and Vigna sinensis seedlings, respectively. These extracts of algae are a suitable source of K+ so that it helps in regulating the relative water content, controls stomatal conductivity, and the gas exchange rate and increase meristematic growth in plants. The presence of Ca2+ in extracts improves some activities and functions of the cell, including enzyme activation, elongation, and stability of the cells.
There are several possible reasons for the opposite results that can associated with the different values of mineral contents present in studied algae can highly be related to various factors, like the season, location, and analytical methods (Salehpour et al. 2021).
Conclusions
The plant growth-promoting ability of liquid extracts from seaweed S. angustifolium and microalga S. platensis as biostimulants priming was studied in C. procera. The biostimulants extract showed promising results in C. procera at lower doses (≤ 1%). Combined algal treatments showed an increment in seed germination percentage, seed germination speed, shoot and root fresh and dry weight, and decreased mean time germination. Our results confirmed that the biostimulants priming increased the seed vigurity indices by increasing the number of germinated seed, shoot, and root length in milkweed. Therefore, this study suggests that the application of S. angustifolium and S. platensis extracts (individual and combined) as seed primings would benhighly potent, eco-friendly, and cost-effective for improving the crop biomass and yield. Hence, we suggest that field experiments need to be conducted to evaluate the effects of algae biostimulants on plants growth to pave a new path towards commercialization.
References
Abd El - Baky HH, El - Baz FK, El - Baroty GS (2010) Enhancing antioxidant availability in wheat grains from plants grown under seawater stress in response to microalgae extract treatments. J Sci Food Agric 90:299-303https://doi.org/10.1002/jsfa.3815
Amin A, Gharib F, El - Awadi M, Rashad E, (2011) Physiological response of onion plants to foliar application of putrescine and glutamine. Sci Hortic 129:353–360. https://doi.org/10.1016/j.scienta.2011.03.052
AOAC (Association of Official Analytical Chemists) (1990) Official methods of analysis, AOAC 15th edn.AOAC. Washington DC
AOSA (Association of Official Seed Analysts), 1981AOSA (Association of Official Seed Analysts) (1981) Rules for testing seeds. J Seed Technol 6:1-126
Atzmon N, Van Staden J (1994) the effect of seaweed concentrate on the growth of Pinus pinea seedlings. New for 8:279–288. https://doi.org/10.1007/BF00025373
Bahmani M, Naghdi R, Kartoolinejad D (2018) Milkweed seedlings tolerance against water stress: Comparison of inoculations with Rhizophagus irregularis and Pseudomonas putida. Environ Tech Innov 10:111–121. https://doi.org/10.1016/j.eti.2018.01.001
Bahmani Jafarlou M, Pilehvar B, Modarresi M, Mohammadi M (2021) Performance of Algae Extracts Priming for Enhancing Seed Germination Indices and Salt Tolerance in Calotropis procera (Aiton) W.T. Iran J Sci Technol Trans Sci 45:493–502. https://doi.org/10.1007/s40995-021-01071-x
Basher AA, Mohammed AJ, Teeb AIH (2012) Effect of seaweed and drainage water on germination and seedling growth of tomato (Lycopersicon spp.). Euphrates J Agri Sci 4:24–39
Battacharyya D, Babgohari MZ, Rathor P, Prithiviraj B (2015) Seaweed extracts as biostimulants in horticulture. Scientia Horti 196:39–48. https://doi.org/10.1016/j.scienta.2015.09.012
Bremner JM (1965) Total nitrogen. Methods of soil analysis: Part 2 chemical and microbiological properties 9: 1149 - 1178.
Carrasco-Gil S, Allende-Montalban R, Hernández-Apaolaza L, Lucena JJ (2019) Application of seaweed organic components increases tolerance to Fe deficiency in tomato plants. Bio Rxiv 10:1–32. https://doi.org/10.3390/agronomy11030507
Dineshkumar R, Kumaravel R, Gopalsamy J, Sikder MNA, Sampathkumar P (2018) Microalgae as bio - fertilizers for rice growth and seed yield productivity. Waste Biomass Valor 9:793–800. https://doi.org/10.1007/s12649-017-9873-5
Dmytryk A, Michalak I, Wilk R, Chojnacka K, Grecka H, Grecki H (2015) Innovative seed treatment with algae homogenate. Waste Biomass Valor 6:441–448. https://doi.org/10.1007/s12649-015-9363-6
Falch BS, Konig GM, Wright AD, Sticher O, Angerhofer CK, Pezzuto JM, Bachmann H (1995) Biological activities of cyanobacteria: evaluation of extracts and pure compounds. Planta Med 61:321. https://doi.org/10.1055/s-2006-958092
