Abstract
Patients with pulmonary sequestration are at risk of life-threatening bleeding during lung resection. To perform safe and adequate lung resection in patients with pulmonary sequestration, we utilized the following combination of techniques: (1) three-dimensional computed tomographic (3D-CT) imaging for preoperative planning and intraoperative identification of blood vessels, including aberrant arteries, and (2) intraoperative intravenous administration of indocyanine green (ICG). We describe our surgical technique through three cases who underwent lung resection for pulmonary sequestration using 3D-CT and fluorescence navigation with ICG. Intraoperative identification and division of the aberrant arteries, draining veins, and resection margins of the lungs were successfully completed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pulmonary sequestration is a rare malformation characterized by nonfunctional (sequestrated) lung tissue with an abnormal tracheobronchial tree supplied by aberrant systemic arteries [1, 2]. Pulmonary sequestration patients are at risk of hemoptysis, respiratory infections, and intrapulmonary hemorrhage. The mainstay of treatment is surgical resection of the sequestrated lung and shutting off aberrant feeding arteries [3, 4].
To avoid life-threatening hemorrhage and imprecise pulmonary resection, accurate intraoperative identification of the aberrant arteries and precise margins of the sequestrated lung is paramount. Therefore, we utilized a combination of two imaging techniques: (1) three-dimensional computed tomographic (3D-CT) imaging for preoperative planning and intraoperative identification of the blood vessels, and (2) intraoperative intravenous administration of indocyanine green (ICG) for demarcating the sequestrated lung. We report three cases who successfully underwent lung resection for pulmonary sequestration using 3D-CT and ICG.
Techniques
All contrast-enhanced 3D-CT examinations (Revolution CT, GE Healthcare, US) were performed at our institute using 0.63-mm-thick full-resolution scans. The images were constructed using a DICOM data workstation (Ziostation2, Ziosoft, Japan).
Preoperatively, the aberrant arteries (including origins and branches) of the pulmonary artery (PA), pulmonary vein (PV), and bronchus of the affected lobe were identified using 3D-CT. We isolated the draining vein from the sequestrated lung, but preserved the PA, PV, and bronchus in the nonsequestrated lungs during resection. In case of inflammatory lung lesions (such as consolidations and cavitary lesions), 3D-CT was used to determine the extent of preservation of structures of the affected lobe to allow adequate resection of the inflamed lung parenchyma.
Intraoperatively, we identified and isolated the aberrant artery and draining vein using the 3D-CT images we obtained. Blood vessels were ligated using a ligature or a vascular stapling device depending on the size of vessels. An anesthesiologist then administered 0.25 mg/kg of ICG. Using real-time VISERA ELITE II (Olympus, Japan) or IMAGE1 S (Karl Stortz, Japan) fluorescent imaging technique, a demarcation margin between the sequestrated and nonsequestrated lungs was identified and marked using an electrocautery. After isolating segmental branches of the PA, PV, and bronchus, we used ICG to identify the intersegmental plane on inflamed lungs requiring parenchymal resection (segmentectomy).
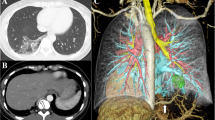
Case 1 was a 33-year-old man, a nonsmoker with a history of pneumonia, diagnosed as having left intrapulmonary sequestration. 3D-CT revealed a mass in the left lower lobe with an aberrant artery arising from the descending aorta and a vein draining into the left inferior PV (Fig. 1a). The sequestrated lung was resected by thoracotomy using ICG. The boundary between the sequestrated and the normal lungs was mostly unclear under the normal thoracoscopic view. However, intravenous ICG injection successfully visualized the boundary which was shown as a complexed bumpy line (Fig. 1b, c). His postoperative course was uneventful.
Case 1 showing intrapulmonary sequestration in the left lower lobe. a A three-dimensional computed tomography image (posterior view) showing the aberrant artery (arrowhead) arising from the descending aorta. b A thoracoscopic view showing unclear boundary between the sequestrated and the normal lungs. c Fluorescence navigation using indocyanine green showing green staining in the normal lung and no staining in the sequestrated lung, thus demarcating a bumpy boundary of the sequestrated lung
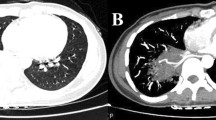
Case 2 was a 38-year-old woman, a former smoker with a history of recurrent pneumonia, diagnosed as having right intrapulmonary sequestration. 3D-CT revealed a cystic mass in the right lower lobe with an aberrant artery arising from the descending aorta and a vein draining into the right inferior PV (Fig. 2a). We resected the sequestrated lung by video-assisted thoracic surgery (VATS) using ICG. Similar to Case 1, the unclear boundary of the sequestrated lung was clearly visualized using ICG injection (Fig. 2b, c). Her postoperative course was uneventful.
