Abstract
Objective
Presenting our experience of direct perfusion of the carotid artery in patients with brain malperfusion secondary to acute aortic dissection.
Patients
Among 381 patients who underwent aortic repair for acute type A aortic dissection from October 1999 to August 2017, brain malperfusion was recognized in 50 patients. Nine patients had direct perfusion of the right carotid artery in patients with brain malperfusion secondary to acute aortic dissection. Age at surgery was 65.7 ± 13.5 years and three patients were male. Preoperative consciousness level was alert in one patients, drowsy in six, and coma in two. Five patients had preoperative hemiplegia. All patients showed a blood pressure difference between the upper extremities and eight patients showed more than 15% difference of rSO2. Seven patients had a temporary external active shunt from the femoral artery to the right common carotid artery preoperatively. Two patients had direct perfusion to the right common carotid artery during cardiopulmonary bypass or in the intensive care unit after surgery because of a sudden decrease of rSO2 and cessation of carotid artery flow. Antegrade cerebral perfusion was used in all patients. Total arch replacement was performed in six patients and hemiarch in three.
Results
The hospital mortality was 33% (3 patients). Causes of death were huge hemispheric brain infarction or anoxic brain damage in two patients and myocardial infarction in one. The postoperative neurological outcome was alert in four, hemiplegia in two, and coma in three, but five patients showed some improvement of neurological signs.
Conclusion
Aggressive direct reperfusion of the carotid artery before the aortic repair may reduce neurological complications in patients with preoperative brain malperfusion secondary to acute aortic dissection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to a survey by the Japanese Association for Thoracic Surgery, since 1984, the annual number of operations for the aortic dissection, has steadily increased from 519 in 1984 [1] to 7733 in 2014 [2] and the 30-day mortality for acute type A aortic dissection in 1984 was reported to be 18.4% (350/1901) [1] and improved to 8.8% (434/4953) in 2014 [2]. Early reperfusion is generally accepted as extremely important to reduce brain ischemic insult. The purpose of this report is to describe our strategy and results of surgery for acute type A aortic dissection complicated with brain malperfusion. This study was approved by our Institutional Review Board of Kobe University Hospital, and the need for individual consent was waived.
Patients and methods
Nine patients required direct carotid artery perfusion. Preoperative consciousness level was alert in one patients, drowsy in six, coma or intubated in two. Six showed left hemiplegia. These patients were sent to our institution 22.1 ± 40.3 h (2–120 h) after an onset of aortic dissection. The true lumen of the right common carotid artery was occluded in four patients and severely stenosed in five patients on duplex scans and none showed opacification from contrast material on CT (Table 1).
Age at surgery was 65.7 ± 13.5 years and three patients were male. Seven patients had a temporary external active shunt from the femoral artery to the right common carotid artery preoperatively. One patient had direct perfusion to the right common carotid artery during cardiopulmonary bypass because of a sudden decrease of rSO2 and cessation of carotid artery flow. One required direct perfusion of the carotid artery in the intensive care unit after repair of the aortic dissection because of a sudden decrease of right rSO2 and loss of the light reflex of the right pupil.
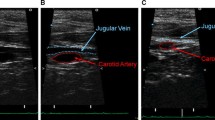
Our indications of direct carotid perfusion in acute type A aortic dissection complicated with brain malperfusion are patients who have at least one sign or symptoms of diminished flow in the carotid arteries, such as dissected carotid arteries with compressed true lumen by CT scan, decreased blood flow in the carotid artery demonstrated by ultrasound, persistent consciousness disorder, hemiparesis, or right to left difference in forehead rSO2 or arm blood pressures (Fig. 1). The right common carotid artery was exposed through the right oblique neck incision anterior to the right sternocleidomastoids muscle in the emergency room in one patient, in the intensive care unit in two patients, and in the operation room in six patients. After limited heparinization (100 IU/kg), an 8 to a 10-Fr pediatric arterial cannula (Stockert; Sorin, Milan, Italy) was inserted directly into the true lumen of the right common carotid artery by cut down technique. The right common femoral artery was chosen for arterial drainage using a double-lumen cannula (connected with each of the dialysis catheters, Blood Access UK-Catheter Kit; Unitica, Aichi, Japan). A simple bypass circuit (priming volume 200 ml, poly-vinyl Prolight 4.5-mm tube; Senko, Tokyo, Japan) containing a small roller pump (HAD 110π, Senko, Tokyo) or a conventional cardiopulmonary bypass circuit was used. Target flow was set at 90 ml/min and blood temperature was set to 30 °C. The durations of right carotid perfusion were 99 ± 36 (29 ± 125) min (Fig. 2).
