Abstract
The aim of this study was to determine the differences between organic and conventional grape seed oils extracted from different grapes (Bordô and Isabel). The physicochemical quality, bioactive compounds and oxidative stability of the oils were investigated. The organic samples exhibited the best color parameters, and all samples were within the limits established by the Codex Alimentarius regarding their quality parameters. Only Bordô grape seed oils presented lutein and the best results regarding α- and β-carotene and α-tocopherol contents. All samples exhibited the same antioxidant activity results, but the Bordô ones exhibited higher oxidative stability. Overall, the results from this study suggest no differences between organic and conventional grape seed oils but between the grape varieties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Grape seed is an important part of the pomace, representing 38–52 % of the dry matter thereof [1], which makes it a significant residue of agribusiness juices and wines [2]. Grape seed may contain 7–20 % oil [3]; thus, its extraction may represent a good option for adding value to a new product.
In addition to the basic function of providing nutrition, vegetable oils contribute to the palatability of food, act as a vehicle for fat-soluble vitamins and are sources of essential fatty acids such as linoleic, linolenic and arachidonic acids [4].
Virgin oils contain bioactive compounds, including carotenoids, tocopherols and phenolic compounds of numerous low and high molecular weights, that may offer beneficial effects to human health [5]. These compounds have been of great interest to the food and pharmaceutical industries due to their anti-inflammatory, anticarcinogenic and antimutagenic effects as well as their association with a decreased risk of cardiovascular disease [6].
Some carotenoids are valued for their pro-vitamin A activity (β-carotene) or their protection against age-related macular degeneration (lutein), and numerous studies have been performed to evaluate these compounds in foods [7]. These carotenoids have also been related to important functional properties, especially antioxidant activity, and have been implicated in preventing cardiovascular disease. These properties render the compounds ideal for promoting the consumption of natural products containing them [8].
Tocopherols are important inhibitors of lipid oxidation in food and biological systems [9]. Each type of oil exhibits a characteristic tocopherol content, which also depends on plant genotype, climatic conditions and crop growth, content of polyunsaturated fatty acids and processing and storage conditions [10]. In addition to exhibiting vitamin E activity, tocopherols occur in seed oils in different forms: α, β, γ and δ-tocopherol, where γ-tocopherol is reported as one of the most highly antioxidant forms [11]. Because of their fat-soluble antioxidant properties, these compounds inhibit the peroxidation processes of polyunsaturated fatty acids and other compounds that affect the cell membrane [12], as well as prevent the rancidity of oils during storage [13].
An increasingly large portion of the population prefers to consume products of organic farming, mainly due to the absence of contaminants in the production process [14]. The number of farms dedicated to organic agriculture has increased worldwide. Consequently, the market is shifting toward the consumption of organic products, and numerous studies have been conducted in this area; however, little attention has been paid to the quality of these food products [15]. Many studies have reported that organic foods provide higher nutritional value, exhibiting a lower nitrate content and better organoleptic quality [16].
The aim of this study was to evaluate the quality of seed oils of organic and conventional grapes (Vitis labrusca cv. Bordô and Isabel) for the identification and quantification of bioactive compounds and to determine the oils’ physicochemical characteristics and oxidative stability.
Materials and Methods
Raw Material
The processed residual material (pomace) of the production of Bordô and Isabel (organic and conventional, respectively) grape juices (Vitis labrusca, vintage 2013) was dried in a horizontal rotary dryer at 70 °C for 4 h until the moisture content was approximately 7 %, avoiding rancidity. A ventilation process was used to separate the seeds from the rest of the waste, and grape seed oil extraction was performed by cold extraction using a worm-type extractor composed of plated carbon steel. The temperature was controlled such that it did not exceed 50 ± 1 °C, preserving the quality of the oil obtained as well as allowing for the greatest possible amount of bioactive compounds to be obtained. The oils were transported to the Laboratory of Bioactive Compounds of the Federal University of Rio Grande do Sul (UFRGS) and were stored in amber vials at room temperature (25 ± 1 °C). The oils were decanted for 72 h to remove the residual sludge formed naturally in crude oils. After this period, the oils were refrigerated (5 ± 1 °C) until analysis. The equipment used in obtaining the raw material stage were designed and built by Econatura Company. The following oils were obtained for analysis: Bordô Conventional Oil (BCO); Bordô Organic Oil (BOO); Isabel Conventional Oil (ICO) and Isabel Organic Oil (IOO).