Case 2 showing intrapulmonary sequestration in the right lower lobe. a A three-dimensional computed tomography image (posterior view) showing an aberrant artery (arrowhead) arising from the descending aorta. b A thoracoscopic view showing unclear boundary between the sequestrated and the normal lungs. c Fluorescence navigation using indocyanine green showing green staining in the normal lung and no staining in the sequestrated lung, thus demarcating a bumpy boundary of the sequestrated lung
Case 3 was a 24-year-old woman, a nonsmoker with a history of recurrent pneumonia, diagnosed as having right intrapulmonary sequestration. 3D-CT revealed an aberrant artery arising from the celiac artery and a large pulmonary consolidation in the right lower lobe which was considered as the sequestrated lung with surrounding inflammatory changes. The central part of the right basal segment was involved with the consolidation, whereas the superior segment (S6) was preserved (Fig. 3a, b). Intraoperatively, inflammatory changes surrounding the sequestrated lung such as interlobar adhesions and inflammatory perivascular tissues in the hilum were observed. Based on both the preoperative radiological and intraoperative findings, we thought that a simple resection of the sequestrated lung without dividing the hilar structures in the basilar segment was difficult and we, therefore, performed basilar segmentectomy of the right lower lobe using VATS on the inflammatory areas of the basilar segment and the sequestrated lung. The intersegmental plane between the superior and basilar segments was identified using ICG (Fig. 3c). Her postoperative course was uneventful.
Case 3 showing intrapulmonary sequestration in the right lower lobe with an inflammatory change of the right basilar segment. a A three-dimensional computed tomography (3D-CT) image (right-anterior view) showing the aberrant artery (arrowhead) arising from the celiac artery running into the right lower lobe. Inflammatory changes in the basilar segment is shown in green. b A 3D-CT image (posterior view) showing a close margin from the inflammatory lung (shown in green) and the right inferior pulmonary vein (arrowhead). c Fluorescence navigation using indocyanine green showing green staining in the superior segment and no staining in the basilar segment and the sequestrated lung. The demarcation line represents the intersegmental plane between the superior segment and the basilar segment of the right lower lobe
Comments
The complications of pulmonary sequestration lung resection include life-threatening intraoperative hemorrhage resulting from an injury of the aberrant artery, postoperative hemothorax, prolonged air leakage, and empyema [5,6,7]. Our proposed technique has the following advantages: (1) 3D-CT imaging allows pre- and intraoperative visualization, especially for intraoperative identification of the vascular structures, and (2) intraoperative ICG administration allows demarcation of the 3D boundaries of the sequestrated lung or the intersegmental plane. In our patients, both the procedures were used without complications, and their application was affordable and safe.
We previously reported on the usefulness of 3D-CT reconstruction during pulmonary segmentectomy [8]. The variations in the anatomy of segmental pulmonary vessels and bronchi make it vital for surgeons to identify anatomical structures during pulmonary segmentectomy. In addition, 3D-CT provided a precise understanding of the spatial relationship between different pulmonary structures, which helped to determine a reasonable and safe surgical procedure preoperatively (such as in case 3).
Demarcating lung boundaries during surgery is more difficult in the sequestered lung than the intersegmental plane due to inflammatory and structural changes (e.g., adhesions). In cases 1 and 2, complex bumpy lines were visible on the boundaries. Thus, fluorescence navigation using ICG might be more useful and accurate than classical methods (inflation of nonsequestrated lung) to demarcate lung boundaries.
When dividing intersegmental plane during segmentectomy for lung cancer, we often utilized electrocautery to dissect the intersegmental plane. In our current 3 cases, however, we utilized endostaplers to divide the lung parenchyma since we thought that the use of electrocautery would be difficult and might cause postoperative complications because: (1) in resection of the sequestrated lungs (cases 1 and 2), the boundaries of the sequestrated lungs were complex and bumpy; and (2) in basilar segmentectomy (case 3), we thought that the intersegmental plane might have been affected by infection spread from the sequestrated lungs.
In conclusion, 3D-CT and ICG-guided lung resection for pulmonary sequestration are useful for surgeons to perform safe and adequate procedures.
References
Clements B, Warner J. Pulmonary sequestration and related congenital bronchopulmonary-vascular malformations: Nomenclature and classification based on anatomical and embryological considerations. Thorax. 1987;42:401–8.
Liechty KW, Flake AW. Pulmonary vascular malformations. Semin Pediatr Surg. 2008;17:9–16.
Walker CM, Wu CC, Gilman MD, Godwin JD, Shepard J-AO, Abbott GF. The imaging spectrum of bronchopulmonary sequestration. Curr Probl Diagn Radiol. 2014;43:100–14.
Savic B, Birtel F, Tholen W, Funke H, Knoche R. Lung sequestration: Report of seven cases and review of 540 published cases. Thorax. 1979;34:96–101.
Li R, Li H, Liang T, Wu C. Undiagnosed pulmonary sequestration results in an unexplained hemorrhagic shock in thoracoscopic pulmonary lobectomy. J Clin Anesth. 2016;35:485–7.
Gezer S, Tastepe I, Sirmali M, et al. Pulmonary sequestration: A single-institutional series composed of 27 cases. J Thorac Cardiovasc Surg. 2007;133:955–9.
Lin CH, Chuang CY, Hsia JY, et al. Pulmonary sequestration-differences in diagnosis and treatment in a single institution. J Chin Med Assoc. 2013;76:385–9.
Shimizu K, Nakazawa S, Nagashima T, Kuwano H, Mogi A. 3D-CT anatomy for vats segmentectomy. J Vis Surg. 2017;3:88.
Funding
The authors received no financial support for this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Matsuoka, S., Eguchi, T., Takeda, T. et al. Three-dimensional computed tomography and indocyanine green-guided technique for pulmonary sequestration surgery. Gen Thorac Cardiovasc Surg 69, 621–624 (2021). https://doi.org/10.1007/s11748-020-01511-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-020-01511-2