A 72-year-old female (case 1) with acute aortic dissection. a CT scan showed occlusion of the right common carotid artery (arrow), b left; complete occlusion of the right common carotid artery, c right; patent true lumen of the left common carotid artery, right; Rt CCA right common carotid artery, Lt left
Results
There were three hospital deaths (33.3%). Two patients died of right hemispheric infarction and the other one died of myocardial infarction. The first patient had no distal flow after anastomosing the left carotid and brachiocephalic artery to the side branches of the main arch graft. He had a sudden decrease of the right forehead rSO2 after surgery and lost light reflex in the ICU. Immediate perfusion of the right carotid artery was done but he never regained consciousness. The second patient had a dissection of the both left and right coronary artery and she had malperfusion of the right carotid artery during the cardiopulmonary bypass. She had perfusion through the right carotid artery but she died of left ventricular failure. The third patient was diagnosed to have an acute stroke and had tissue plasminogen activator in the local hospital. She was referred to us after diagnosis of acute aortic dissection with cardiac tamponade. She was resuscitated and had open drainage in the emergency room. During cardiopulmonary bypass, she had malperfusion on the right carotid artery and the direct perfusion was immediately done. However, right rSO2 did not respond initially. After grafting the brachiocephalic artery in 2 h the right rSO2 suddenly equalized to the left one. We speculated that some thrombi might resolve and resultant recanalization of the blood flow was ensued. She had huge brain infarction. Consciousness level improved in three patients and showed no change in three. Three had permanent neurological dysfunction with left hemiparesis. Four patients had no neurological sequelae and two had hemiplegia or hemiparesis. The preoperative difference was noticed in right and left brain rSO2 in eight patients and the right rSO2 was dramatically improved after staring direct perfusion of the right carotid artery in seven patients (Fig. 3, Table 2).
There was no late death among six survivors who had direct carotid perfusion. Actuarial survival at 5 years was 75.2 ± 12.5% and 60.5 ± 23.4% at 10 years.
Discussion
Although early surgical results for acute type A aortic dissection have recently improved [2], brain malperfusion caused by acute type A aortic dissection represents a major detrimental risk factor for early death or disability [3, 4]. The incidence of the malperfusion has been reported as 20–40% and the results of aortic dissection repair in patients who have a concomitant branch malperfusion are poor, and the early mortality rate is reported to be 30–50% [5, 6].
The pathophysiology of brain complications in acutely dissected patients is largely due to compressed true lumen by the false lumen, static or dynamic. However, it is often multifactorial, since anatomical factors, such as head–neck vessels involvement, frequently coexist with circulatory collapse (due to tamponade, low output, and acute aortic valve regurgitation), sequelae of acute hypoxia (due to stroke or cardiorespiratory failure) and thromboembolism originating from the false lumen. The surgery itself, requiring anticoagulation and hypothermic circulatory arrest, additionally increases the risk of a pre-existing neurological dysfunction. In addition, coma manifests more often in elderly patients, with greater comorbidities and splanchnic or limb malperfusion, all phenomenal risk factors for hospital mortality and morbidity.
Diagnosis of malperfusion should be started on suspicion of the existence of malperfusion syndrome based on patients’ signs and symptoms, such as consciousness, neurological deficits, or pain. The ultrasound is mostly utilized clinical tools because malperfusion circulation changes time to time and ultrasound is the only objective tool,which can instantaneously track accordingly to the altered hemodynamics and perfusion status of the true or false lumen. Every patient should have carotid artery ultrasound simultaneously in the emergency room before going to CT scan room (Fig. 4).