Fleming H, Haselkorn R (1973) Differentiation in Nostoc muscurum - Nitrogenase is synthesized in heterocysts. Proc Natl Acad Sci 70:2727–2731. https://doi.org/10.1073/pnas.70.10.2727
Fornes F, Sanchez-Perales M, Guadiola JL (2002) Effect of a seaweed extract on the productivity of ‘de Nules’ Clementine mandarin and Navelina orange. Bot Mar 45:486–489. https://doi.org/10.1515/BOT.2002.051
Garcia - Gonzalez J, Sommerfeld M (2016) Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus .J Appl Phycol 28:1051-1061https://doi.org/10.1007/s10811-015-0625-2
Geries LSM, Elsadany AY (2021) Maximizing growth and productivity of onion (Allium cepa L.) by Spirulina platensis extract and nitrogen-fixing endophyte Pseudomonas stutzeri. Arch Microbiol 203:169–181. https://doi.org/10.1007/s00203-020-01991-z
Guzman - Murillo MA, Ascencio F, Larrinaga Mayoral JA (2013) Germination and ROS detoxification in bell pepper (Capsicum annuum l.) under NaCl stress and treatment with microalgae extracts. Protoplasma 250:33:42https://doi.org/10.1007/s00709-011-0369-z
Hernandez-Herrera RM, Santacruz-Ruvalcaba F, Ruiz-Lopez MA, Norrie J, Hernandez-Carmona G (2013) Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.). J Appl Phycol 26:619–628. https://doi.org/10.1007/s10811-013-0078-4
Hindi SS (2013) Calotropis procera: The miracle shrub in the Arabian Peninsula. Int J Sci & Eng Invest 16:48–57
Hussein MH, Eltanahy E, Al Bakry AF, Elsafty N, Elshamy MM (2021) Seaweed extracts as prospective plant growth bio - stimulant and salinity stress alleviator for Vigna sinensis and Zea mays. J Appl Phycol 33:1273–1291. https://doi.org/10.1007/s10811-020-02330-x
Ibrahim EA (2016) Seed priming to alleviate salinity stress in germinating seeds. J Plant Physiol 192:38–46
Jisha KC, Vijayakumari K, Puthur JT (2012) Seed priming for abiotic stress tolerance: an overview. Acta Physiol Plant 35:1381–1396. https://doi.org/10.1016/j.jplph.2015.12.011
Kakkar RK, Bhaduri S, Rai VK, Kumar S (2000) Amelioration of NaCl stress by arginine in rice seedlings: changes in endogenous polyamines. Biol Plant 43:419–422. https://doi.org/10.1023/A:1026715032115
Kemka HO, Rebecca AA, Gideon OA (2007) Influence of temperature and pH bioresource and protein biosynthesis in putative Spirulina sp. Bioresource Technol 98:2207–2211. https://doi.org/10.1016/j.biortech.2006.08.028
Khalilzadeh R, Tajbakhsh M, Jalilian J (2012) Effect of foliar application of bio-organic fertilizers and urea on yield and yield components characteristics of mung bean. Int J Agric Res Rev 2:639–645
Khan W, Rayirath UP, Subramanian S, Jithesh MN, Rayorath P, Hodges DM, Critchley AT, Craigie JS, Norrie J, Prithiviraj B (2009) Seaweed extracts as biostimulants of plant growth and development. J Plant Growth Regul 28:386–399. https://doi.org/10.1007/s00344-009-9103-x
Kocira S, Szparaga A, Kubon M, Czerwińska E, Piskier T (2019) Morphological and biochemical responses of Glycine max (l.) merr to the use of seaweed extract. Agron 9:1–23. https://doi.org/10.3390/agronomy9020093
Kulkarni MG, Sparg SG, Van Standen J (2007) Germination and post - germination response of Acacia seeds to smoke - water and butenolide, a smoke - derived compound. J Arid Environ 69:177–187. https://doi.org/10.1016/j.jaridenv.2006.09.001
Kumari R, Kaur I, Bhatnagar AK (2011) Effect of aqueous extract of Sargassum johnstonii Setchell & Gardner on growth, yield and quality of Lycopersicon esculentum Mill. J Appl Phycol 23:623–633. https://doi.org/10.1007/s10811-011-9651-x
Latique S, Mohamed Aymen E, Halima C, Che´rif H, Mimoun EK, (2017) Alleviation of salt stress in durum wheat (Triticum durum L.) seedlings through the application of liquid seaweed extracts of Fucus spiralis. Commun in Soil Sci Plant Anal 48:2582–2593. https://doi.org/10.1080/00103624.2017.1416136
Liu S, Li B, Chen X, Qin Y, Li P (2018) Effect of polysaccharide from Enteromorpha prolifera on maize seedlings under NaCl stress. J Oceanol Limnol 37:1372–1381. https://doi.org/10.1007/s00343-019-8150-9
Marcos Filho J (2015) Seed vigor testing: an overview of the past, present and future perspective. Sci Agric 72:363–374. https://doi.org/10.1590/0103-9016-2015-0007
Norrie J, Branson T, Keathley PE (2002) Marine plant extracts impact on grape yield and quality. Acta Hortic 594: 315 - 319. https://doi.org/10.17660/ActaHortic.2002.594.38
Panwar P, Bhardwaj SD (2005) Handbook of Practical Forestry. Agro bios p.191, India
Priyadarshani I, Rath B (2012) Commercial and industrial applications of microalgae — a review. J Algal Biomass Utln 3:89–100
