Abstract
Bixa orellana L. seeds possess a resinous lipid (6.3 %), which has a pungent and spicy odour. The seed is known for its medicinal properties such as anti-inflammatory, antipyretic activity and as a cure for tonsilitis. Trachyspermum copticum L. seed is a well known digestive aid and relief from colic pain. T. copticum possesses essential oil rich in thymol (>50 %) and lipid (15.6 %). The present study was aimed to quantify lipid classes of these two species by silicic acid chromatography and analyze their fatty acid composition by gas chromatography (GC) and gas chromatography–mass spectrometry (GC–MS). It was observed that the seed lipids are rich in neutral lipids with 98.1 and 95.2 % and lower quantities of glycolipids of 1.5 and 3.8 % and phospholipids of 0.36 and 1.0 % in B. orellana and T. copticum, respectively. The fatty acid composition of B. orellana seed lipid showed major quantities of palmitic (26.9 %), linoleic (26.1 %), oleic (17.5 %), linolenic (15.1 %), stearic acid (10.8 %) and small quantities of eicosanoic acid (3.6 %). In T. copticum seed lipids, petroselinic acid (68.3 %) and linoleic acid (25.3 %) together constituted 93 % of the total lipid. The results revealed that the lipids after recovery of the essential components namely, bixin and volatile oil from B. orellana and T. copticum, respectively can be further explored for industrial applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oils or fats are present in the seed portion of most plants. In general, triglycerides are the storage lipids in plants [1]. Several seed lipids are reported to possess medicinal value and functional characteristics such as hypercholesterolemic, anti-diabetic and anti-arthritic [2] activities. Several conventional and unconventional seed lipids useful for food and medicinal or cosmetic applications were characterised for their fatty acid composition. Fatty acids such as oleic acid (23–52 %), linolenic acid (7–47.3 %), and linoleic acid (20–65 %) were reported in few seed lipids [3–8]. In general, cocoa butter, coconut oil or hydrogenated palm oil forms the major industrially used oils/fats that are rich in saturated fatty acids.
Bixa orellana L. (B. orellana) also known as annatto, is an ancient crop of tropical lowlands of Latin America, where it is grown extensively for bixin. The literature on B. orellana is extensively concentrated on the bixin pigment and its extraction methods. Bixin is the principle colouring component in the annatto seeds. Peru is the world’s largest producer and exporter of annatto seeds, followed by Brazil and Kenya. Annatto is a naturally occurring tree in the hotter parts of India and is cultivated to some extent in the states of Orissa, Andhra Pradesh and Maharashtra for its seeds (Anonymous, 1988) [9]. More than 80 % of the carotenoids in the annatto seed coat consist of cis-bixin [(2E,4E,6E,8E,10E,12E,14E,16Z,18E)—20-methoxy-4,8,13,17-tetramethyl-20-oxoicosa-2,4,6,8,10,12,14,16,18 nonaenoic-acid]. Commercial preparations consist of a solution or suspension of the pigment in vegetable oil or in water-soluble form in dilute alkaline solution. Three main commercial processes are used to extract the pigment from dried annatto seeds. These are (i) extraction into oil, (ii) extraction with organic solvents and (iii) extraction into aqueous alkali (Preston & Rickard, 1980) [10]. A detailed review of literature on annatto chemistry, processing methods, toxicological aspects and degradation products was made by Satyanarayana, Prabhakara Rao, and Rao (2003) [11].
The seeds and leaves of annatto are used in traditional medicine [9]. The seeds are edible, but slightly purgative and said to be effective against fever, dysentery and kidney diseases. The paste of fruits and seeds is applied against itching; the leaf decoction is taken to stop vomiting, used as a gargle for sore throat and tonsillitis; and as a bath against muscular pain; used in the cosmetic industry in the production of nail gloss, hair oil, lipstick, soap; used in household products like floor wax, furniture polish, shoe polish, brass lacquer and wood stain [9]. Extraction methods, purification, quantification, analysis, stability studies and applications of principle component of B. orellana in foods are available in the literature [12–17].
