Abstract
This study investigated the physicochemical properties of ternary mixtures of palm mid-fraction (PMF):refined bleached deodorized palm kernel oil (RBDPKO):refined bleached deodorized palm stearin (RBDPS) for cocoa butter substitute (CBS). Fatty acid constituents, triacylglycerol constituents, solid fat contents (SFCs), melting behavior, polymorphism and crystal morphology were determined using gas chromatography (GC), high-performance liquid chromatography (HPLC), differential scanning calorimetry (DSC), pulsed nuclear magnetic resonance (p-NMR), X-ray diffraction (XRD) and polarized light microscopy (PLM), respectively. Eight blends of various ratios of ternary mixtures were investigated based on the previously studied binary fat mixtures. The composition of palmitic (P) and oleic (O), POP, and crystal morphology (size and shape) of the PMF/RBDPKO/RBDPS [14.9/59.6/25.5 (%w/w)] mixture were comparable to cocoa butter (CB), while its melting profile (18.5 and 37 °C), SFC at 20 °C and polymorphism were different from CB. The iso-solid diagrams of the mixture displayed a monotectic effect at 20–25 °C. Therefore, the 14.9/59.6/25.5 PMF/RBDPKO/RBDPS mixture could be used as a CBS in confectionery fillings because of the crystal morphology and monotectic behaviors comparable to those of CB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cocoa butter (CB) is traditionally used for the formulation of chocolates, coatings, confectionery fillings and other confectionery products. CB is solid at room temperature and melts quickly above 30–32 °C [1]. This behavior produces a stable confectionery product that releases flavors in the mouth at body temperature without any undesirable waxy texture. CB consists of three main triacylglyceols (TAGs): glycerol-1,3-dipalmitate-2-oleate (POP; P = palmitic, O = oleic; 13.6–15.5%), glycerol-1-palmitate-2-oleate-3-stearate (POSt; St = stearic; 33.7–40.5%) and glycerol-1,3-distearate-2-oleate (StOSt, 23.8–31.2%) [1, 2]. These TAGs dominate the melting characteristic and solid fat content as a function of temperature and polymorphic transformations of chocolate, providing chocolate’s textural and sensory properties [3]. CB exhibits complex polymorphic forms, namely γ, α, β′ and β in ascending stability [4]. The stable polymorph (β) is typically preferred in chocolates and coatings because it melts at high melting temperature with small to moderate crystal sizes, allowing for smooth mouth-feel products [5]. The metastable β′ polymorph is desirable for confectionery fillings and compound chocolates because it melts at low temperature with a fine arrangement and a large surface area of solid crystals [5, 6].
CB is expensive compared to other commercial vegetable fats and oils because of its limited supply and high market demand. Therefore, manufacturers are looking for alternatives to CB. CB alternatives (CBAs) can be classified into three groups: CB substitutes (CBSs) derived from lauric fats, CB replacers (CBRs) obtained from hydrogenation of vegetable oils, and CB equivalents (CBEs) derived from polymorphic and non-lauric fats. Among them, CBSs are generally used in confectionery fillings such as truffles, compound chocolates and other confectionery products [5]. CBSs contain high amounts of lauric acid (12:0, 54.6%), followed by myristic acid (14:0, 20.7%), palmitic acid (16:0, 9.2%) and stearic acid (18:0, 8.7%), resulting in short-chain TAGs [7]. CBSs show solid fat content (monotectic behavior) as a function of temperature and crystal morphology (size and shape) similar to those of CB, while the polymorphism, melting profile, TAG composition and fatty acid profile are different from CB [8–10]. In addition, CBSs with a low melting temperature of 21–23 °C are used in confectionery fillings [5].
Palm mid-fraction (PMF) is a fraction of palm oil (Elaeis guineensis) with melting points between 9.8 and 32.8 °C [2]. PMF contains 51.8% of POP among three main TAGs. Most importantly, PMF shows a steep solid fat content versus temperature curve, which is similar to that of CB. Moreover, PMF tends to crystallize in the β′ form and is, therefore, an attractive option for producing confectionery fillings and fat spreads [11].
Refined, bleached and deodorized palm kernel oil (RBDPKO) refers to extract palm kernel oil that has been refined, bleached and deodorized. It is a semi-solid fat at room temperature, with a melting point of approximately 26–28 °C. The oil contains a high percentage of short-chain fatty acids such as lauric (12:0, 47.7%) and myristic acids (14:0, 22.6%) [10]. Although this oil contains high levels of undesirable short-chain fatty acids, it is broadly used as a suitable raw material for the production of confectioneries [12]. In this application, RBDPKO shows melting characteristic and crystallization behavior similar to those of CB, but the two chemical formulations differ considerably. In CB, the constituents of long-chain fatty acids such as palmitic (16:0, 24.4%), stearic (18:0, 33.6%), and oleic acids (18:1, 37.0%) are high, whereas short-chain fatty acids such as lauric acid and myristic acid constituents occur in very low amounts [2]. In RBDPKO, the concentration of short-chain fatty acids is high, whereas the concentration of long-chain fatty acids is relatively low compared to that of CB. Hence, the high short-chain fatty acid content and low long-chain fatty acids (palmitic/oleic) content of RBDPKO makes it unsuitable as a direct CBS. Blending RBDPKO with palmitic and oleic acid-rich fats such as PMF and/or palm stearin, however, makes it possible to decrease the short-chain fatty acid concentration whilst increase the long-chain fatty acid concentration. This process may produce high-quality CBSs with fatty acid compositions more similar to that of CB. RBDPKO is now gaining more interest as a suitable CBS in confectionery products [13].
Refined, bleached and deodorized palm stearin (RBDPS) is obtained by fractionating refined palm oil to separate olein from palm stearin. The physical properties of RBDPS differ from other palm oil products because it is a solid fat with a high melting point (44–56 °C), that needs to be mixed with lower melting point oils such as PMF and RBDPKO to yield a melting profile similar to that of CB. In addition, this oil contains a low amount of linoleic acid (18:2), making it less prone to oxidation [12]. The applications of RBDPS have been well-reviewed, and the stearin has also been reported to be a suitable coating material [14, 15].
In compound chocolates, coatings and fillings, different vegetable fats such as palm kernel oil, palm kernel stearin, palm oil or cocoa butter are commonly modified or blended. The modification of palm kernel oil to CBS is traditionally performed through hydrogenation. This process, however, has always been regarded to produce trans-fatty acids that increase undesirable low-density lipoprotein cholesterol [16]. Therefore, many studies have been performed to produce for CBS-based compound chocolates and coatings by blending different proportions of palm kernel oil, palm kernel stearin, palm oil or cocoa butter [6, 11, 17, 18]. Vereecken et al. studied the crystallization behavior, microstructure and macroscopic properties of the lauric-based and palm-based fats for confectionery fillings [19]. However, no information has been obtained with respect to the melting behavior, polymorphisms and crystal morphology of ternary mixtures of PMF/RBDPKO/RBDPS, which are important in producing CBSs in confectionery fillings.
Iso-solid phase diagrams have been used to illustrate the eutectic and monotectic behavior of binary/ternary fat mixtures because they are useful in understanding the compatibility of mixed fat systems [6]. For instance, the eutectic effect occurs, when the contour lines of constant solid fat content are not straight, with some blend compositions having lower melting temperatures than expected. This indicates that the fat blends are not compatible (eutectic). The monotectic effect (dilution effect) is manifested by a straight line connecting the SFC of each pure component, indicating fats in a blend are mixed well (monotectic) [20].
PMF, RBDPKO and RBDPS oils are less expensive than other vegetable oil products, making them cost-effective ingredients in confectionery fillings and compound chocolates. The present study aims to evaluate the physical and chemical characteristics of ternary fat mixtures of PMF/RBDPKO/RBDPS in terms of their fatty acid profiles, triacylglycerol constituents, melting behavior, solid fat content, polymorphism and crystal morphology to produce better CBSs in confectionery fillings. The ternary fat mixtures were combined based on our preliminary study of binary fat mixtures [21].
Materials and Methods
Materials
PMF, RBDPKO and RBDPS were obtained from Sime Darby Research Sdn. Bhd. CB was purchased from Le Bourne Sdn. Bhd (Selangor, Malaysia). All chemicals and solvents used were of analytical reagent or HPLC grades (Fisher Scientific Comp., USA). Fatty acid methyl ester (FAME) standards and TAG standards were obtained from Lab Science Solution Sdn. Bhd. and Sigma-Aldrich.
Preparation of Fat Blend
Blends (%w/w) of PMF, RBDPKO and RBDPS were mixed in various ratios (Table 1) according to binary fat mixtures in our preliminary study [21]. The blends were melted at 80 °C for 30 min to erase crystal memory. The samples were kept at 25 °C for further analysis.
Example of ternary relation from binary mixtures: PMF:RBDPKO:RBDPS = (PMF:RBDPKO) × (PMF:RBDPS). Where, PMF:RBDPKO = 2:3 (40/60); RBDPKO:RBDPS = 1:1 (50/50); therefore, PMF:RBDPKO:RBDPS = 2:3:3 (25/37.5/37.5).
Fatty Acid Analysis
Fatty acid composition of the investigated fat mixtures was determined in terms of FAME, following the method developed by Saberi et al. [22]. The samples (50 mg) were weighed and dissolved in 1 ml of heptane inside a 1.5-ml centrifuge tube. The mixtures were then added to 50 μl of 1 M sodium methoxide in anhydrous methanol and then mixed vigorously for 1 min by using a vortex mixer. After the sedimentation of sodium glycerolate, 1 μl of the clear supernatant was injected into a gas chromatograph (Perkin Elmer Clarus 500 GC, Waltham, USA) fitted with an elite-FFAP column (30-m length × 0.32-mm i.d. × 0.25-μm film thickness). A flame ionization detector (FID) was used to detect the FAME. The injection and detection temperatures were both at 250 °C. The oven temperature was programmed as follows: heat from 110 to 140 °C (30 °C/min), hold at 140 °C for 1 min, heat from 140 to 240 °C (15 °C/min) and hold for 7 min at 240 °C. The carrier gas (helium) flow rate was 0.9 ml/min. The peaks were identified by comparing retention times with FAME standards and quantified by using a peak area normalization method.
Triacylglycerol (TAG) Analysis
The TAG profiles of the selected samples were analyzed using high-performance liquid chromatography (Agilent HPLC series 1260, CA, USA) according to AOCS Official Method Ce 5b-89. The column used was a ZORBAX C-18 (4.6 × 250 mm, 5 μm, Agilent Technologies, CA, USA) and maintained at 35 °C by a column oven. Isocratic elution was carried out at a flow rate of 1.5 ml/min with a mixture of acetone/acetonitrile (70:30, v/v) as the mobile phase. A refractive index detector (RID 1260 Infinity, CA, USA) was used. The injection volume with an auto-injector was 10 μL of 5% (w/v) oil in acetone. TAG peaks were identified based on the retention time of TAG standards. The percentage of TAGs was determined by using a peak area of the chromatogram.
Solid Fat Content (SFC)
SFC of the selected fats was determined by using pulsed nuclear magnetic resonance (p-NMR) with a Bruker Minispec PC 120 NMR analyzer (Karlsruhe, Germany), according to the AOCS Official Method Cd 16b-93 for stabilizing confectionery fats. The method was also used in previous researches [19, 23, 24]. Samples were melted at 100 °C for 15 min and filled into NMR tubes (10-mm o.d. × 75-mm length, up to 3 cm in height). Samples were tempered at 60 °C for 5 min, followed by 0 °C for 90 min, 26 °C for 40 h, 0 °C for 90 min, and finally kept for 60 min at the desired measuring temperatures of 5, 10, 15, 20, 25, 30, 35, 37, 40, and 45 °C before SFC was measured. The melting profiles were drawn by plotting SFC against temperature. Iso-solid phase diagrams of the fat mixtures were constructed with OriginPro 9.1 software (OriginLab Crop., Northampton, MA, USA) based on SFC values obtained from NMR at 10, 20 and 25 °C.
Melting Behaviour
Melting behavior of the fat mixtures was determined using differential scanning calorimetry (DSC, Pyris 4000 DSC, Perkin-Elmer Ltd., USA). Nitrogen gas was used at a flow rate of 20 ml/min. The instrument was calibrated with indium and n-dodecane. The samples (5–8 mg) were hermetically sealed in an aluminum pan. An empty, covered aluminum pan was used as the reference. The samples were cooled to −50 °C at 10 °C/min, held at −50 °C for 5 min and then heated to 80 °C at 5 °C/min [25].
Polymorphism
The polymorphic forms of the fat crystals were determined at room temperature (24 °C) with a D8 Discover X-ray diffractometer (Bruker, Germany) fitted with Cu-Kα radiation (k = 1.5418 Å, voltage 40 kV and current 40 mA). The samples were analysed at 2θ angles of 10°–30° with a scan rate of 1.5°/min. Short (d) spacing (Å) was determined using the EVA-diffraction software (Bruker, Germany). Assignments of polymorphs were based on the following short spacing characteristics of CB: α form (d = 4.15 Å); β′ forms (d = 3.8–4.3 Å) and β forms (d = 4.5–4.6 Å) [4].
Crystal Morphology
Polarized light microscopy (PLM, Olympus BX51, Tokyo, Japan) equipped with a digital camera (Nikon, DS-Filc, Tokoyo, Japan) at 24 °C was used to observe the crystal network microstructure of individual CB, PMF, RBDPKO, RBDPS and ternary mixtures of PMF/RBDPKO/RBDPS. The method described by Narine and Marangoni [26] was used for the crystallization of fat blends. The sample was melted at 80 °C for 20 min to destroy crystal memory. About 15 μl of melted sample was placed on a glass slide, heated to 80 °C, and covered carefully by a coverslip. The slides were then stored in a temperature-controlled cabinet at 24 ± 1 °C for 48 h to ensure proper crystallization. The liquid phase appears black, while the solid phase appears grey. NIS-Element Imaging Software (Version 4.20, Nikon Instruments Inc. Melville, USA) was used to obtain images.
Statistical Analysis
Data were statistically analysed by one-way analysis of variance (ANOVA) using the OriginPro 9.1 software (OriginLab Crop., Northampton, MA, USA). Tukey’s test was applied to determine the significant differences at a P < 0.05 level. NMR analysis was performed in duplicate. DSC diagrams, XRD and PLM analyses were conducted in triplicate.
Results and Discussion
Fatty Acid Composition
Table 2 shows the fatty acid profiles of ternary mixtures of PMF/RBDPKO/RBDPS. When the RBDPKO decreased in the mixtures, the concentration of short-chain fatty acids (lauric and myristic) significantly (P < 0.05) decreased along with a gradual increase in the concentration of long-chain fatty acids such as palmitic and oleic (Table 2). The fatty acid concentrations in all ternary blends were significantly (P < 0.05) affected by the ratios of the mixtures. Calliauw and co-workers [10] also produced CBSs via two-stage static fraction of palm kernel oil, and reported the fatty acid profiles: lauric (56.3%), myristic (19.6%), palmitic (8.9%) and stearic (2.0%). Zaidul et al. also studied the fatty acids in different blends of supercritical carbon dioxide extracted palm kernel oil fractions and palm oil in various ratios to obtain CB replacers, and they reported that the fatty acid profiles of certain blends were comparable to those of commercial CB [27]. In the present study, the concentrations of long-chain fatty acids such as palmitic, oleic and linoleic with the exception of stearic and short-chain fatty acids in blend (c) were observed to be comparable to those of commercial CB (Table 2).
Triacylglycerol (TAG) Composition
The TAG profiles of the different PMF/RBDPKO/RBDPS mixtures are shown in Table 3. CB contains predominantly monounsaturated TAGs: POP (18.1%), POSt (39.2%) and StOSt (29.7%). PMF contains high concentrations of unsaturated TAGs: POP (50.7%), POO (12.9%), PLP (10.2%; L = linoleic) and POSt (9.1%). RBDPS has high concentrations of both saturated and unsaturated TAGs: PPP (28.4%) and POP (32.1%). RBDPKO is rich in saturated TAGs: LaLaLa (La = lauic), LaLaM (M = myristic) and LaMM at 27.2, 17.0 and 15.1%, respectively. These findings are consistent with the results reported in previous studies [2, 28]. Blending of PMF/RBDPKO/RBDPS shows variations in TAG constituents (Table 3). All eight mixtures of the PMF/RBDPKO/RBDPS blend contained a mixture of SSS (trisaturated), SUS (monounsaturated), SUU (diunsaturated) and UUU (polyunsaturated) TAGs. In the mixtures, the trisaturated SSS TAGs, CLaLa (C = capric), LaLaLa, LaLaM, LaMM and MMM, decreased, whereas the unsaturated SUS TAGs, especially POP and PLP, increased gradually (P < 0.05) with the reduction of RBDPKO. This can be explained by the presence of a decreasing amount of short-chain saturated fatty acids and increasing amounts of both long-chain saturated and unsaturated fatty acids (Table 2). The most remarkable difference between the ternary mixtures and CB was the content of SUS TAGs, especially POSt and StOSt. Sabariah et al. reported that CBS contains a mixture of short-chain fatty acids: lauric, myristic, and long-chain fatty acids: palmitic, stearic with corresponding TAGs [7]. In the present study, the constituent of POP with the exception of POSt and StOSt TAGs in blend (c) was found to be comparable to that of CB.
Melting Behavior
DSC melting curves of ternary mixtures of PMF/RBDPKO/RBDPS are shown in Fig. 1. All ternary mixtures demonstrated two broad endothermic peaks, with T 1 ranging from 17.1–19.7 °C and T 2 ranging from 36.7–37.5 °C. Only small differences in melting temperatures were observed among all the eight blends. These differences are most likely caused by the variation in fatty acid constituents (Table 2) and TAG contents in PMF, RBDPKO and RBDPS (Table 3). When the RBDPS was reduced in mixtures A–H, the first broad endothermic peak (T 1) slowly increased towards a temperature of 19.7 °C and the second peak (T 2) decreased to 36.7 °C. For instance, blend A with 44.4% RBDPS showed its first broad endotherm (T 1) at 17.1 °C and second endotherm (T 2) at 37.5 °C, whereas the first endotherm (T 1) was observed at 19.7 °C and the second endotherm (T 2) was observed at 36.7 °C for mixture H with 11.4% RBDPS. This observation is consistent with a previous report by Jahurul et al. [25], who successfully produced high melting profiles of CB replacers by blending supercritical carbon dioxide-extracted mango seed fat with palm stearin. The authors reported the melting profiles of mango seed fat/palm stearin blends that resemble CB with two endotherms at 17.6 and 36.9 °C. Sonwai et al. also produced CBE by blending mango kernel fat and PMF, and reported two maxima at 22.8 and 36.5 °C [9]. In the current study, blend E of 14.9/59.6/25.5 PMF/RBDPKO/RBDPS contains 25.74% palmitic, 24.51% oleic and 3.95% linoleic acids (Table 2). The melting profile of blend E spanning from 18.5 to 37 °C (Fig. 1) is reasonably different from commercial CB, leading to its potential use as a CBS in confectionery fillings [5, 29].
Solid Fat Content (SFC)
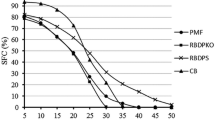
The SFC profiles of the investigated fat samples are shown in Fig. 2. CB showed a high SFC (≥70%) up to 20 °C, followed by a steep decline between 25 and 35 °C and 0% SFC was detected at or above 37 °C (Fig. 2). This finding is in agreement with previous studies by Kadivar et al. [30], who reported there was more than 75% SFC up to 20 °C, followed by a steep decrease between 25 and 35 °C and no solids above the body temperature. It was observed from Fig. 2 that the SFC of all the eight ternary mixtures decreased gradually with increasing temperature. The SFC of the ternary mixtures showed a maximum decline at temperatures ranging from 15 to 25 °C. This behavior was likely caused by the decreased proportion of unsaturated fatty acids (Table 2) and SUS TAGs (Table 3), which melted over this temperature range. The SFCs for mixtures A–E were found to be different from those of mixtures F to H (Fig. 2). In the present study, blend E showed approximately 40% SFC at 20 °C and 30% SFC at 25 °C which are comparatively lower than that of CB (Fig. 2). This was close to a report by Timms [31] who suggested that confectionery fat (e.g., chocolate) should exhibit approximately 63% SFC at 20 °C, 40% SFC at 25 °C and 0% SFC at 37 °C. In another study of Talbot [5], fat with less than 50% SFC at 20 °C is suitable as confectionery fillings. In this regard, blend E could potentially be used as a CBS in confectionery fillings.
Iso-Solid Diagrams of Ternary Blends
Ternary iso-solid phase diagrams of PMF/RBDPKO/RBDPS mixtures at 10, 20 and 25 °C are shown in Fig. 3. The ternary blends showed slightly curvatures at 10 °C, which indicated the eutectic effect (Fig. 3a). This behavior could be caused by the differences in SSS TAGs and SFC at low temperature among PMF, RBDPKO and RBDPS (Fig. 2). As the temperature increased from 10 to 25 °C, the eutectic effect gradually shifted to a monotectic effect. The contour lines of all the 8 mixtures were nearly straight at 25 °C (Fig. 3c), indicating the fats are compatible. In the case of blend E (14.9/59.6/25.5 PMF/RBDPKO/RBDPS), approximately 30% of SFC was observed at 25 °C (Fig. 2), which is within the monotectic area of the iso-solid diagram (Fig. 3c).
Polymorphism
The polymorphic structures of individual PMF, RBDPKO, RBDPS, their ternary mixtures (A–H) and commercial CB were identified by XRD at 24 °C (Fig. 4). CB showed multiple diffraction peaks at d = 3.8–4.3 and 4.5 Å, indicating a mixture of β′ and β polymorphs. Two diffraction peaks at d = 3.8 and 4.3 Å indicate the β′ forms were observed for individual PMF. Similar XRD patterns were found for RBDPS with a major diffraction peak near 4.5 Å, representing the β form. RBDPKO exhibited mainly three diffraction peaks at d = 3.8, 4.1 and 4.3 Å which are characterized by the β′ forms. These findings are in agreement with previous studies [4, 11, 31, 32].
Sato [33] reported that fat with high levels of PPP and SSS TAGs was responsible for β forms and POP/POSt were responsible for β′ forms. RBDPS had a high percentage of PPP while PMF was rich in POP (Table 3), so RBDPS tends to have β crystals whereas PMF tends to have β′ crystals. Timms [31] stated that palm kernel oil tends to crystalize in the stable β′ form due to the presence of a high percentage of LaLaLa TAG. In the present study, it was observed that with decreasing the concentration of RBDPS in the blends A–H resulted in disappearance of the major diffraction peak at d = 4.5 Å (β formation; Fig. 4). This could be explained by the decreased proportion of PPP in RBDPS (Table 3). Blends A to E exhibited diffraction peaks at d = 3.8, 4.2 and 4.3 Å with a major peak at d = 4.5 Å, representing combinations of β′and β polymorphs. For blends F–H, peaks at d = 3.8–4.3 Å (only β′ formation) were observed. Vereecken et al. [19] reported that the β′ form is desired for confectionery fillings because it melts at low temperature with a fine arrangement, whilst the β form gives hardness as well as rough and sandy textures. Blend E with 14.9/59.6/25.5 PMF/RBDPKO/RBDPS showed comparable fatty acid composition to CB, but its melting profile (18.5–37 °C) and 30% SFC at 25 °C with polymorphism are different from CB, making it potentially useful as a CBS in confectionery fillings.
Crystal Morphology
The crystal morphologies of individual PMF, RBDPKO, RBDPS, their ternary blend and CB were observed using PLM at 24 °C (Fig. 5). Commercial CB displayed spherulitic crystals (10–100 μm in diameter) consisting of needle-like crystals branching outward from the central nuclei (Fig. 5a). The microstructure of individual PMF showed continuous granular crystals in the shape of very small spherulites (10–50 μm in diameter) with an orderly packed structure (Fig. 5b). Similar granular crystals (densely packed) were observed for RBDPS with a size of less than 20 μm in diameter (Fig. 5d). Large spherulitic crystals (100–300 μm in diameter) with a tight nucleus were observed in RBDPKO (Fig. 5c). The morphology of RBDPKO was found to be consistent with that observed by Schmelzer et al. [34], who reported large spherulitic crystals.
The crystal network morphologies of all ternary blends were found to be a mixture of tightly packed spherulites and granular structures. With the addition of RBDPKO in the formulations, the spherulitic granular crystals shifted to large needle-like crystals. This variation could be related to the differences in the fatty acid composition (Table 2) and TAG species (Table 3). Another possible reason for the differences in crystalline structure (size and shape) could be the differences in textural properties among PMF, RBDPKO and RBDPS [35]. A blend of 14.9/59.6/25.5 PMF/RBDPKO/RBDPS showed small spherulites (≥50 μm in diameter) consisting of needle-like crystals radiating and branching outward from the central nuclei (Fig. 5e). This behavior was explained by Jahurul et al. who reported spherulites exhibiting needle-like structures when blending mango seed fat and PMF [36]. No drastic change in microstructure in terms of size and shape was observed between CB and the 14.9/59.6/25.5 PMF/RBDPKO/RBDPS blend. However, CB still had densely and orderly packed crystals compared to the ternary blend (Fig. 5).
Conclusion
The crystal morphology, palmitic acid and oleic acid with POP content of blend E (14.9/59.6/25.5 PMF/RBDPKO/RBDPS) closely approximated those of CB, although its melting profile and polymorphism are different from CB. In addition, blend E showed a monotectic behavior at 20–25 °C with less than 50% SFC at 20 °C, making it suitable to be used as a CBS in confectionery fillings.
References
Gunstone F (2011) Vegetable oils in food technology: composition, properties and uses. Wiley, CRC Press, pp 291–343
Bootello MA, Hartel RW, Garcés R, Martínez-Force E, Salas JJ (2012) Evaluation of high oleic-high stearic sunflower hard stearins for cocoa butter equivalent formulation. Food Chem 134(3):1409–1417
Afoakwa EO (2010) Chocolate production and consumption patterns. In: Afoakwa EO (ed) Chocolate science and technology. Wiley & Blackwell, West Sussex, pp 1–10
D’Souza V (1990) Short spacings and polymorphic forms of natural and commercial solid fats: a review. J Am Oil Chem Soc 67(11):835–843
Talbot G (2009) Fats for confectionery coatings and fillings. In: Science and technology of enrobed and filled chocolate, confectionery and bakery products. Woodhead Publishing, Cambridge, UK, pp 53–79
Wang F, Liu Y, Jin Q, Meng Z, Wang X (2011) Characterization of cocoa butter substitutes, milk fat and cocoa butter mixtures. Eur J Lipid Sci Technol 113(9):1145–1151
Sabariah S, Ali AM, Chong C (1998) Chemical and physical characteristics of cocoa butter substitutes, milk fat and Malaysian cocoa butter blends. J Am Oil Chem Soc 75(8):905–910
Lippe M, Anklam E (1998) Review of cocoa butter and alternative fats for use in chocolate—part A. Compos Data Food Chem 62(1):73–97
Talbot G (2006) Application of Fats in Confectionery. Kennedy’s Publications, Loughton, UK, pp 125–137
Calliauw G, Foubert I, De Greyt W, Dijckmans P, Kellens M, Dewettinck K (2005) Production of cocoa butter substitutes via two-stage static fractionation of palm kernel oil. J Am Oil Chem Soc 82:783–789
Sonwai S, Kaphueakngam P, Flood A (2014) Blending of mango kernel fat and palm oil mid-fraction to obtain cocoa butter equivalent. J Food Sci Technol 51(10):2357–2369
Soares FASDM, da Silva RC, da Silva KCG, Lourenço MB, Soares DF, Gioielli LA (2009) Effects of chemical interesterification on physicochemical properties of blends of palm stearin and palm olein. Food Res Int 42(9):1287–1294
Williams SD, Ransom-Painter KL, Hartel RW (1997) Mixtures of palm kernel oil with cocoa butter and milk fat in compound coatings. J Am Oil Chem Soc 74(4):357–366
Kellens M, Gibon V, Hendrix M, De Greyt W (2007) Palm oil fractionation. Eur J of Lipid Sci Technol 109(4):336–349
Lonchampt P, Hartel RW (2004) Fat bloom in chocolate and compound coatings. Eur J Lipid Sci Technol 106(4):241–274
Kummerow FA (2009) The negative effects of hydrogenated trans fats and what to do about them. Atherosclerosis 205:458–465
Wang F, Liu Y, Shan L, Jin Q, Wang X, Li L (2010) Blooming in cocoa butter substitutes based compound chocolate: investigations on composition, morphology and melting behavior. J Am Oil Chem Soc 87(10):1137–1143
Pease JJ (1985) Confectionery fats from palm oil and lauric oil. J Am Oil Chem Soc 62(2):426–430
Vereecken J, Foubert I, Smith KW, Dewettinck K (2007) Relationship between crystallization behavior, microstructure, and macroscopic properties in trans-containing and trans-free filling fats and fillings. J Agric Food Chemistry 55(19):7793–7801
Timms R (1984) Phase behaviour of fats and their mixtures. Prog Lipid Res 23(1):1–38
Biswas N, Cheow Y, Tan C, Siow L (2016) Blending of palm mid-fraction. J Am Oil Chem Soc 93:1415–1427
Saberi AH, Lai O-M, Toro-Vázquez JF (2011) Crystallization kinetics of palm oil in blends with palm-based diacylglycerol. Food Res Int 44(1):425–435
Osborn H, Akoh C (2002) Enzymatically modified beef tallow as a substitute for cocoa butter. J Food Sci 67:2480–2485
NorAini I, Embong M, Aminah A, Maimon C (1995) Physical characteristics of shortenings based on modified palm oil, milkfat and low melting milkfat fraction. Lipid/Fett 97:253–260
Jahurul M, Zaidul I, Norulaini NN, Sahena F, Abedin M, Mohamed A, Omar AM (2014) Hard cocoa butter replacers from mango seed fat and palm stearin. Food Chem 154:323–329
Narine SS, Marangoni AG (1999) The difference between cocoa butter and Salatrim® lies in the microstructure of the fat crystal network. J Am Oil Chem Soc 76(1):7–13
Zaidul I, Norulaini NN, Omar AM, Smith R (2007) Blending of supercritical carbon dioxide (SC-CO 2) extracted palm kernel oil fractions and palm oil to obtain cocoa butter replacers. J Food Eng 78(4):1397–1409
Tan C, Man YC (2002) Differential scanning calorimetric analysis of palm oil, palm oil based products and coconut oil: effects of scanning rate variation. Food Chem 76(1):89–102
Rodríguez A, Castro E, Salinas MC, López R, Miranda M (2001) Interesterification of tallow and sunflower oil. J Am Oil Chem Soc 78(4):431–436
Kadivar S, De Clercq N, Mokbul M, Dewettinck K (2016) Influence of enzymatically produced sunflower oil based cocoa butter equivalents on the phase behavior of cocoa butter and quality of dark chocolate. LWT-Food Sci Technol 66:48–55
Timms RE (2003) Production and characteristic properties. In: Timms RE (ed) Confectionery fats handbook: properties, production and application. The Oily Press, Bridwater, pp 191–254
Zhou SL, Zhang FQ, Jin QZ, Liu YF, Shan L, Zhang T, Zou XQ, Wang XG (2010) Characterization of palm kernel oil, palm stearin, and palm olein blends in isosolid diagrams. Eur J Lipid Sci Technol 112(9):1041–1047
Sato K (2001) Molecular aspects in fat polymorphism. AOCS Press, Champaign, pp 1–15
Schmelzer JM, Hartel R (2001) Interactions of milk fat and milk fat fractions with confectionery fats. J Dairy Sci 84(2):332–344
Çiftçi ON, Fadıloğlu S, Göğüş F (2009) Conversion of olive pomace oil to cocoa butter-like fat in a packed-bed enzyme reactor. Biores Technol 100(1):324–329
Jahurul M, Zaidul I, Norulaini N, Sahena F, Abedin M, Ghafoor K, Omar AM (2014) Characterization of crystallization and melting profiles of blends of mango seed fat and palm oil mid-fraction as cocoa butter replacers using differential scanning calorimetry and pulse nuclear magnetic resonance. Food Res Int 55:103–109
Acknowledgements
The authors would like to thank the School of Science for supporting the current study and are grateful for grant number FRGS/1/2013/SG01/MUSM/03/2. The authors are also grateful for the SFC determinations conducted by the Malaysia Palm Oil Board, and Mr. Nasrun from the School of Engineering for XRD technical support.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Biswas, N., Cheow, Y.L., Tan, C.P. et al. Cocoa Butter Substitute (CBS) Produced from Palm Mid-fraction/Palm Kernel Oil/Palm Stearin for Confectionery Fillings. J Am Oil Chem Soc 94, 235–245 (2017). https://doi.org/10.1007/s11746-016-2940-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-016-2940-4