Raghunandan BL, Vyas RV, Patel HK, Jhala YK (2019) Perspectives of Seaweed as Organic Fertilizer in Agriculture. In: Panpatte D, Jhala Y (eds) Soil Fertility Management for Sustainable Development. Springer, Singapore. https://doi.org/10.1007/978-981-13-5904-0_13
Rehmat Y, Jabeen R, Hameed S, Ejaz M, Khattak MI (2021) Effects of Cyanobacterium, Leptolyngbya sp. and green Microalga, Chlorella Sorokiniana as biofertilizers on In vitro seed priming and seedling growth of some economically important vegetables from Pakistan. Pak J Bot 53: 343 - 350. https://doi.org/10.30848/PJB2021-1 (12)
Rouphael Y, De Micco V, Arena C, Raimondi G, Colla G, De Pascale S (2017) effect of Ecklonia maxima seaweed extract on yield, mineral composition, gas exchange, and leaf anatomy of zucchini squash grown under saline conditions. J Appl Phycol 29:459–470. https://doi.org/10.1007/s10811-016-0937-x
Salehpour R, Biuki NA, Mohammadi M, Dashtiannasab A, Ebrahimnejad P (2021) The dietary effect of fucoidan extracted from brown seaweed, Cystoseira trinodis (C. Agardh) on growth and disease resistance to WSSV in shrimp Litopenaeus vannamei. Fish Shellfish Immunol 119:84–95. https://doi.org/10.1016/j.fsi.2021.09.005
Sànchez MC, Castillo B, Rodriguez I (2003) (Spirulina Arthrospira): An edible microorganism. A review. Universitas Scientiarum 8, 1. Bogotà, Colombia.
Seifikalhor M, Hassani SB, Aliniaeifard S (2020) Seed priming by cyanobacteria (Spirulina platensis) and salep gum enhances tolerance of maize plant against cadmium toxicity. J Plant Growth Regul 39:1009–1021. https://doi.org/10.1007/s00344-019-10038-7
Sohrabipour J, Rabiei R (1996) new records of algae for Persian Gulf and flora of Iran. Iran J Bot 8:53–61
Souri MK, Hatamian M (2019) Aminochelates in plant nutrition: a review. J Plant Nutr 42:67–78. https://doi.org/10.1080/01904167.2018.1549671
Taghvaei M, Khaef N, Sadeghi H (2012) the effects of salt stress and prime on germination improvement and seedling growth of Calotropis procera L seeds. J Ecol Environ Sci 35:73–78. https://doi.org/10.5141/JEFB.2012.011
Vasantharaja R, Abraham LS, Inbakandan D, Thirugnanasambandam R, Senthilvelan T, Jabeen SA, Prakash P (2019) Influence of seaweed extracts on growth, phytochemical contents and antioxidant capacity of cowpea (Vigna unguiculata L. Walp). Biocatal Agric Biotechnol 17:589–594. https://doi.org/10.1016/j.bcab.2019.01.021
Woodell SRJ (1985) Salinity and seed germination patterns in coastal plants. In: Beeftink WG Rozema J, Huiskes AHL (eds) Ecology of coastal vegetation. Advances in vegetation science, vol 6. Springer, Dordrecht. https://doi.org/10.1007/978-94-009-5524-0_24
Yanni YG, Elashmouny AA, Elsadany AY (2020) Differential response of cotton growth, yield and fiber quality to foliar application of Spirulina platensis and urea fertilizer. Asian J Adv Agric Res 29–40https://doi.org/10.9734/ajaar/2020/v12i130072
Yildiztekin M, Tuna AL, Kaya C (2018) Physiological effects of the brown seaweed Ascophyllum nodosum and humic substances on plant growth, enzyme activities of certain pepper plants grown under salt stress. Acta Biol Hung 69:325–335. https://doi.org/10.1556/018.68.2018.3.8
Yousefi S, Kartoolinejad D, Bahmani M, Naghdi R (2017) Effect of Azospirillum lipoferum and Azotobacter chroococcum on germination and early growth of hopbush shrub (Dodonaea viscosa L.) under salinity stress. J Sustain Forest 36:107–120. https://doi.org/10.1080/10549811.2016.1256220
Zarrouk C (1966) Contribution a l’etude d’une Cyanophycee. Influence de Divers Facteurs Physiques et Chimiques sur la croissance et la photosynthese de Spirulina mixima. Thesis University of Paris, France.
Acknowledgements
The authors of this article sincerely thank and appreciate the cooperation and sponsorship of the Iran National Science Foundation (INSF) from the Office of President Vice-Presidency for Science and Technology from the research project number 96005573 and also thank the Vice-Chancellor of Research and Technology of Lorestan University. Moreover, the authors highly appreciate the help of Dr. Razieh Salehpour for her critical reviewing and editing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that we have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jafarlou, M.B., Pilehvar, B., Modaresi, M. et al. Interactive effects of seaweed and microalga extract priming as a biostimulant agent on the seed germination indices and primary growth of milkweed (Calotropis procera Ait.). Biologia 77, 1283–1293 (2022). https://doi.org/10.1007/s11756-021-00996-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-021-00996-3