Reagents and Standards
The chromatographic analyses were performed using solvents of HPLC grade: methanol, methyl tert-butyl and acetonitrile (Panreac). The standards used for the construction of calibration curves were: (±)-α-tocopherol (99.5 % HPLC, SULPECO), (+)-γ-tocopherol (≥96 % HPLC, Sigma-Aldrich). The carotenoids α-carotene (purity >93 %), β-carotene (purity >95 %) and zeaxanthin (purity >95 %) were purchased from Sigma Chemical (USA). Lutein (purity >95 %) was purchased from Indofine Chemical Company Inc. Hillsborough (USA). All the analysis were performed in triplicate.
Chromatograph
The HPLC analysis was performed with an Agilent 1100 Series HPLC system equipped with a quaternary solvent pumping system (G1311A – DE14917573 Agilent 1100 Series, Waldbronn, Germany) and a UV/Vis detector (G1314B – DE71358944 Agilent 1100 Series, Waldbronn, Germany).
Color Analysis
Color analysis was performed via a MINOLTA CR 310 187 colorimeter using the color parameters L* a* b*, Illuminant D65 and a factor observer angle of 10°.
Physicochemical Quality Analyses
The refraction and acidity indices of the oils were determine according to the methodology of the Instituto Adolfo Lutz [17]. The peroxide value and unsaponifiable matter were determined by the Cd 8-53 and Ca 6a-40 methods according to AOCS [18], respectively.
Carotenoids
The carotenoid extract was prepared according to the method described by Mercadante and Rodriguez-Amaya [19]. A 250 mm × 4.6 mm i.d., 3 μm, C30 reversed phase polymeric column was used (YMC, Japan) in HPLC and the wavelength was adjusted to 450 nm. The mobile phase used was water:methanol:tert-methyl butyl ether (MTBE) (J.T. Baker – Mallinckrodt, EUA) starting at a ratio of 5:90:5 and reaching 0:95:5 in 12 min, 0:89:11 in 25 min, 0:75:25 in 40 min and 0:50:50 after a total of 60 min, with a flow rate of 1 mL min−1 and a injection volume of 5 µL at 33 °C [20]. The carotenoids were quantified using standard curves of lutein (1–65 µg mL−1), β-carotene (5–50 µg mL−1) and α-carotene (2–25 µg mL−1). The limits of detection (LD) and quantification (LQ) were, respectively, 6.9 × 10−3 and 1.15 × 10−2 mg kg−1 for lutein; 4.46 × 10−2 and 7.43 × 10−2 mg kg−1 for β-carotene, and 1.97 × 10−2 and 3.28.10−2 mg kg−1 for α-carotene. The results were expressed in micrograms per 100 g of sample.
α and γ-Tocopherol
The oil tocopherol extraction of was carried out with methanol at room temperature. Five grams of seeds and 25 mL of methanol were placed in ultrasonic bath for 15 min. The methanolic portion were separated by centrifugation. This step was repeated and the supernatants were combined and injected on HPLC. For chromatographic separation, a polymeric Vydac C18 column (218TP54) (250 mm × 4.6 mm id) containing 5 mM methanol was used at a wavelength of 292 nm. The analysis was performed in isocratic mode, and a methanol/water solution (96:4, v/v) was used as the mobile phase at a flow rate of 1 mL min−1 for 10 min. The column temperature was maintained at 30 °C, and an injection volume of 10 μL was used. For the quantification of α-tocopherol, an external standard method involving the construction of a calibration curve determined by individual diluted solutions of α-tocopherol in methanol (from 2 to 303 mg L−1) was employed. The limits of detection and quantification were, respectively, 0.09 and 0.17 mg kg−1. The quantification of γ-tocopherol was carried out in the same way but with individual solutions of γ-tocopherol diluted in methanol (from 7062 to 12,600 mg L−1). The limits of detection and quantification were, respectively, 0.10 and 0.18 mg kg−1. The results were expressed in micrograms per 100 g of sample.
Phenolic Compounds and Antioxidant Activity
The determination of the total phenolic compounds of the grape seed oils was performed according to the method used by Capannesi et al. [21]. Quantification was based on a calibration curve using gallic acid dissolved in methanol as a standard. The results were expressed in mg gallic acid g oil−1. The TRAP was measured and calculated as previously described by Dresch et al. [22] with a methanolic extract diluted with water and DMSO. The results were converted to percentile ranks, and the area under the curve (AUC) was calculated by utilizing the GraphPad software program (San Diego, CA, USA). The smaller the AUC is (relative to that of the system), the higher the total reactive antioxidant potential of a sample becomes.