The lipid fraction from B. orellana contains mainly tocotrienols of which δ-tocotrienol at 140–147 mg/100 g dry seeds and to an extent of 5.2–5.5 % on a lipid basis [18]. Palmitic, oleic and linoleic acids were found to be major fatty acids, which accounted for 16.4, 33.9, 34.3 %, respectively.
Trachyspermum copticum L. is also known as azwain. In India, the states of Rajasthan and Gujarat are the major producers with Rajasthan alone accounting for 90 % of the total harvest. The monoterpene, thymol is the main component in the essential oil of raw T. copticum seeds and its flavour is aromatic similar to thyme. In India, T. copticum is used as a digestive relief for abdominal pain, indigestion and as an antiseptic. In southern India, powdered T. copticum seeds soaked in milk are fed to babies to provide relief from colic pains. In northern India, people serve it after dinner [19]. Essential oils from T. copticum L. obtained by hydro-distillation were studied for antifungal activities with special reference to the inhibition of Aspergillus parasiticus growth and aflatoxin production. Aflatoxin production was inhibited at 450 ppm of T. copticum. Essential oils were shown to contain thymol (37.2 %), p-cymene (32.3 %), γ-terpinene (27.3 %). Preservative effects of essential oils of azwain were confirmed and the natural materials can safely be applied to protect food materials from toxigenic fungal infections [20]. Several therapeutic effects including carminative, diuretic and anti-vomiting effects were reported for Carum copticum. GC–MS analysis of essential oil from C. copticum fruits showed thymol (54.5 %), γ-terpinene (26.1 %) and p-cymene (22.1 %) as the major terpenoid compounds [21]. Thymol, present in the essential oil was characterised by the chemical shift method using spectral properties, which was confirmed by optical microscopy and gas chromatography-mass spectrometry (GC–MS), respectively [22].
However, the analysis of the total lipid portion of B. orellana and T. copticum had not been carried out in detail till now and hence, the present study was taken up with the main objective to evaluate residual lipid after recovering the principal components namely, bixin from B. orellana seed and the essential oil from T. copticum.
Materials and Methods
Materials
B. orellana seeds (10 kg) were procured from M/s. Girijan Cooperative Corporation Ltd., during the three successive seasons of 2009–11, which were collected by tribal population form the Paderu forests of the Visakhapatnam district of Andhra Pradesh, India, where the average temperature ranged between 15 and 22 °C during 2009 and 2011. The plants wildly growing at an altitude of 900–1200 m were harvested for annatto seed during the season of November to December. T. copticum seeds (10 kg) were purchased from a grower in the Kurnool district of Andhra Pradesh, India, located at an altitude of 270 m in three consecutive seasons of 2010–2012, where the average temperature was 12–30 °C. The seed was harvested in the months of December and January. Solvents and chemicals used in the study were of laboratory grade obtained from M/s. Loba Chemie, Mumbai, India. Standard fatty acid methyl esters (FAME, C4–C24) were procured from M/s. Sigma-Aldrich, St Louis, USA.
Sample Preparation
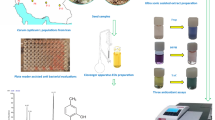
Seeds of B. orellana and T. copticum were manually cleaned from dust, fibre and stones. The seeds were powdered using a high speed laboratory mixer (Sumeet Food Processor, Nasik, India) and passed through a 30 mesh (500 µm) sieve to obtain uniform sample.
Chemical Composition of Seed Samples
Estimation of Moisture
Moisture was determined using a hot air oven method [23]. The sample weighed in a glass dish was dried in a hot air oven maintained at 103 ± 2 °C and the drying was continued for 16–18 h. The dish was cooled in a desiccator, and weighed. The sample was reheated and checked for weight until two consecutive weights show no significant variation. Loss on drying is expressed as moisture present in sample.
