Abstract
Background
Acute respiratory failure is a serious issue that occasionally occurs after weaning from cardiopulmonary bypass (CPB) after heart surgery. This condition can be refractory to mechanical ventilation and the mortality rate is high. Venovenous extracorporeal membrane oxygenation (VV-ECMO) is applied to treat acute lung failure after CPB at our institution. This report describes the use of VV-ECMO after cardiac surgery at a single institution.
Methods
We analyzed the outcomes of 11 patients who developed severe acute respiratory failure requiring VV-ECMO after undergoing heart surgery with a cardiopulmonary bypass.
Results
Four (36.4 %) patients died in hospital. One patient required conversion from VV- to venoarterial (VA-) ECMO because of circulatory instability. One patient each died of respiratory failure and heart failure and two died of ischemic colitis. Lung damage secondarily developed in these four patients to other disabled organs. Seven (63.6 %) patients whose lungs were primarily disabled were weaned from VV-ECMO upon recovery from respiratory failure and were ambulatory at the time of discharge from hospital. The ratio of PaO2/FIO2 (P/F) at 24 h after starting VV-ECMO did not significantly differ between survivors and non-survivors (187.9 ± 57.7 vs. 135.5 ± 20.5, p = 0.10), but tended to be higher in survivors. Non-survivors were significantly older than survivors.
Conclusion
Patients who develop severe acute respiratory failure after undergoing heart surgery using cardiopulmonary bypass derive a survival benefit from VV-ECMO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of acute respiratory damage is increasing, with an approximate occurrence of 190,000 per year in the USA [1]. The clinical picture of this condition is mostly an acute respiratory distress syndrome (ARDS), but it might also be related to cardiac disease [2]. The mortality rate of severe acute lung failure that sometimes occurs after cardiopulmonary bypass is high [3], because mechanical ventilation alone cannot maintain the respiratory functions of such patients.
Extracorporeal membrane oxygenation (ECMO) provides powerful circulatory and respiratory support in patients with severe acute cardiac and respiratory failure that is refractory to mechanical ventilation [4]. It also decreases mortality rates among neonates with severe respiratory failure [5, 6] and the findings of other studies of ECMO in respiratory failure have been encouraging [7, 8]. Respiratory support is considered comparable between venovenous (VV) and venoarterial (VA) ECMO [9], but the outcomes of VV-ECMO for patients who develop severe acute respiratory failure after heart surgery are unknown. Therefore, the present study analyzes the value of VV-ECMO to such patients.
Patients and methods
Eleven patients (age, 63.2 ± 17.1; range, 35–83 years; male, n = 8) developed severe acute respiratory failure that required VV-ECMO after heart surgery at the New Tokyo Hospital between December 2009 and September 2011. During this period, 706 heart surgeries were performed at our hospital. We analyzed differences between survivors and non-survivors.
Indication for venovenous ECMO
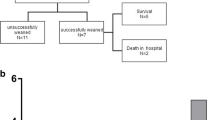
We apply VV-ECMO under the following conditions: PaO2 < 80 mmHg and exacerbation of oxygenation or the appearance of foamy expectoration under maximum mechanical ventilation [FiO2, 1.0; positive end-expiratory pressure (PEEP), 10 mmHg]. Nitric oxide is not used to optimize V/Q matching. The selection of VV or VA ECMO depends on the absence of circulatory collapse (Fig. 1). During this surveyed period, VA-ECMO was performed in 2 patients after surgeries.
Cardiac functions were measured by transesophageal echocardiography. We evaluated whether hemodynamic states could be preserved using inotropes and intra-aortic balloon pumping (IABP) without conversion to VA-ECMO. Table 1 shows hemodynamic parameters [cardiac index (CI; L/min/m2), pulmonary artery pressure (PAP, mmHg), pulmonary capillary wedge pressure (PCWP, mmHg), and central venous pressure (CVP, mmHg)] at the time of starting VV-ECMO in each patient. The CI was maintained at >2.2 in most patients, but was <2.2 in one patient (No. 9) who was converted to VA-ECMO four hours after starting VV-ECMO.
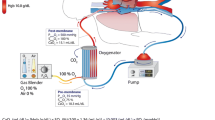
We established VV-ECMO under venous drainage from the femoral vein using a 20–24-Fr catheter. The tip of a long catheter was inserted into the inferior vena cavae and arterialized blood was returned to the right atrium via a 17–20 Fr catheter inserted into the femoral vein on the opposite side, or via a 14 Fr catheter from the right jugular vein (Fig. 2). Pump flow was 2.0–4.0 L/min and adjusted to allow lung protective ventilation and sufficient gas exchange.
Conventional therapies included continuous hemodiafiltration (CHDF), lung recruitment maneuvers, and prone positioning while VV-ECMO maintained the respiratory status of the patients.
Statistical analysis
Continuous variables and categorical variables are expressed as mean ± standard deviation and frequency (%), respectively. The preoperative and operative data of the patients were assessed using a t test for continuous variables and the χ2 test or Fisher’s exact test for categorical variables. All data were statistically analyzed using Dr SPSS II software (SPSS Inc., Chicago, IL, USA). A probability value of < 0.01 was considered statistically significant.
Results
Eleven patients required VV-ECMO. Table 2 shows the background of the patients and Table 3 shows their surgical features and outcomes. Four (36.4 %) patients died in hospital due to uncontrollable respiratory failure (n = 1), ischemic colitis (n = 2), and heart failure related to systolic anterior movement (SAM; n = 1). The latter patient required conversion from VV to VA ECMO because of hemodynamic instability. All seven (63.6 %) remaining patients who recovered from respiratory failure were weaned from VV-ECMO and discharged while ambulatory.
Non-survivors
Patient 1. An 82-year-old man with a history of severe chronic obstructive pulmonary disease (COPD) requiring full medical treatment underwent total arch and descending thoracic aorta replacement, tricuspid annular plasty (TAP), and the Maze procedure.
Patient 6. A 76-year-old man with a history of aortic valve replacement (AVR) was re-admitted with ongoing sepsis and active prosthetic valve endocarditis with an aortic root abscess that communicated with the right atrium. Emergency redo-Bentall procedure and tricuspid valve replacement were performed. This patient required mechanical ventilation, inotropes, and CHDF before surgery.
Patient 8. A 78-year-old woman who underwent aortic valve plasty and CABG developed ischemic colitis on POD3 followed by aspiration pneumonia. Although VV-ECMO considerably improved oxygenation, she did not recover from the ischemic colitis.
Patient 9. An 83-year-old man developed severe acute respiratory failure immediately after complicated triple valve surgery, the Maze procedure and CABG. Because his hemodynamic status was maintained reasonably well, VV-ECMO was applied. However, transesophageal echocardiography revealed worsening SAM that causes hemodynamic instability and he was converted from VV to VA-ECMO.
Patient 1 died 10 days after weaning from VV-ECMO and the others died under VV-ECMO support.
Table 4 compares the characteristics between the survivors and non-survivors. The non-survivors were significantly older (79.8 ± 3.3 vs. 53.7 ± 14.0 years, p < 0.01) and had a significantly higher ejection fraction (67.0 ± 1.8 vs. 46.6 ± 12.9 p = 0.01) than the survivors. The duration of surgery, cardio-pulmonary bypass (CPB), aortic cross clamping (ACC), and ECMO did not significantly differ between the groups (non-survivors vs. survivors: 582 ± 178 vs. 536 ± 126 min, p = 0.63; 325 ± 110 vs. 307 ± 80 min, p = 0.76; 249 ± 83 vs. 235 ± 73 min, p = 0.95; 99 ± 115 vs. 88 ± 33 min, p = 0.81, respectively).
Table 5 and Fig. 3 show changes in PaO2, PaCO2, and PaO2/FiO2 (P/F) ratios. Although PaO2 improved after starting VV-ECMO (from 68.5 ± 11.4 to 180.8 ± 75.1), the values did not significantly differ between non-survivors and survivors (65.7 ± 16.2 to 182.9 ± 103.4 vs. 70.2 ± 8.7 to 179.5 ± 63.7).
Changes in PaO2, PaCO2 and PaO2/FiO2 (P/F) ratios. The P/F ratio did not significantly differ between the groups after introducing venovenous ECMO (non-survivors vs. survivors: 65.7 ± 16.0 vs. 71.2 ± 8.5, p = 0.55). Although the difference did not reach significance, the P/F rate was higher in the survival group at 24 h after starting VV-ECMO (187.9 ± 57.7 vs. 135.5 ± 20.5, p = 0.10)
The P/F ratio also did not significantly differ between the groups after applying VV-ECMO (non-survivors vs. survivors: 71.2 ± 8.5 vs. 65.7 ± 16.0, p = 0.55). Although the difference did not reach significance, the P/F rate was higher in the survivors at 24 h after starting VV-ECMO (187.9 ± 57.7 vs. 135.5 ± 20.5, p = 0.1).
Comment
Mortality rates are very high among patients who undergo heart surgery and develop severe acute lung damage after CPB [3]. Applying VV-ECMO improved the survival of such patients.
Powerful circulatory and respiratory support can be provided by EMCO to patients with severe acute cardiac and respiratory failure that is refractory to mechanical ventilation [4]. The extracorporeal life support organization (ELSO) registry reports survival rates of >80 % in neonates and of 40–50 % in older children who have received ECMO support [10]. Adults with severe respiratory failure have received ECMO since the 1970s. Although two randomized trials did not find any particular benefit of ECMO [11, 12], others have found that ECMO is helpful for adults with severe ARDS [8, 13]. Although the benefit of VV-ECMO for adults remains controversial because convincing results have never been demonstrated in a large patient cohort [14–16], Schmid has demonstrated an overall survival rate of 56 % in 176 patients [17].
Criteria regarding the choice of VV- or VA-ECMO at our institution have not been established. Schmid described general indications for VV-ECMO of partial oxygen pressure (PO2)/fraction of inspired oxygen (FiO2) < 80 mmHg under FiO2 1.0, PEEP 18 cm H2O, and refractory to respiratory acidosis (pH < 7.25) despite optimized conservative therapy [17].
The risk of bleeding after heart surgery by using anticoagulation drug is a serious issue. We use anticoagulation clotting time as an index and maintain it between 180 and 200 s during VV-ECMO and no bleeding re-exposure has occurred. However, deep venous thrombosis in one patient required placement of an inferior vena cava filter.
As for the value of respiratory support, Oshima et al. [15] found a comparable effect of respiratory support between VV- and VA-ECMO. Their study compared patients who received VV- (n = 9) and VA- (n = 7) support and found ECMO removal rates of 50 and 43 %, and hospital discharge rates of 33 and 14 %, respectively (*9).
The present study analyzed the outcomes of 11 patients who received VV-ECMO after weaning from CPB (n = 6) within 3 days after surgery (n = 5). The hemodynamics of these patients was maintained using inotropes and IABP, and VV-ECMO was needed only to support respiratory function. The duration of ECMO did not differ between survivors and non-survivors. Only one (25.0 %) of the four deaths was associated with respiratory failure. The others were ischemic colitis (n = 2) and heart failure related to SAM (n = 1). One patient died after weaning from VV-ECMO and the remaining died under VV-ECMO support. However, lung damage developed secondarily in these four patients after other organs became disabled. To rescue patients under such circumstances is difficult if the primary disabled organ has not fully recovered. On the other hand, the primary disabled organ in all survivors was also the lung. While VV-ECMO maintained respiratory status in these patients, we frequently assessed cardiac function and applied conventional therapies such as CHDF, lung recruitment maneuvers, and prone positioning. All survivors who were weaned from VV-ECMO without conversion to VA-ECMO were discharged while ambulatory.
The non-survivors were significantly older than the survivors (79.8 ± 3.3 vs. 53.7 ± 14.0, p < 0.01). Age is a powerful predictor of mortality although underpowered due to the small study cohort. Schmid indicated that the risk factors affecting survival were advanced age, multiple organ failure assessed by the sequential organ failure assessment score, renal failure, and minute ventilation, whereas sex, body mass index, duration of mechanical ventilation before ECMO, and the degree of impaired gas exchange (hypoxia, hypercapnia) were not significant risk factors for death. [17].
Limitations
Several limitations are associated with this study. This small, retrospective investigation included only 11 patients. An algorithm has not been established for selecting VV or VA-ECMO at the beginning and decisions to use VV-ECMO are made by a senior surgeon. However, supporting our patients using other mechanical devices was not a viable option, and this procedure improved mortality in patients who developed respiratory failure after heart surgery using CPB.
Conclusion
Venovenous ECMO supports survival in patients who develop severe acute respiratory failure after heart surgery with CPB when the primary disabled organ is the lung.
References
Tsushima K, King LS, Aggarwal NR, De Gorordo A, D’Alessio FR, Kubo K. Acute lung injury review. Intern Med. 2009;48(9):621–30.
Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;369(9572):1553–64.
Hammermeister KE, Burchfiel C, Johnson R, Grover FL. Identification of patients at greatest risk for developing major complications at cardiac surgery. Circulation 1990;82(5 Suppl):IV380–9.
Hemmila MR, Napolitano LM. Severe respiratory failure: advanced treatment options. Crit Care Med. 2006;34(9 Suppl):S278–90.
Bartlett RH, Roloff DW, Cornell RG, Andrews AF, Dillon PW, Zwischenberger JB. Extracorporeal circulation in neonatal respiratory failure: a prospective randomized study. Pediatrics. 1985;76(4):479–87.
UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation. UK Collaborative ECMO Trail Group. Lancet 1996;348(9020):75–82.
Lindén V, Palmér K, Reinhard J, Westman R, Ehrén H, Granholm T, Frenckner B. High survival in adult patients with acute respiratory distress syndrome treated by extracorporeal membrane oxygenation, minimal sedation, and pressure supported ventilation. Intensive Care Med. 2000;26(11):1630–7.
Hemmila MR, Rowe SA, Boules TN, Miskulin J, McGillicuddy JW, Schuerer DJ, Haft JW, Swaniker F, Arbabi S, Hirschl RB, Bartlett RH. Extracorporeal life support for severe acute respiratory distress syndrome in adults. Ann Surg. 2004;240(4):595–607.
Oshima K, Kunimoto F, Hinohara H, Ohkawa M, Mita N, Tajima Y, Saito S. Extracorporeal membrane oxygenation for respiratory failure: comparison of venovenous versus venoarterial bypass. Surg Today 2010;40(3):216–22. (Epub 2010 Feb 24).
Bohn D. Pushing the boundaries for the use of ECMO in acute hypoxic respiratory failure. Intensive Care Med. 2005;31(7):896–7.
Zapol WM, Snider MT, Hill JD, Fallat RJ, Bartlett RH, Edmunds LH, Morris AH, Peirce EC 2nd, Thomas AN, Proctor HJ, Drinker PA, Pratt PC, Bagniewski A, Miller RG Jr. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA. 1979;242(20):2193–6.
Morris AH, Wallace CJ, Menlove RL, Clemmer TP, Orme JF Jr, Weaver LK, Dean NC, Thomas F, East TD, Pace NL, Suchyta MR, Beck E, Bombino M, Sittig DF, Böhm S, Hoffmann B, Becks H, Butler S, Pearl J, Rasmusson B. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149(2 Pt 1):295–305.
Oto T, Rosenfeldt F, Rowland M, Pick A, Rabinov M, Preovolos A, Snell G, Williams T, Esmore D. Extracorporeal membrane oxygenation after lung transplantation: evolving technique improves outcomes. Ann Thorac Surg. 2004;78(4):1230–5.
Schmid C, Philipp A, Mueller T, Hilker M. Extracorporeal life support—systems, indications, and limitations. Thorac Cardiovasc Surg. 2009;57(8):449–54.
Kolla S, Awad SS, Rich PB, Schreiner RJ, Hirschl RB, Bartlett RH. Extracorporeal life support for 100 adult patients with severe respiratory failure. Ann Surg. 1997;226(4):544–64.
Anderson HL 3rd, Delius RE, Sinard JM, McCurry KR, Shanley CJ, Chapman RA, Shapiro MB, Rodriguez JL, Bartlett RH. Early experience with adult extracorporeal membrane oxygenation in the modern era. Ann Thorac Surg. 1992;53(4):553–63.
Schmid C, Philipp A, Hilker M, Rupprecht L, Arlt M, Keyser A, Lubnow M, Müller T. Venovenous extracorporeal membrane oxygenation for acute lung failure in adults. J Heart Lung Transplant. 2012;31(1):9–15. (Epub 2011 Sep 1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakamura, H., Yamaguchi, H., Amano, A. et al. Venovenous extracorporeal membrane oxygenation is effective against post-cardiotomy acute respiratory failure in adults. Gen Thorac Cardiovasc Surg 61, 402–408 (2013). https://doi.org/10.1007/s11748-013-0226-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-013-0226-4