Abstract
Background
Postoperative fluid management is important after open heart surgery, because cardiopulmonary bypass evokes an abnormal inflammatory response and increases vascular permeability, especially in pediatric patients. We assessed the safety and effectiveness of tolvaptan for management of postoperative fluid retention after congenital heart surgery.
Methods and results
This retrospective study analyzed data from 43 children with uncomplicated congenital heart disease who underwent open heart surgery between September 2013 and August 2016. The patients were divided into two groups. Group N (n = 18; September 2013 through May 2014) received the conventional oral diuretics alone, and Group T (n = 25; June 2014 through August 2016) received a single dose of tolvaptan (0.45 mg/kg) in addition to the conventional oral diuretic therapy. Data were collected, while patients who received intensive care were assessed and compared between groups. Add-on tolvaptan use was associated with increased urinary output standardized by body weight (54.3 ± 4.5 vs 47.3 ± 19.1 mL/kg; p = 0.043), decreased additional intravenous diuretic dose standardized by body weight (0.26 ± 0.23 vs 0.62 ± 0.48 mg/kg; p = 0.001), and a smaller decrease in central venous pressure (1.3 ± 2.7 vs 1.9 ± 3.8 mmHg; p = 0.019). Laboratory analysis showed that electrolyte concentrations in blood and urine did not significantly differ between groups.
Conclusions
Tolvaptan appears to be effective and safe for management of postoperative fluid retention after congenital heart surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Open heart surgery results in a heavy fluid load, because cardiopulmonary bypass (CPB) triggers an abnormal inflammatory response and increases vascular permeability. Appropriate fluid management is required during the early postoperative period, especially in pediatric patients. Diuretics are the mainstay of treatment, and intermittent doses of diuretics and fluids are commonly administered as primary therapy during the early postoperative stage. Loop diuretics are usually the first option for pediatric patients, followed by aldosterone blockers, and human atrial natriuretic peptide.
Tolvaptan (TLV)—a selective oral vasopressin V2-receptor antagonist—has been approved in Japan since 2010 for treatment of heart failure. TLV promotes aquaresis without adverse effects such as low blood pressure, electrolyte abnormalities, renal dysfunction, or increased renin–angiotensin–aldosterone system activity [1]. Recent studies indicate that TLV results in symptomatic improvement in pediatric patients with worsening heart failure and does not cause the above adverse effects [2,3,4]. Although diuretics are routinely used after cardiac surgery, a few studies have examined the effects of TLV on postoperative fluid retention after open heart surgery for pediatric patients. Therefore, this study can assess the safety and effectiveness of TLV for management of postoperative fluid retention after congenital heart surgery.
Methods
Study protocol
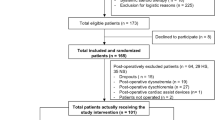
This retrospective study investigated data from patients who underwent congenital heart surgery under CPB. The inclusion criteria were the presence of a simple congenital left-to-right shunt requiring surgical treatment and a body weight greater than 4 kg. Among patients who underwent primary congenital heart surgery at Toho University Omori Medical Center from September 2013 through August 2016, data from 43 patients were analyzed in this study. An institutional ethics committee approved the protocol for the study (protocol number: M16050).
We introduced TLV for postoperative fluid retention after June 2014. Before June 2014, 18 patients (Group N) received furosemide (0.67–1 mg/kg) and spironolactone (0.67 mg/kg by interatrial shunt; 1 mg/kg by interventricular shunt) once per 8 h as the conventional diuretics, which were started after extubation. After June 2014, 25 patients (Group T) received a single dose of TLV (0.45 mg/kg) in combination with the initial administration of the conventional oral diuretics. The common criteria in both groups for additional intravenous administration of furosemide are indicated below: (1) sufficient peripheral perfusion, (2) decreased urine output standardized body weight (<1 ml/kg/h) for couple hours, and (3) stable vital signs. In addition, the doses of administrated furosemide are 1 mg for less than 10 kg, 2 mg for 10–20 kg, and 3 mg for more than 20 kg. The final decision about the administration of inotropic agents and additional diuretic injection was left up to the experienced intensivists. No patient received human atrial natriuretic peptide.