Estimation of Total Ash
The ash content of a food material determines the inorganic metals after combustion of organic matter. High ash or low alkalinity of ash, may suggest the presence of adulterants. The acid-insoluble ash is a measure of sand or silica. About 5 g of the sample was weighed into each silica dish and the contents were ignited on a Bunsen burner. Further, the material was exposed to 525 °C for 4–6 h in a muffle furnace (Adair Dutt and Company, Chennai, India) [23]. The crucibles were cooled and weighed and the difference in weight gave the total ash content, which was expressed as a percentage.
Estimation of Protein
Nitrogen content was estimated by the Kjeldahl method, which is based on determination of the amount of reduced nitrogen (NH2 and NH) present in the sample. Various nitrogenous compounds are converted into ammonium sulphate, when digested with concentrated sulphuric acid in the presence of a digestion mixture consisting of copper sulphate, potassium sulphate and selenium dioxide. Ammonia was liberated by boiling with an alkali (NaOH), which was absorbed in neutral boric acid solution and was titrated with a standard acid. Percentage protein was calculated using a general conversion factor N × 6.25. B. orellana and T. copticum seed powders from three consecutive seasons were analysed for moisture, total ash, and protein contents following standard methods (Ranganna 1986) [23].
Extraction of Total Lipids
Extraction of total lipids from B. orellana and T. copticum powder was conducted using the solvent mixture chloroform:methanol (2:1, v/v) at room temperature (RT, 27 ± 2 °C) under magnetic stirring [24]. Extraction was repeated 3 times for maximum recovery of lipids. Recovery of solvent was conducted at <50 °C in a rotary film evaporator.
Estimation of Volatile Oil in T. copticum
The volatile oil content of T. copticum seed was estimated by hydro-steam distillation using a Clevenger apparatus. The powdered sample (25 g) was dispersed in water in a round-bottomed flask, which was connected to a Clevenger apparatus and a water cooled condenser. The contents of the flask were heated to reflux for collecting the volatile oil in the graduated side arm. The percent volatile oil (v/w) in the sample was calculated by measuring the volume of volatile oil.
Determination of Bixin Content in the Total Lipid of B. orellana
Bixin content in the total lipid of B. orellana was analysed using a spectrophotometric method [25] by dissolving in chloroform and suitably diluting aliquots for measuring optical density at 470 nm. The optical measurements were conducted in a Shimadzu (Kyoto, Japan), UV-160 A UV–Vis spectrophotometer. The percentage of bixin was calculated using the following expression:
where \(E_{{_{{ 1\;{\text{cm}}}} }}^{{^{ 1 \% } }}\) at the 470 nm value of bixin is 3230
Lipid Quality Analysis
The total lipids of B. orellana and T. copticum were analysed for chemical parameters by standard AOCS methods such as free fatty acids (FFA) Cd 3d-63 [26], saponification value (SV) Cd 3–25 [27], peroxide value (PV) Cd 8–53 [28] and iodine value Cd 1–25 [29].
Acid value (AV) and Free Fatty Acid Content (FFA)
The fat sample (2 g) was put into a 250-ml Erlenmeyer flask and 50 ml petroleum ether was added. The contents were thoroughly swirled until the sample dissolved. To the contents, 50 ml neutral ethyl alcohol was added and mixed. Phenolphthalein indicator (1 % in 95 % ethyl alcohol) was added and titrated with 0.1 N KOH solution till the pink color appeared. The acid value was calculated as:
where, N = normality of KOH; (% FFA as oleic acid = Acid value/2)
Iodine Value
Wijs Procedure: the iodine value is the number of grams of iodine absorbed per 100 g of the oil or fat, when determined using Wijs solution. The material is treated in carbon tetrachloride medium with a known excess of iodine monochloride in glacial acetic acid. The excess of iodine monochloride is treated with potassium iodide, and the liberated iodine is estimated by titrating with standard sodium thiosulphate (Na2S2O3) solution using starch indicator. A blank determination was carried out along side without the addition of fat.
Peroxide Value
The peroxide value of an oil or fat is the amount of peroxides present and expressed as milli-equivalents of peroxide per 1000 g of sample. The sample was dissolved in solvent (2 volumes of glacial acetic acid and one volume of chloroform), treated with potassium iodide, and the iodine liberated by the peroxides present in rancid fat or oil was titrated with standard sodium thiosulphate solution.
Saponification Value
When a fat is boiled with an excess of alcoholic KOH, the triglycerides become hydrolysed resulting in glycerol and soap. The alkali consumed for this hydrolysis is a measure of the saponification value (SV) and is defined as the number of milligrams of KOH required to saponify one gram of oil or fat completely. A blank determination was carried out along side without the addition of fat.
Chromatographic Separation of Lipid Classes
The neutral, glyco- and phospholipids of the total lipids were quantitatively separated by successively eluting with chloroform, acetone and methanol using silicic acid column chromatography [30]. The separation was carefully monitored by thin layer chromatography for ascertaining the complete elution of each class. Neural lipids, glycolipids and phospholipids were monitored by TLC using solvent systems namely, 90:10 (hexane:ethyl acetate, v/v), 170:25:24:4 (chloroform:methanol:aceticacid:water, v/v/v/v) and 65:25:4 (chloroform:methanol:water, v/v/v), respectively [30].
Determination of the Fatty Acid Composition of Lipid Classes
The total lipids and the lipid classes were converted into their respective fatty acid methyl esters (FAME) by refluxing with methanol containing 2 % sulphuric acid for 6 h ensuring the complete conversion [30] of the triacylglycerol by testing with TLC using a solvent system of 90:10 (hexane:ethyl acetate, v/v). After completion of the reaction, the reaction mixture was concentrated by distillation under a vacuum and the FAME were extracted into ethyl acetate and the excess acid was removed by repeatedly washing with water [30]. The extracts were concentrated by removing the solvent for conducting GC and GC–MS analysis.
The FAME were analysed by GC and GC–MS for fatty acid composition by applying the following conditions.
-
The GC-FID analysis was performed with an Agilent 6850 (Agilent Technologies, Palo Alto, CA, USA) series gas chromatograph equipped with an FID detector, DB-225 capillary column (30 m × 0.25 mm i.d. and 0.25 µm film) with temperature programming maintaining 160 °C for 2 min, increasing to 230 °C at 6 °C/min, and finally maintained for 10 min at 230 °C [31]. The carrier gas was nitrogen at a flow rate of 1.5 ml/min.
The injector and detector temperatures were maintained at 230 and 250 °C, respectively with a split ratio of 50:1.
The GC–MS analysis of FAME was conducted with an Agilent (Palo Alto, USA) 6890 N gas chromatograph equipped with a HP-5 MS capillary column (30 m × 0.25 mm i.d. 0.25 µm film) connected to an Agilent 5973 mass spectrometer operating in the EI mode (70 eV; m/z 50–550; source temperature 230 °C and a quadruple temperature 150 °C).
-
The column temperature was programmed with an initial temperature of 200 °C for 2 min, increasing to 300 °C at a rate of 4 °C/min, and maintained for 20 min at 300 °C.
-
Helium was used as the carrier gas with a flow rate of 1.0 mL/min. The inlet temperature was maintained at 300 °C and a split ratio of 50:1 [31].
Structural assignments were based on comparison of retention times as well as the fragmentation pattern of authentic compounds and confirmed by the spectral data obtained from the Wiley and NIST libraries.
Results and Discussion
The photographs of the fruiting branch depicting the pods and seed of B. orellana and T. copticum are presented in Figs. 1 and 2 respectively.
Chemical Characterisation of the Total Lipids
Results indicated that not much variation in chemical composition with change of season was observed. The results of chemical analyses of seed powders are presented in Table 1. The ash and protein contents in B. orellana were 5.3 and 11.2 % respectively. The yield of total lipids in B. orellana was 6.3 %, which possessed a bixin content of 17 %. The lipids with bixin can be applied to fatty foods such as butter and cheese to yield an orange yellow colour in the products. Higher peroxide (10.3 mequiv O2/kg) and iodine values (146 g I2/100 g fat) were noted in B. orellana lipids. The quantity of FFA present in the seed oils was reported to have a significant effect on the formation of soap or biodiesel manufacture wherein a free fatty acid content of <1 % is recommended [32, 33]. Therefore, in the present study, B. orellana with a lower FFA content of 0.2 % is in good agreement with the reported literature and hence can be suggested for examining its compatibility in industrial applications.
The ash and protein contents in T. copticum were 7 and 16.3 %, respectively. The yield of total lipid from T. copticum was 15.6 % and the essential oil was found to an extent of 10 %. A higher free fatty acid content of 1.5 % and a saponification value of 188 mg KOH/g was observed in T. copticum total lipid (Table 2). The essential oil can be recovered using a Clevenger apparatus and may find application as a flavourant in sweets and fried foods.
Lipid Classes and Fatty Acid Composition of Total Lipids
Silicic acid column chromatography revealed that both the plant seeds were rich in neutral lipids (Table 2). Neutral, glyco- and phospholipids were 98.12, 1.52 and 0.36 %, respectively in B. orellana. Neutral, glyco- and phospholipids were found to be 95.17, 3.76 and 1.07 % in T. copticum. The presence of low PL content indicates that the lipid of T. copticum can be used as a frying oil. Similarly, the higher glycolipid content suggests its use in the preparation of bio-surfactants. Glycolipid based bio-surfactants are the most promising ones due to versatile biochemical properties in the food, cosmetic, and pharmaceutical industries and in environmental protection [34].
Based on GC and GC–MS data, the fatty acid composition of the seed lipids and their lipid classes are tabulated (Tables 3 and 4). The total lipid from B. orellana was characterised by substantial amounts of palmitic (26.9 %), linolenoic (26.1 %), oleic (17.5 %), linolenic (15.1 %) and stearic acids (10.8 %). In the case of phospholipids, palmitic and linoleic acids were 25.6 and 43.8 %, respectively, making up to 69.4 % of total lipids.
The lipids from Bixa orellana were reportedly used in cosmetic application [11]. However, the polyunsaturated fatty acid (PUFA) content of the total lipid of B. orellana (41.2 %) was comparable to the PUFA contents of sesame seed oil (38 %) and Sterculia urens cotyledons (36.7 %) [35, 36]. Palm oil with higher saturated fatty acids (~50 %) was reported as being used for food applications and the spent fried oil for bio-diesel production [37]. Saturated fatty acid content was noted as being higher in glycolipids (42.9 %) compared to neutral or phospholipid fractions of B. orellana. Higher PUFA of 39.1, 35.7 and 51.5 % were found in neutral, glyco- and phospholipids, respectively in B. orellana.
The fatty acid composition of T. copticum was characterised by lower amounts of palmitic (4.5 %), stearic (0.9 %), linolenic (1.0 %) acids, and substantial amounts of linoleic (25.3 %) and petroselinic acids (68.3 %). The total lipid content of T. copticum was highly aromatic with the flavour of thymol. The total lipid and other lipid classes were typically composed of high concentrations of oleic and linoleic acids, which together constituted 93.6 % of the total lipids. Such a high percentage of unsaturation is similarly observed in the non-edible oil, jatropha seed oil, oleic (44.7 %) and linoleic acid (32.8 %), which is one of the important sources for bio-diesel production [38]. Hence T. copticum can also be exploited for biodiesel preparation. In case of glyco- and phospholipids of T. copticum, palmitic and stearic acid contents were higher and a corresponding decrease in oleic and linoleic acids was observed. Seasonal variation was not observed to be significant in the lipid or fatty acid composition of the seed samples. Higher monounsaturated (68.3 %) fatty acids were observed in T. copticum lipids. The glycolipid fraction possessed higher saturated fatty acids (11.8 %) compared to phosphor- or neutral lipids. In addition, the T. copticum lipid classes were found to be rich in monounsaturated fatty acids (Table 2).
The ratio of polyunsaturated to saturated fatty acids (PUFA/SFA) and the ratio of polyunsaturated to monounsaturated fatty acids (PUFA/MUFA) were found to be 1.0 and 2.35 in Bixa orellana total lipids, respectively. The ratio of PUFA/SFA is generally used to evaluate the nutritional value of fat. Chang and Huang [39] studied the effects of monounsaturated fatty acids (MUFA) and the ratio of the sum of polyunsaturated and monounsaturated fatty acids to saturated fatty acids (PUFA + MUFA/SFA) on plasma and liver lipid concentrations in rats. They suggested that a low MUFA/SFA ratio, high PUFA/MUFA ratio and PUFA + MUFA/SFA ratio not to exceed 2 are the prerequisites for keeping low plasma and liver lipid concentration. In the present study, low MUFA/SFA (0.42) ratio and high PUFA/MUFA (2.35) ratio, and PUFA + MUFA/SFA (1.42) ratio of total lipid of B. orellana were observed. The findings of Chang and Huang [39] are in agreement with the fatty acid profile of B. orellana seed lipid. Similarly highly nutritious foxtail millet bran oils were found to be rich in polyunsaturated fatty acids (83.47 %) with very low saturated fatty acids [40].
In the present study T. copticum lipid was found to be rich in monounsaturated fatty acids (68.3 %) and PUFA (26.3 %) and low quantities of saturated fatty acids (5.4 %). The ratio of PUFA to SFA was found to be 4.87 and the ratio of PUFA to MUFA was 0.45 in T. copticum lipid. In the total lipid, higher MUFA/SFA ratio (12.65) and lower PUFA/MUFA ratio (0.38) and very high PUFA + MUFA/SFA ratio (17.52) was observed. In this context, B. orellana seed lipid can be exploited for nutritional applications. Jojoba seeds with major monounsaturated fatty acid (20:1) (66.3 %) [41], was employed in biodiesel preparation. Hence, T. copticum seed lipid with high yields of lipid and rich in monounsaturated fatty acids can be employed in industrial applications especially for the production of biodiesel.
Conclusions
The seed samples collected during three consecutive seasons did not show considerable variation in physico-chemical, lipid and fatty acid composition. This study showed that the by-product of resin after extraction of the principle natural dye from B. orellana, could be a good source of lipids for the food, pharmaceutical, cosmetic and varnish industries. Similarly, T. copticum oil a rich source of monounsaturated fatty acids could be nutritionally good for minimising the risks of cardiovascular diseases. The results revealed that the lipids after recovery of the essential components of bixin and volatile oils rich in thymol, respectively from B. orellana and T. copticum can be further explored for industrial applications.
References
Murphy DJ (1990) Storage lipid bodies in plants and other organisms. Progr Lipid Res 29:299–324
Anilakumar KR, Saritha V, Khanum F, Bawa AS (2009) Ameliorative effect of ajwain extract on hexachlorocyclohexane-induced lipid peroxidation in rat liver. Food Chem Toxicol 47:279–282
Anonymous (2012) Fat content and fatty acid composition of seed oils http://curezone.com/foods/fatspercent.asp
Shin EC, Pegg RB, Phillips RD, Eitenmiller RR (2010) Commercial Runner peanut cultivars in the USA: fatty acid composition. Eur J Lipid Sci Technol 112:195–207
Berrin B, Feral T (2008) Chemical composition and oxidative stability of flax, safflower and poppy seed and seed oils. Bioresource Technol 99:6354–6359
Anonymous (2008) Flax nutrition profile. http://www.flaxcouncil.ca/english/index. Retrieved from CA:2008-05-08
Prabhakara Rao PG, Narsing Rao G, Jyothirmayi T, Karuna MSL, Prasad RBN (2009) Analysis of lipid classes and fatty acid composition of jangle jalebi (Pithecellobium dulce L.) seed oil. J Lipid Sci Technol 41:5–7
Prabhakara Rao PG, Narsing Rao G, Jyothirmayi T, Karuna MSL, Prasad RBN (2010) Analysis of lipid classes and fatty acid composition of adavichinta (Entada pursaetha) seed oil. J Lipid Sci Technol 42:66–68
Anonymous (1998) The wealth of India: raw materials. Council of Scientific and Industrial Research (CSIR), New Delhi, India, Vol. 2B (Rev):157–160
Preston HD, Rickard MD (1980) Extraction and chemistry of annatto. Food Chem 5:47–56
Satyanarayana A, Prabhakara Rao PG, Rao DG (2003) Chemistry, processing and toxicology of annatto (Bixa orellana L.). J Food Sci Technol 40:131–141
Scotter M (2009) The chemistry and analysis of annatto food colouring: a review. Food Addit Contam 26:1123–1145
Prabhakara Rao PG, Satyanarayana A, Balaswamy K, Jyothirmayi T, Nagender A, Rao DG (2007) Application of annatto dye formulation in bakery and extruded food products. J Foodservice 18:53–58
Balaswamy K, Prabhakara Rao PG, Satyanarayana A, Rao DG (2006) Stability of bixin in annatto oleoresin and dye powder during storage. LWT Food Sci Technol 39:952–956
CFTRI Patent (DEL No. 552/2004) A process for the preparation of high bixin dye from annatto (Bixa orellana L.) seed, CFTRI, Mysore, CSIR, India
Reith JF, Gielen JW (1971) Properties of bixin and norbixin and the composition of annatto extracts. J Food Sci 36:861–864
Silva GF, Gamarra FMC, Oliveira AL, Cabral FA (2008) Extraction of bixin from annatto seeds using supercritical carbon dioxide. Brazilian J Chem Eng 25:419–426
Frega N, Mozzon M, Bocci F (1998) Identification and estimation of tocotrienols in annatto lipid fraction by gas chromatography-mass spectrometry. J Am Oil Chem Soc 75:1723–1727
Hill T (2004) Ajwain in the contemporary encyclopedia of herbs and spices: seasonings for the global kitchen. Wiley, New York, pp 21–23
Rasooli I, Fakoor MH, Yadegarinia D, Gachkar L, Allameh A, Rezaei MB (2008) Antimycotoxigenic characteristics of Rosmarinus officinalis and Trachyspermum copticum L. essential oils. Int J Food Microbiol 122:135–139
Mohagheghzadeh A, Faridi P, Ghasemi Y (2007) Carum copticum (Benth. and Hook) essential oil chemotypes. Food Chem 100:1217–1219
Gersbach PV, Reddy N (2002) Non-invasive localization of thymol accumulation in Carum copticum (Apiaceae) fruits by chemical shift selective magnetic resonance imaging. Annals Botany 90:253–257
Ranganna S (1986) Hand book of analysis and quality control for fruits and vegetable products, 2nd edn. Tata McGraw-Hill Publishing Company limited, New Delhi
Folch J, Lees M, Stanley GS (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Reith JF, Gielen JW (1971) Properties of bixin and norbixin and the composition of annatto extracts. J Food Sci 36:861–864
Official Methods and Recommended Practices of the AOCS (2003) Fifth edition, Edited by Firestone, AOCS Press, Champaign Illinois, USA. Method for determination of acid value, Cd 3a–63
Official Methods and Recommended Practices of the AOCS (2003) