Abstract
Brachial artery aneurysm (BAA) following long-standing arteriovenous fistula ligation after renal transplantation is uncommon. Herein, we describe the case of a 64-year-old man who developed a giant symptomatic BAA 21 years after ligation of the fistula. He was submitted to surgical excision of the aneurysm followed by interposition prosthetic graft.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
True aneurysms of the brachial artery are rare and are usually caused by infection, congenital defects, and iatrogenic injuries. Since the pioneering work of Hunter over two centuries ago on arterial aneurysm formation proximal to arteriovenous fistulas (AVF), few cases of brachial artery aneurysm (BAA) after arteriovenous fistula ligation have been described in the literature [1–8]. The aim of this study is to report a case of giant BAA in a patient who had undergone renal transplantation followed by AVF ligation and to review the relevant literature.
Case report
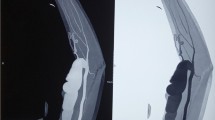
A 64-year-old man came to our attention because of a progressive increase in the volume of his right arm associated with pain and paresthesia in his right hand during the last month. On clinical examination, a large pulsatile mass was observed medial to the bicipital groove. A duplex scan revealed an aneurysm about 8 cm in diameter. The patient had a history of renal failure due to IgA nephropathy and as a result had hemodialysis therapy via a Cimino–Brescia AVF when he was 30 years old. The AVF was ligated after a successful cadaveric renal transplant which he underwent 13 years later. His past medical history included type 2 diabetes, hypertension, chronic obstructive pulmonary disease, cirrhosis due to chronic hepatitis C, and deforming arthrosis of the left hip treated with a hip replacement when he was 54 years old. Ever since he underwent renal transplantation, the patient had been on steroids and immunosuppressive drugs to prevent biocompatibility problems. Because of a history of an allergy to contrast agent that had caused a severe respiratory crisis, the patient underwent a computed tomography (CT) scan without contrast. The images confirmed the presence of a BAA measuring 8.4 × 14 cm (Fig. 1). Endovascular treatment was not indicated because of the patient’s allergy and the large size of the aneurysm, which caused symptoms of nerve compression. The aneurysm was therefore treated surgically.
With the patient under general anesthesia, a longitudinal incision was made in the bicipital groove to expose the brachial artery. The aneurysm was isolated and an incision was made in the aneurysm wall. A large concentric thrombus was removed. Since an autologous vein was not available as prosthetic material, a reinforced polytetrafluoroethylene (PTFE) prosthesis was used to re-establish blood flow in the arm (Fig. 2a, b). A duplex scan performed in the postoperative period showed that the prosthetic graft was patent and that there was no perianastomotic stenosis. Histological examination using hematoxylin and eosin stain of the aneurysm wall showed that it consisted of intimal and medial degeneration with fibrous and inflammatory chronic tissue without signs of infection (Fig. 3). No complications were observed on clinical examination and duplex scanning performed 6 months after surgery.
Discussion
An arterial aneurysm is defined as a focal dilation that increases normal arterial diameter by at least 50 %. The normal diameter of the brachial artery is 3.5–4.3 mm in women and 4.1–4.8 mm in men. In a recent study, Eugster et al. describe various factors that cause a significant increase in the diameter of the brachial artery after closure of an AVF following renal transplantation. The most important of these are an increase in blood flow inside the vessels that causes stress to the vessel walls resulting in the release of endothelial factors like nitric oxide (NO), and an increase in wall resistance caused by ligation of the AVF [9]. Even more recently, it was demonstrated that a further consequence of wall stress is the production of reactive oxygen species (ROS), which play a key role in vessel enlargement caused by an increase in blood flow since ROS lead to an elevated production of superoxide ions (O2 −). These ions combined with NO form peroxynitrates that stimulate the up-regulation of metalloproteinases, which in turn cause the fragmentation of the internal elastic lamina and thus lead to vascular dilation [10]. It was also demonstrated that treatment with steroids and immunosuppressive drugs can damage the muscular layer of the arteries, which leads to a significant increase in the incidence of aneurysms [11]. Our patient had been taking immunosuppressants (cyclosporin 100 mg/day) and steroids (prednisone 5 mg/day) for 20 years. In spite of these hypotheses, the etiology of these aneurysms remains unclear. Large BAA is associated with various symptoms including arm pain due to ischemia, embolism, or a mass effect involving nerve compression. The mass effect can also be a result of compromised arteriovenous exchange which causes edema and thus compression. Allen’s test should be performed on all patients with BAA. Usually, in those with a history of AVF ligation the test shows scarce collateral blood supply from the palmar arcade, and therefore revascularization is required rather than simple ligation of the aneurysm. The result of Allen’s test is confirmed with a duplex scan [6]. A CT scan with contrast is useful for visualizing kinking or coiling of blood vessels, for evaluating distal run-off, and for planning an endovascular treatment [7, 8]. Magnetic resonance imaging (MRI), with or without gadolinium, is also helpful for planning surgery. Both these imaging techniques were contraindicated in our patient because of allergic diathesis and the presence of a metal hip prosthesis. Indications for surgery are based on the characteristics of the aneurysm and the patient’s neurological symptoms. Autologous veins are preferred as prosthetic material. Our review of the literature showed that a PTFE prosthetic graft was successfully used in two patients besides our patient and that was a valid alternative to autologous veins. The data available in the literature regarding clinical characteristics of patients and the prosthetic material used are summarized in Table 1.
Conclusions
The case reported above and a review of the relevant literature have shown that patients who have had hemodialysis via AVF for many years followed by kidney transplantation and AVF ligature are at risk of complications. Long-term immunosuppression and corticosteroid therapy may promote the development of these aneurysms. Careful follow-up with duplex scanning is needed to prevent the formation of giant or symptomatic aneurysms.
References
Hunter W. The history of an aneurysm of the aorta with some remarks on aneurysms in general. Trans Med Obstet Soc Phys. 1757;1:323.
Schunn CD, Sullivan TM. Brachial arteriomegaly and true aneurysmal degeneration: case report and literature review. Vasc Med. 2002;7:25–7.
Nguyen DQA, Ruddle AC, Thompson JF. Late axillo-brachial arterial aneurysm following ligated Brescia-Cimino haemodialysis fistula. Eur J Vasc Endovasc Surg. 2001;22:381–2.
Adachi I, Matsuda H, Ogino H, Ishibashi-Ueda H, Minatoya K, Sasaki H, et al. A case true aneurysm of the brachial artery in a patient with a radio-cephalic fistula and cholesterol apheresis. EJVES Extra. 2005;10:117–9.
Battaglia L, Bucci F, Battaglia M, Reddler A. Late occurrence of a large brachial artery aneurysm following closure of a hemodialysis arteriovenous fistula. Ann Vasc Surg. 2006;20:533–5.
Ventura M, Perilli L, Pisani F, Cucciolillo L, Franceschini E. True aneurysm of the brachial artery in a kidney transplant patient. J Vasc Endovasc Surg. 2006;13:15–9.
Murphy J, Bakran A. Late, acute presentation of a large brachial artery aneurysm following ligation of a Brescia-Cimino arteriovenous fistula. EJVES Extra. 2009;18:73–5.
Basile C, Antonelli M, Libutti P, Teutonico A, Casucci F, Lomonte C. Is there a link between the late occurrence of a brachial artery aneurysm and the ligation of an arteriovenous fistula? Semin Dial. 2011;24:341–2.
Eugster T, Wigger P, Bölter S, Bock A, Hodel K, Stierli P. Brachial artery dilatation after arteriovenous fistulae in patients after renal transplantation. A 10-year follow-up ultrasound scan. J Vasc Surg. 2003;37:564–7.
Castier Y, Lehoux S, Hu Y, Foteinos G, Tedgui A, Xu Q. Characterization of neointima lesions associated with arteriovenous fistulas in a mouse model. Kidney Int. 2006;70:315–20.
Reilly JM, Savage EB, Brophy CM, Tilson MD. Hydrocortisone rapidly induces aortic rupture in a genetically susceptible mouse. Arch Surg. 1990;125:707–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dinoto, E., Bracale, U.M., Vitale, G. et al. Late, giant brachial artery aneurysm following hemodialysis fistula ligation in a renal transplant patient: case report and literature review. Gen Thorac Cardiovasc Surg 60, 768–770 (2012). https://doi.org/10.1007/s11748-012-0075-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-012-0075-6