Oxidative Stability Index
Three grams of grape seed oil was used placed in a Rancimat 743 (Metrohm AG, Switzerland) instrument to determine the oxidative stability of the samples. The oxidation process was assessed by response surface methodology (RSM). A full-rotational 22 experimental design with 4 axial points and 3 central points was used. The variables studied were temperature (T from 50 to 210 °C) and flow rate (F from 8 to 22 L h−1). At each time point, eight oil samples were analyzed simultaneously by the equipment. Each sample was analyzed in duplicate. Statistical analysis was performed using the software Statistica 12.0 (Statisoft Inc.), allowing for the evaluation of the experimental data fitting to mathematical models obtained by the F test and the determination of the response surface as a function of the variables.
Results and Discussion
Color Analysis
Table 1 shows the results of the colorimetric analysis and shows that all samples presented color angles (h) smaller than 90°, suggesting a tendency toward yellow and brown colors.
IOO showed high rates for L, b*, C and h as the lightest sample (high L*); although more saturated (higher C*), on the other hand, ICO showed lower levels for a, b*, C* and h. With respect to BCO, BOO also showed more significant results, suggesting that the organic samples exhibited more favorable color parameters.
The color measurement of a sample is of great importance to food production due to the relationship between sensory attributes and the acceptability of a product for consumers [23]. Lee et al. [24] observed values similar to those obtained in this work for parameters L* and b* in studying ginseng oils, and the extraction method (pressing, solvent or supercritical fluid) did not affect these characteristics of the samples.
Investigating eight varietal cultivars of conventional Spanish olive oils, Moyano et al. [25] observed values ranging from 61.94 to 99.28 for L*, from −14.96 to 9.96 for a*, from 11.98 to 128.68 to b*; 12.20 to 128.96 for C* and from 85.03 to 100.84 to h*. Although similar to those explored in this study, the cultivars studied by Moyano et al. [25] exhibited very different values for the same parameter, indicating a tendency toward lighter and more saturated samples with less reddish colors.
The color parameters of plant products can provide indications of quality and can be determined in terms of sensory and physicochemical analyses. The values of the parameter a* may decrease during storage, tending toward green (−a*), whereas b* values may decrease at temperatures near 20 °C, showing a greater tendency toward yellow (+b*). The possible variations of these parameters can be related to the reactions of lipid oxidation and decomposition of antioxidants, which are common during the storage of oils [26].
Physicochemical Quality Analyses
The results of physicochemical analyses of the conventional and organic grape seed oils Bordô and Isabel are shown in Table 2.
The physicochemical properties of the oils are related to preservation and quality by parameters such as acidity and peroxide values, which depend on the nature and quality of the raw material. The results indicate the level of conservation of the oils considering effects of deterioration by light, temperature and oxygen, which accelerate the decomposition of glycerides, the development of rancidity and the release of fatty acids [26].
IOO showed the lowest and therefore best acidity index among the analyzed samples, followed by ICO, BCO and BOO.
With respect to the refractive index, all oils showed equal values within the limits established by the Codex Stan (1.467–1.477).
The peroxide value is one of the main parameters in the quality analysis of oils because it indicates the oxidation state of a sample. BCO and BOO showed the best results. During auto-oxidation, the peroxide value can reach its maximum, decrease during later stages and varying according to the fatty acid composition of an oil and the oxidation conditions [27].
The oils did not differ significantly in the analysis of unsaponifiable matter, all being within the standard set of values indicated by Codex Stan (<20 g kg−1). The saponification reaction can help establish the degree of deterioration and stability of oils, verify that the properties of the oils are in accordance with specifications and identify potential fraud and adulteration [28]. Waxes, sterols and hydrocarbons in oils are generally determined as unsaponifiable matter. Water contributes to the hydrolysis of oils during the various handling and processing steps that generate products such as free fatty acids and glycerol [27].
Carotenoids
BCO and BOO showed values that were statistically equivalent to the content of lutein. The presence of lutein was not detected in ICO or IOO (Table 3).
BCO presented the most significant contents of α- and β-carotene, followed by ICO and IOO, which showed no significant difference in content.
β-Carotene is particularly interesting due to its pro-vitamin A structure of, providing 100 % activity [29]. Lutein is an important component of the human retina and is associated with a reduction in age-related macular degeneration [30]. These properties, combined with an attractive color, have led to the increased use and demand for β-carotene, lutein and zeaxanthin in particular as supplements and food additives [31].
Quantifying chlorophylls and carotenoids in varietal conventional olive oil of two consecutive harvests, Criado et al. [32] state that during the extraction of oil, mass transfer phenomena occur and determine the distribution of pigment between the olive solid (pomace) and liquid phase (oil) and wastewater. The lipophilic nature of chloroplasts can determine their affinity for the oil phase, and the more hydrophilic nature of anthocyanins determines their retention in pomace and wastewater. Thus, the chloroplast pigments (chlorophyll and carotenoids) are primarily responsible for the color of virgin olive oil, which ranges from yellow to green.
The ingestion of oils and fats is known to increase the bioavailability of lipophilic vitamins. Many studies have clearly demonstrated that incorporating these substances into the diet increases the bioavailability of plant-derived carotenoids in human subjects [33]. The effects of oils and fats in the diet are manifested by the dispersion of carotenoids in the digestive tract or the indirect promotion of pancreatic juice secretion. Moreover, the secretion of chylomicrons promoted by oils and fats facilitate the transfer of lymph micellar carotenoids. The effects of free oils and fats, unsaponifiable matter and other classes of lipids on bioavailability fatty acids have not been fully elucidated [34].
The red palm oil obtained from the fruit of palm (Elaeis guineensis) also has a high content of carotenoids and is one of the richest natural sources of β-carotene, containing approximately 550 μg g−1 of total carotenes and 375 μg g−1 of β-carotene [35].
Investigating carotenoids, polyphenols and antioxidant activity in organic and conventional grapes [36] reported more significant levels of lutein in organic grapes compared to those observed in conventionally cultivated grapes; however, the latter showed more favorable results regarding β-carotene content. Despite studying six different cultivars, the authors noted that it was not possible to discern any specific trend in the results obtained for the samples, i.e., a cultivar may have higher levels of a carotenoid under organic farming, but the opposite behavior for another carotenoid may occur within the same farming unit.
Studying the effects of type of cultivation in carotenoids, antioxidant activity and vitamin C in organic and conventional Brazilian fruits, Cardoso et al. [37] reported statistically equivalent contents of lycopene and β-carotene between organic and conventional khaki crops. In acerola fruit, conventional cultivation showed more significant results for levels of β-carotene. In strawberry fruits, organic farming did not differ significantly from conventional cultivation with respect to the content of β-carotene. According to the authors, organic farming has environmental and social impacts related to the health of workers and consumers, increasing socioeconomic viability when compared to conventional farming, but does not necessarily imply a better nutritional value of food.
α and γ-Tocopherol
Regarding the content of α-tocopherol, BOO showed the highest level, followed by ICO and IOO (Table 3). The oils analyzed did not differ significantly with respect to the content of γ-tocopherol.
Investigating the extraction of grape seed oil with conventional pressurized liquid, Freitas et al. [13] reported less than 1 mg of α-tocopherol in 100 g of oil in Isabel grapes. The authors stated that Isabel, Herbemont and Seibel grape cultivars are unsatisfactory for producing quality wine but are widely consumed fresh in Brazil and are therefore used for the production of juices.
Studying 10 different conventional varieties of grape seed oils from Portugal, Fernandes et al. [2] reported values above those observed in this study: 85.5–244 mg kg−1 of oil for α-tocopherol and 2.50–45.0 mg kg−1 of oil for γ-tocopherol. It is noteworthy that Vitis vinifera grapes were used, which seeds may contain more bioactive compounds by having undergone fermentation in the wine making process. Furthermore, these authors used an extraction method and separations quite different as proposed in this paper (hexane).
Analyzing seven conventional flaxseed oils marketed in New Zealand, Choo et al. [27] observed very significant levels of tocopherols. The amounts of α-tocopherol varied from 9.11 mg kg−1 of oil and those of γ-tocopherol varied from 10.56 to 15.0 mg kg−1 of oil.
Studying quality parameters of organic and conventional extra virgin olive oils for three consecutive years, Ninfali et al. [38] reported that it was not possible to observe a trend in the results obtained between organic and conventional crops with respect to tocopherol content, for example. The authors state that the differences observed in 1 year are not guaranteed to occur in the coming years. Moreover, they suggest that differences between the cultures could not be observed or proved inconsistent over the analysis period due to factors such as cultivar type and conditions and storage time.
Phenolic Compounds and Antioxidant Activity
IOO, BCO and BOO showed no significant differences in the content of total polyphenols (Table 3).
Organic farming is generally characterized by the absence of pesticides and synthetic fertilizers during the cultivation period. The literature suggests that organic agriculture could result in foods with high levels of polyphenols, mainly due to two reasons. The first is that the use of synthetic fertilizers could provide more bioavailable nitrogen sources, accelerating plant growth and thereby leading to a reduction in resources used in the production of secondary metabolites. The second reason is the absence of synthetic pesticides that could result in greater exposure of the plant to stress, leading to a natural increase in the amount of defense substances such as phenolic compounds [16]. Both cases result in foods with high antioxidant capacity as a result of a higher amount of polyphenols. The literature reports mixed results regarding the antioxidant activity and phytochemicals of organic and conventional vegetable composition, which vary according to the bioactive compounds and the type of food analyze d [39].
Bail et al. [5] studied conventional grape seed oils (Welschriesling, Chardonnay, Schilcher, Merlot, Cabernet-Sauvignon, Zweigelt and unknown cultivars) derived from wine production, reporting total polyphenol contents that ranged from 69.5 to 115.5 μg g of oil−1. These oils were obtained by cold pressing, and the highest content was observed in red grapes.
Characterizing grape seed oils obtained by the cold pressing of different conventional cultivars of Turkey (IFTA, Mazruna, Black Kerküş, Zeyti, Verdani, Karfoki, and Kerküş), Demirtas et al. [40] reported phenolic compound contents ranging from 2.19 to 4.70 mg GAE 100 g of oil−1.
Lutterodt et al. [41] studied the antioxidant properties of oils of different cultivars of conventional grapes from the wine industry (Chardonnay, Muscadine, Ruby Red and Concord) and reported phenolic compound contents between 0.16 and 0.80 mg GAE g of oil−1. The authors attribute the low values to the low solubility of low-molecular-weight polyphenols in the oil.
Comparing conventional and organic peaches and pears, Carbonaro et al. [42] observed a higher content of polyphenols in both organic fruits. The authors suggest that changes occur in the metabolism of polyphenols as a result of the practice of organic farming but reported that the total content of polyphenols, as well as the proportion of several food components, are subject to variations according to the variety and species of plants studied.
Investigating the phenolic compound content and antioxidant activity of organic and conventional grape juices, Bordô and Niagara, Dani et al. [43] observed higher levels of polyphenols in organic juices. The authors reported that the choice of method of organic farming resulted in different levels of resveratrol, anthocyanins and tannins in the juices.
Antioxidant compounds suppress oxidation reactions and quench light emission; therefore, it is possible for chemiluminescence to be applied during the analysis of antioxidant activity. The extracts of the oils analyzed were able to reduce chemiluminescence, i.e., the extracts showed antioxidant activity, but the activities were not significantly different (Fig. 1). The measurement of chemiluminescence in the gradual extinction of the consumption of antioxidants depends on the reactivity of a substance toward the concentration of free radicals [44].
According to Desmarchelier et al. [45], the ability of antioxidant compounds to scavenge free radicals relative to that of synthetic standards, e.g., Trolox, allows for the determination of the antioxidant activity of substances such as plant extracts.
The antioxidant content of a food is an important parameter with respect to the relationship between nutrition and human health, in addition to affecting the shelf life of a product [42].
The harmful effects caused by oxidative stress may be delayed or reversed by increased levels of antioxidants, particularly phytochemicals such as polyphenols [46]. Therefore, many studies have suggested an inverse relationship between the consumption of foods rich in polyphenols and the risk of degenerative diseases, cancers and cardiovascular diseases [47].
Faller and Fialho [39] studying the relationship between phenolics and antioxidant activity of six vegetables and six different organic and conventional fruits, reported that it was not possible to discern a trend for the types of cultivation studied. The authors observed that in this food, the high content of soluble polyphenols observed in onions did not manifest as being high on antioxidant activity. Furthermore, the authors suggest that other components can be attributed to antioxidant capacity, such as vitamin C, carotenoids and glucosinolates.
Oxidative Stability Index (OSI)
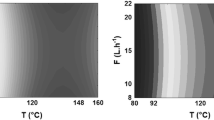
The results of the experimental design for the OSI are shown in Table 4 and Fig. 2.
The models were tested by analysis of variance (ANOVA) for a confidence level of 95 % (P < 0.05), yielding F values of 58.57 and 60.45 for BCO and BOO, respectively. For ICO and IOO, the calculated F values were 52.83 and 51.61, respectively. OSI is highly dependent on the linear and quadratic effects of temperature for both oils. The quadratic models adequately predict the influence of the experimental factors studied, with an R 2 value of 0.9482 for BCO, 0.9497 for BOO, 0.9429 for ICO and 0.9416 for IOO.
The experimental data were fitted to a full quadratic model. Polynomials fitted to the surface responses of BCO, BOO, ICO and IOO (Eqs. 1, 2, 3, and 4, respectively) were calculated by multiple regression:
OSI decreased with increasing temperature, and air flow showed a linear effect on the stability index. BCO and BOO did not differ significantly at a temperature of 50 °C and air flow of 15 L h−1, yielding the same OSI of 95 h. At 73 °C and 10 L h−1, the samples showed no significant differences. Under more extreme conditions, i.e., higher heat (73 °C) and air flow (20 L h−1), BOO may be considered more stable than BCO, obtaining an OSI of 86.45 h. In other experiments, BOO showed the same behavior as BCO, with no significant difference. Thus, it was observed that BCO and BOO retained the same trend of decreasing OSI. At 73 °C, an increase of 10 L h−1 in airflow caused a drastic reduction in the OSI of BCO and BOO, from 77.12 to 19.25 h and 86.45 to 20.52 h, respectively. For ICO and IOO, these values decreased from 70.07 to 18.72 h and 70.30 to 18.77 h, respectively.
The optimal parameters determined by the experimental design for BCO and BOO are a temperature of 50 °C and an air flow of 15 L h−1. For IOO and ICO, the optimal conditions are 73 °C and 10 L h−1. Therefore, BOO and BCO are the most stable samples.
Investigating the oxidative stability of cranberry, carrot, cumin and flaxseed seed oils compared to that of soy and corn seed oils, Parker et al. [48] observed that cumin obtained the most significant OSI (151 h) using 4 mL of sample at 80 °C and 7 L h−1. The authors state that the Rancimat is a device widely used to automate the determination of OSI, which is directly related to the stability of oils.
According to Santos et al. [49], to compare the OSI results reported in other studies for seed oils can be difficult due to the different conditions analyzed and parameters employed, such as temperature, air flow, sample size and equipment, and other factors that are peculiar to each sample.
Studying the quality and oxidative stability of “Pará” nut obtained by supercritical fluid extraction, Santos et al. [49] obtained an OSI of 14.85 h using 5 g of sample at 100 °C and 10 L h−1. The oxidation curves obtained by the authors showed that the more the oil was oxidized, the greater the amount of volatile compounds derived from fatty acids was released and the shorter the induction period or the lower the OSI became.
In determining the quality of virgin olive oil during the ripening of Arbequina olives, Benito et al. [50] obtained OSI ranging from 1.7 to 2.3 h using 3 g of sample at 120 °C and 20 L h−1. The authors reported that, although the OSI is not considered a parameter indicative of quality, it is useful in providing information regarding the shelf life of an oil, demonstrating its resistance to oxidation, as characterized by reactions with free radicals.
Although the current literature suggests that exposure of food products to stress can lead to the synthesis of defense substances such as polyphenols, the benefits of organic farming, such as the increase in the amounts of this type of substance, were not observed in this study. IOO showed the most significant results for color analysis and acidity, whereas BCO and BOO showed the lowest values for peroxides. Regarding the analysis of carotenoids and α-tocopherol, BCO and BOO stood out by yielding the most significant results. When analyzing the content of polyphenols, IOO, BCO and BOO showed no significant differences, but when analyzing the antioxidant activity, all samples showed the same behavior. The determination of oxidative stability indicated better performance by BCO and BOO, which may be due to the greater amount of bioactive compounds present in these samples, such as lutein and α- and β-carotene and α-tocopherol. The results showed no significant differences between the types of cultivation but between different cultivars, where the Bordô oil exhibited the most significant amounts of bioactive compounds.