Multiple routes of blood perfusion should be used when there is pre-existed organ malperfusion [7]. Organ malperfusion can occur even after the CPB started, particularly at the time of starting the bypass from the femoral arterial cannulation, or onset of left ventricular fibrillation, or aortic cross-clamping. We always try to cannulate into the true lumen of the ascending aorta by the Seldinger’s method and closely monitor the blood pressure on the both upper limbs and bilateral rSO2 on the foreheads to minimize this complication. When the malperfusion syndrome occurs during the CPB, the aortic clamp can be released if the aorta is not opened. Or additional arterial cannulas are inserted into the axillary arteries, in the femoral arteries, in the ascending aorta, or even in the apex pf the left ventricle. Core cooling should be progressed and earlier installation of the ACP started. There was a debate [8] regarding arterial cannulation sites, such as the carotid artery, axillary artery, brachiocephalic artery, ascending aorta, or femoral artery. In the particular cases with brain malperfusion, we would use direct carotid artery perfusion of the affected side as a first choice.
A few successful maneuvers to reduce brain damage have been reported. Some endovascular techniques [9] to relive malperfusion syndrome of the carotid artery system, however, reported that experience are very limited and outcomes of the patient are nor predictable. The IRAD reported [10] that brain injury reversal occurred in 84.3% of patients with cerebrovascular accident and 78.8% of those with coma after simple surgery of central aorta repair. Even spontaneous regression of symptoms has been recognized in many reports [10, 11]. Urbanski et al. [12] first reported in 2006 two cases of acute aortic dissection with bilateral circumferential dissection of the common carotid artery, resulting in a severely narrowed true lumen and clinical signs of cerebral malperfusion. A vascular prosthesis was interposed in the carotid artery to restore the true lumen perfusion and the interposition graft of the right CCA and a femoral artery was cannulated with a bifurcated arterial line. Direct perfusion of the carotid artery is not a new technique. In 1984, Bachet et al. [13] reported six cases of acute type A aortic dissection who underwent aortic arch replacement using direct cannulation in the both carotid arteries as a brain protection. Luehr et al. [14] reported that extra-anatomic aorto-carotid bypass was performed in 23 patients who suffered from cerebral malperfusion due to occlusion of the carotid artery secondary to aortic dissection. They reported that new stroke was found in 34.8% and that hospital mortality was 13.0%. They cannulated or bypassed equally in the right or left carotid arteries but we had a great dominance on the right side.
We [15] reported our first successful case as a simple bypass circuit between the common femoral artery and right common carotid artery performed in the emergency room (Fig. 3). Although she arrived 20 h after onset of aortic dissection, she survived with minimum neurological sequelae (Fig. 4). We selected the amount of flow to the carotid artery and temperature of the perfusate based on our canine experimental data [16]. We found that lower reperfusion flow to the carotid artery after 15 min of global brain ischemia was beneficial in terms of electroencephalographic recovery and brain water contents. We also applied reperfusion flow at 1.8 ml/kg/min, determined according to recovery on near-infrared spectroscopy. Optimal reperfusion flow at tepid blood temperature (30 °C) was set between half and one-third of the normal common carotid artery flow of 4–6 ml/kg/min under normothermia (36–37 °C).
Our indications of direct carotid perfusion in acute type A aortic dissection complicated with brain malperfusion should be for patients who have at least one sign of diminished flow in the carotid arteries, such as dissected carotid arteries with compressed true lumen by CT scan, decreased blood flow in the carotid artery demonstrated by ultrasound, persistent consciousness disorder, hemiparesis, or right to left difference in forehead rSO2 or arm blood pressures. Unfortunately, we lost three patients who had direct carotid perfusion. One died of cardiac problems and two died of reperfusion injury. Appropriate timing of this procedure has not yet been determined, the universal rule, “the sooner, the better”, in the neurological emergency should be applied in these patients. We may try to perfuse directly the carotid arteries when the blood flow was very scarce in the setting of acute aortic dissection. Brain injury, per se, should not contraindicate surgery, especially if patients do not present with signs of neurologic devastation.