After admission to the intensive care unit (ICU), patient clinical data, including urine output, blood pressure, heart rate, and central venous pressure (CVP), were recorded every hour, and data on laboratory variables including serum electrolyte levels were recorded every 4 h. Cumulative urine output and in–out balance including infusion and water intake were measured at 7 a.m. on postoperative day 1 (POD1).
We analyzed urine output and hemodynamic status, changes in serum electrolyte concentrations and renal function, incidence and dose of additional diuretic administration, and changes in blood pressure, heart rate, and CVP.
Statistical analysis
The data were analyzed using IBM SPSS 20 statistical analysis software (SPSS Inc., Chicago, IL, USA). For continuous variables, descriptive statistics were calculated and reported as mean ± SD. Categorical variables are described using frequency distributions and are presented as frequency (%). The Mann–Whitney U test was used to compare continuous variables, and the Fisher’s exact test was used to compare frequencies between groups. Statistical significance was defined as a p value of <0.05.
Results
Patient enrollment
The background and demographic data of the 43 patients analyzed are shown in Table 1. The mean age of the patients was 27.5 ± 37.8 months (median 12 months, range 2–192 months), and mean body weight was 12.2 ± 9.6 kg (median 9.6 kg, range 4.2–52 kg). All 43 patients received the prescribed doses of furosemide and spironolactone; the mean dose of TLV was 4.69 ± 1.9 mg. Laboratory parameters (hematocrit, serum albumin, urea nitrogen, serum creatinine level, and serum electrolyte concentrations) and preoperative catheter data did not significantly differ between the study groups (Table 1).
Surgery, CPB, and perioperative management
In accordance with our institutional protocol, all patients underwent median sternotomy under normothermic or moderate hypothermic CPB. These operations were all performed by the same surgeon, and anesthetic management was performed by an experienced anesthetist and did not substantially vary. All patients were successfully extubated on the day of surgery and the extubation time after surgery was similar in both groups (3.94 ± 2.73 h in Group N, and 3.52 ± 1.85 h in Group T; p = 0.547). There were no significant differences in intraoperative data and postoperative data (Table 1) (Fig. 1).
Urine output, additional diuretics administered, and hemodynamic status
Cumulative urine output standardized body weight was significantly higher for Group T than for Group N (p = 0.043; Fig. 2a), while the additional intravenous diuretic dose standardized by body weight was significantly lower in Group T (p = 0.001; Fig. 2b). The mean change in cumulative in–out balance including infusion and water intake between admission to the ICU and POD1 did not significantly differ between groups (−46.9 ± 243 mL in Group N and −23.5 ± 194 mL in Group T; p = 0.245). The decrease in CVP between admission to the ICU and POD1 was significantly lower in Group T (p = 0.019; Fig. 2c). Inotropic use in ICU was similar in both groups (16 cases in Group N and 23 cases in Group T; p = 0.876).
Changes in serum sodium and renal function
Mean values for laboratory variables (hematocrit, serum albumin, urea nitrogen, serum creatinine level, and serum electrolytes level) upon ICU admission and POD1 were similar in the two groups. Although urea nitrogen and urinary gravity values increased significantly among the 43 patients (8.6 ± 3.5–16.6 ± 6.1 mg/dL, p = 0.004, and 1.014 ± 0.011–1.025 ± 0.009, p = 0.023, respectively), the changes were not significant in either group. Serum electrolyte concentrations were measured every 4 h in the present patients. Two extreme values for serum sodium concentration were between 131 and 149 mEq/L among patients in Group T. The maximum value was 149 mEq/L; however, the minimum value for the same patient was 145 mEq/L, upon admission to the ICU.
Discussion
TLV is used for treatment of heart failure and symptomatic congestion [5, 6]. Moreover, the current guidelines for management of acute heart failure in Japan recommend TLV for treatment of volume overload and symptoms of right and light left failure [7]. In addition, Regan et al. reported that TLV increased urinary volume in pediatric patients with heart failure [8].
A previous report found that TLV was effective for patients after cardiac surgery [9] and recent reports have confirmed this earlier finding [10,11,12]. For postoperative fluid management, pediatric cardiac surgeons and cardiologists prefer vasodilators, which tend to increase preload and greater fluid administration. We hypothesized that the aquaretic effect of TLV after cardiac surgery would be greater in children than in adults. In June 2014, we introduced TLV for postoperative fluid retention at our center. Before that, only a few case reports had described TLV administration for pediatric patients in Japan. In one such report, TLV was uptitrated from 0.5 to 1 mg/kg/day, in addition to administration of the conventional oral diuretics [2]. In another case report, the increase was from 0.17 to 0.42 mg/kg/day [3]. To our knowledge, this is the first study of TLV use in pediatric patients after congenital heart surgery. TLV use was limited to a single dose, which was administered to patients in the ICU to allow detailed monitoring of dynamics and adverse side effects, especially electrolyte abnormalities. We thus decided on a TLV dose of 0.45 mg/kg under conditions suitable for generous amount of TLV. Short-term TLV treatment improved hemodynamic parameters in patients with chronic heart failure [13]. In the present study, we found that TLV increased urinary volume and limited the decrease in CVP. These findings indicate that TLV acts as an aquaretic drug in the specific phase of increased capillary permeability after cardiovascular surgery.
Chronic use of loop diuretics is associated with worse outcomes in patients with chronic heart failure [14]. However, several reports indicate that TLV is beneficial for renal function. TLV does not reduce renal blood flow or glomerular filtration rate (GFR), even in patients with advanced chronic kidney disease [15]. Administration of TLV to patients undergoing cardiac surgery was reported to improve renal perfusion [16]. Stephan et al. reported that TLV increased urine output and GFR, while furosemide increased urine output but decreased GFR. When TLV was given in combination with furosemide, urine volume increased, and GFR remained stable [17]. In our patients, add-on TLV increased urine output and decreased the need for additional administration of intravenous loop diuretics, without worsening renal function.
Kinugawa et al. reported that 18.7% of adults receiving TLV developed adverse drug reactions [18]. Higashi et al. reported that adverse drug reactions developed in 20.6% of children receiving TLV [4]. Elevation in serum sodium concentration is a serious adverse effect of TLV. However, none of the present patients developed a marked elevation in sodium concentration. None of the present patients was treated with a combination of TLV and sodium-excreting loop diuretics, which can lead to extreme hypernatremia.
This study has several limitations. First, the number of patients was small, and selection bias is a concern in a retrospective, observational study. Second, the period of comparison was short, and it remains uncertain whether a single-dose TLV (0.45 mg/kg) regimen is appropriate.
In conclusion, add-on TLV appears to be safe and effective for managing postoperative fluid retention after congenital heart surgery.
References
Onogawa T, Sakamoto Y, Nakamura S, Nakayama S, Fujiki H, Yamamura Y. Effects of tolvaptan on systemic and renal hemodynamic function in dogs with congestive heart failure. Cardiovasc Drugs Ther. 2011;25(Suppl 1):S67–76.
Murakami T, Horibata Y, Morimoto Y, Tateno S, Kawasoe Y, Niwa K. Syndrome of inappropriate secretion of antidiuretic hormone associated with angiotensin-converting enzyme inhibitor administration. Pediatr Cardiol. 2013;34:1261–3.
Horibata Y, Murakami T, Niwa K. Effect of the oral vasopressin receptor antagonist tolvaptan on congestive cardiac failure in a child with restrictive cardiomyopathy. Cardiol Young. 2014;24:155–7.
Higashi K, Murakami T, Ishikawa Y, Itoi T, Ohuchi H, Kodama Y, et al. Efficacy and safety of tolvaptan for pediatric patients with congestive heart failure. Multicenter survey in the working group of the Japanese Society of PEdiatric Circulation and Hemodynamics (J-SPECH). Int J Cardiol. 2016;205:37–42.
Gheorghiade M, Konstam MA, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA. 2007;297:1332–43.
Decaux G, Soupart A, Vassart G. Non-peptide arginine-vasopressin antagonists: the vaptans. Lancet. 2008;371:1624–32.
JCS Joint Working Group. Guidelines for treatment of acute heart failure (JCS 2011). Circ J. 2013;77:2157–201.
Regen RB, Gonzalez A, Zawodniak K, Leonard D, Quigley R, Barnes AP, et al. Tolvaptan increases serum sodium in pediatric patients with heart failure. Pediatr Cardiol. 2013;34:1463–8.
Nishi H, Toda K, Miyagawa S, Yoshikawa Y, Fukushima S, Kawamura M, et al. Effects of tolvaptan in the early postoperative stage after heart valve surgery: results of the STAR (Study of Tolvaptan for fluid retention AfteR valve surgery) trial. Surg Today. 2015;45:1542–51.
Kido T, Nishi H, Toda K, Ueno T, Kuratani T, Sakaki M, et al. Predictive factors for responders to tolvaptan in fluid management after cardiovascular surgery. Gen Thorac Cardiovasc Surg. 2016;. doi:10.1007/s11748-016-0712-6.
Yamada M, Nishi H, Sekiya N, Horikawa K, Takahashi T, Sawa Y. The efficacy of tolvaptan in the perioperative management of chronic kidney disease patients undergoing open-heart surgery. Surg Today. 2016;. doi:10.1007/s00595-016-1406-5.
Matsuyama K, Koizumi N, Nishibe T, Iwasaki T, Iwahasi T, Toguchi K, et al. Effects of short-term administration of tolvaptan after open heart surgery. Int J Cardiol. 2016;220:192–5.
Watanabe K, Dohi K, Sugimoto T, Yamada T, Sato Y, Ichikawa K, et al. Short-term effects of low-dose tolvaptan on hemodynamic parameters in patients with chronic heart failure. J Cardiol. 2012;60:462–9.
Miura M, Sugimura K, Sakata Y, Miyata S, Tadaki S, Yamauchi T, et al. Prognostic impact of loop diuretics in patients with chronic heart failure—effects of addition of renin–angiotensin–aldosterone system inhibitors and beta-blockers. Circ J. 2016;80:1396–403.
Tominaga N, Kida K, Matsumoto N, Akashi YJ, Miyake F, Kimura K, et al. Safety of add-on tolvaptan in patients with furosemide-resistant congestive heart failure complicated by advanced chronic kidney disease: a sub-analysis of a pharmacokinetics/pharmacodynamics study. Clin Nephrol. 2015;84:29–38.
Kato TS, Ono S, Kajimoto K, Kuwaki K, Yamamoto T, Amano A. Early introduction of tolvaptan after cardiac surgery: a renal sparing strategy in the light of the renal resistive index measured by ultrasound. J Cardiothorac Surg. 2015;10:143-015-0372-0.
Gottlieb SS, Brater DC, Thomas I, Havranek E, Bourge R, Goldman S, et al. BG9719 (CVT-124), an A1 adenosine receptor antagonist, protects against the decline in renal function observed with diuretic therapy. Circulation. 2002;105:1348–53.
Kinugawa K, Sato N, Inomata T, Shimakawa T, Iwatake N, Mizuguchi K. Efficacy and safety of tolvaptan in heart failure patients with volume overload. Circ J. 2014;78:844–52.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose regarding this article.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Katayama, Y., Ozawa, T., Shiono, N. et al. Safety and effectiveness of tolvaptan for fluid management after pediatric cardiovascular surgery. Gen Thorac Cardiovasc Surg 65, 622–626 (2017). https://doi.org/10.1007/s11748-017-0798-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-017-0798-5