References
Maier T, Schieber A, Kammerer DR, Carle R (2009) Residues of grape (Vitis vinifera L.) seed oil production as a valuable source of phenolic antioxidants. Food Chem 112:551–559
Fernandes L, Casal S, Cruz R, Pereira JA, Ramalhosa E (2013) Seed oils of ten traditional Portuguese grape varieties with interesting chemical and antioxidant properties. Food Res Int 50:161–166
Matthäus B (2008) Virgin grape seed oil: is it really a nutritional highlight? Eur J Lipid Sci Technol 110:645–650
Pardauil JJR, Souza LKC, Molfetta FA, Zamian JR, Rocha Filho GN, Costa CEF (2011) Determination of the oxidative stability by DSC of vegetable oils from the Amazonian area. Bioresour Technol 102:5873–5877
Bail F, Stuebiger G, Krist S, Unterweger H, Buchbauer G (2008) Characterisation of various grape seed oils by volatile compounds, triacylglycerol composition, total phenols and antioxidant capacity. Food Chem 108:1122–1132
Porto C, Porretto E, Decorti D (2013) Comparison of ultrasound-assisted extraction with conventional extraction methods of oil and polyphenols from grape (Vitis vinifera L.) seeds. Ultrason Sonochem 20:1076–1080
Carvalho E, Fraser PD, Martens S (2013) Carotenoids and tocopherols in yellow and red raspberries. Food Chem 139:744–752
Murillo E, Giuffrida D, Menchaca D, Dugo P, Torre G, Meléndez-Martinez AJ, Mondello L (2013) Native carotenoids composition of some tropical fruits. Food Chem 140:825–836
Kamal-Eldin A, Appelqvist L (1996) The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 31:671–701
Tasan M, Demirci M (2005) Total and individual tocopherol contents of sunflower oil at different steps of refining. Eur Food Res Technol 220:251–254
Chu YH, Lin JY (1993) Factors affecting the content of tocopherol in soybean oil. J Am Oil Chem Soc 10:1263–1268
Abidi SL (2000) Chromatographic analysis of tocol-derived lipid antioxidants. J Chromatogr A 881:197–216
Freitas LS, Jacques RA, Richter MF, Silva AL, Caramão EB (2008) Pressurized liquid extraction of vitamin E from Brazilian grape seed oil. J Chromatogr A 1200:80–83
Pussemier L, Larondelle Y, Van Peteghem C, Huyghebaert A (2006) Chemical safety of conventionally and organically produced foodstuffs: a tentative comparison under Belgian conditions. Food Control 17:14–21
Siderer Y, Maquet A, Anklam E (2005) Need for research to support consumer confidence in the growing organic food market. Trends Food Sci Technol 16:332–343
Winter CK, Davis SF (2006) Organic foods. J Food Sci 71:117–124
Instituto Adolfo Lutz (1985) Normas analíticas do Instituto Adolfo Lutz: Métodos Químicos e Físicos para Análise de Alimentos, 3rd edn. IMESP, São Paulo
American Oil Chemists’ Society (1990) Official methods and recommended practices of the American Oil Chemists’ Society [AOCS Official method Cd 8-53], 4th edn. AOCS Press, Champaign
Mercadante AZ, Rodriguez-Amaya DB (1998) Effects of ripening, cultivar differences, and processing on the carotenoid composition of mango. J Agric Food Chem 46:128–130
Zanatta CF, Mercadante AZ (2007) Carotenoid composition from the Brazilian tropical fruit camu-camu (Myrciaria dubia). Food Chem 101:1526–1532
Capannesi C, Palchetti I, Mascini M, Parenti A (2000) Electrochemical sensor and biosensor for polyphenols detection in olive oils. Food Chem 71:553–562
Dresch MTK, Rossato SB, Kappel VD, Biegelmeyer R, Hoff MLM, Mayorga P, Zuanazzi JAS, Henriques AT, Moreira JCF (2009) Optimization and validation of an alternative method to evaluate total reactive antioxidant potential. Anal Biochem 385:107–114
Calvo C, Salvador A, Fiszman SM (2001) Influence of colour intensity on the perception of colour and sweetness in various fruit-flavoured yoghurts. Eur Food Res Technol 213:99–103
Lee M, Kim S, Cho C, Choi S, In G, Kim K (2013) Quality and characteristics of ginseng seed oil treated using different extraction methods. J Ginseng Res 37:468–474
Moyano MJ, Meléndez-Martínez AJ, Alba J, Heredia FJ (2008) A comprehensive study on the colour of virgin olive oils and its relationship with their chlorophylls and carotenoids indexes (II): CIELUV and CIELAB uniform colour spaces. Food Res Int 41:513–521
Santos OV, Corrêa NCF, Carvalho RN Jr, Costa CEF, Lannes SCS (2013) Yield, nutritional quality, and thermal-oxidative stability of Brazil nut oil (Bertholletia excelsa H.B.K) obtained by supercritical extraction. J Food Eng 117:499–504
Choo W, Birch J, Dufour J (2007) Physicochemical and quality characteristics of cold-pressed flaxseed oils. J Food Compos Anal 20:202–211
Ribeiro EP, Seravalli EAG (2004) Química de alimentos. Edgard Blücler, São Paulo
Rodriguez-Amaya DB (1999) A guide to carotenoid analysis in foods. ILSI Press, Washington
Kachik F, Bernstein PS, Garland DL (1997) Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest Ophtalmol Vis Sci 38:1802–1811
Akhtar MH, Bryan MH (2008) Extraction and quantification of major carotenoids in processed foods and supplements by liquid chromatography. Food Chem 111:255–261
Criado M, Romero M, Casanovas M, Motilva M (2008) Pigment profile and colour of monovarietal virgin olive oils from Arbequina cultivar obtained during two consecutive crop seasons. Food Chem 110:873–880
Goltz SR, Campbell WW, Chitchumroonchock C, Failla ML, Ferruzzi MG (2012) Meal triacylglycerol profile modulates postprandial absorption of carotenoids in humans. Mol Nutr Food Res 56:866–877
Nagao A, Kotake-Nara E, Hase M (2013) Effects of fats and oils on the bioaccessibility of carotenoids and vitamin E in vegetables. Biosci Biotechnol Biochem 77:1055–1060
Manorama R, Rukmini C (1991) Effect of processing on β-carotene retention in crude palm oil and its products. Food Chem 42:253–264
Bunea C, Pop N, Babes AC, Matea C, Dulf FV, Bunea A (2012) Carotenoids, total polyphenols and antioxidant activity of grapes (Vitis vinifera) cultivated in organic and conventional systems. Chem Cent J 6:1–9
Cardoso PC, Tomazini APB, Stringheta PC, Ribeiro SMR, Pinheiro-Sant’Ana HM (2011) Vitamin C and carotenoids in organic and conventional fruits grown in Brazil. Food Chem 126:411–416
Ninfali P, Bacchiocca M, Biagiotti E, Esposto S, Servili M, Rosati A, Montedoro G (2008) A 3-year study on quality, nutritional and organoleptic evaluation of organic and conventional extra-virgin olive oils. J Am Oil Chem Soc 85:151–158
Faller ALK, Fialho E (2010) Polyphenol content and antioxidant capacity in organic and conventional plant foods. J Food Compos Anal 23:561–568
Demirtas I, Pelvan E, Özdemir IS, Alasalvar C, Ertas E (2013) Lipid characteristics and phenolics of native grape seed oils grown in Turkey. Eur J Lipid Sci Technol 115:641–647
Lutterodt H, Slavin M, Whent M, Turner E, Yu L (2011) Fatty acid composition, oxidative stability, antioxidant and antiproliferative properties of selected cold-pressed grape seed oils and flours. Food Chem 128:391–399
Carbonaro M, Mattera M, Nicoli S, Bergamo P, Cappeloni M (2002) Modulation of antioxidant compounds in organic vs conventional fruit (Peach, Prunus persica L., and Pear, Pyrus communis L.). J Agric Food Chem 50:5458–5462
Dani C, Oliboni LS, Vanderlinde R, Bonatto D, Salvador M, Henriques JAP (2007) Phenolic content and antioxidant activities of white and purple juices manufactured with organically- or conventionally-produced grapes. Food Chem Toxicol 45:2574–2580
Fedorova GF, Trofimov AV, Vasil’ev RF, Veprintsev TL (2007) Peroxy-radical-mediated chemiluminescence: mechanistic diversity and fundamentals for antioxidant assay. Arkivoc 8:163–215
Desmarchelier C, Romão RL, Coussio J, Ciccia G (1999) Antioxidant and free radical scavenging activities in extracts from medicinal trees used in the ‘Caatinga’ region in northeastern Brazil. J Ethnopharmacol 67:69–77
Burin MV, Falcão LD, Gonzaga LV, Fett R, Rosier JP, Bordignon-Luiz MT (2010) Colour, phenolic content and antioxidant activity of grape juice. Cienc Tecnol Alim 30:1027–1032
Cardozo MG, Medeiros N, Lacerda DS, Almeida DC, Henriques JAP, Dani C, Funchal C (2013) Effect of chronic treatment with conventional and organic purple grape juices (Vitis labrusca) on rats fed with high-fat diet. Cell Mol Neurobiol 33:1123–1133
Parker TD, Adams DA, Zhou K, Harris M, Yu L (2006) Fatty acid composition and oxidative stability of cold-pressed edible seed oils. J Food Sci 68:1240–1243
Santos OV, Corrêa NCF, Soares FASM, Gioielli LA, Costa CEF, Lannes SCS (2012) Chemical evaluation and thermal behavior of Brazil nut oil obtained by different extraction processes. Food Res Int 47:253–258
Benito M, Lasa JM, Gracia P, Oria R, Abenoza M, Varona L, Sánhez-Gimeno AC (2012) Olive oil quality and ripening in super-high-density Arbequina orchard. J Sci Food Agric 93:2207–2220
Acknowledgments
The authors are grateful to the Brazilian Research Agency (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-CAPES) for its financial support, to Agrobusiness Econatura and Co-op Garibaldi for supplying raw material.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Assumpção, C.F., Nunes, I.L., Mendonça, T.A. et al. Bioactive Compounds and Stability of Organic and Conventional Vitis labrusca Grape Seed Oils. J Am Oil Chem Soc 93, 115–124 (2016). https://doi.org/10.1007/s11746-015-2742-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-015-2742-0