Conclusion
Our brain-saving system for patients with brain malperfusion system secondary to acute aortic dissection was considered to be effective.
References
Yasuda K, Ayabe H, Ide H, Uchida Y. Thoracic and cardiovascular surgery in Japan during 1998. Annual report by the Japanese Association for Thoracic Surgery. Committee of Science. Jpn J Thorac Cardiovasc Surg. 2000;48:401–15.
Masuda M, Okumura M, Doki Y, et al. Thoracic and cardiovascular surgery in Japan during 2014: annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg. 2016;64:665–97.
Bonser RS, Ranasinghe AM, Loubani M, et al. Evidence, lack of evidence, controversy, and debate in the provision and performance of the surgery of acute type A aortic dissection. J Am Coll Cardiol. 2011;58:2455–74.
Czerny M, Schoenhoff F, Etz C, et al. The impact of pre-operative malperfusion on outcome in acute type A aortic dissection: results from the GERAADA registry. J Am Coll Cardiol. 2015;65:2628–35.
Okita Y, Takamoto S, Ando M, Morota T, Kawashima Y. Surgical strategies in managing organ malperfusion as a complication of aortic dissection. Eur J Cardio Thorac Surg. 1995;9:242–6 (discussion 7)
Di Eusanio M, Trimarchi S, Patel HJ, et al. Clinical presentation, management, and short-term outcome of patients with type A acute dissection complicated by mesenteric malperfusion: observations from the international registry of acute aortic dissection. J Thorac Cardiovasc Surg. 2013;145:385–90 (e1)
Most H, Reinhard B, Gahl B, et al. Is surgery in acute aortic dissection type A still contraindicated in the presence of preoperative neurological symptoms? Eur J Cardio Thorac Surg. 2015;48:945–50 (discussion 50).
Rylski B, Urbanski PP, Siepe M, et al. Operative techniques in patients with type A dissection complicated by cerebral malperfusion. Eur J Cardio Thorac Surg. 2014;46:156–66.
Casana R, Tolva V, Majnardi AR, et al. Endovascular management of symptomatic cerebral malperfusion due to carotid dissection after type A aortic dissection repair. Vasc Endovasc Surg. 2011;45:641–5.
Di Eusanio M, Patel HJ, Nienaber CA, et al. Patients with type A acute aortic dissection presenting with major brain injury: should we operate on them? J Thorac Cardiovasc Surg. 2013;145:S213–21 (e1).
Geirsson A, Szeto WY, Pochettino A, et al. Significance of malperfusion syndromes prior to contemporary surgical repair for acute type A dissection: outcomes and need for additional revascularizations. Eur J Cardio Thorac Surg. 2007;32:255–62.
Urbanski PP. Carotid artery cannulation in acute aortic dissection with malperfusion. J Thorac Cardiovasc Surg. 2006;131:1398–9.
Bachet J, Goudot B, Dreyfus G, et al. Surgery of acute type A dissection: what have we learned during the past 25 years? Z Kardiol. 2000;89:47–54.
Luehr M, Etz CD, Nozdrzykowski M, et al. Extra-anatomic revascularization for preoperative cerebral malperfusion due to distal carotid artery occlusion in acute type A aortic dissection. Eur J Cardio Thorac Surg. 2016;49:652–8 (discussion 8–9).
Okita Y, Matsumori M, Kano H. Direct reperfusion of the right common carotid artery prior to cardiopulmonary bypass in patients with brain malperfusion complicated with acute aortic dissection. Eur J Cardio Thorac Surg. 2016;49:1282–4.
Munakata H, Okada K, Kano H, et al. Controlled earlier reperfusion for brain ischemia caused by acute type A aortic dissection. Ann Thorac Surg. 2009;87:e27–e8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okita, Y., Ikeno, Y., Yokawa, K. et al. Direct perfusion of the carotid artery in patients with brain malperfusion secondary to acute aortic dissection. Gen Thorac Cardiovasc Surg 67, 161–167 (2019). https://doi.org/10.1007/s11748-017-0873-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-017-0873-y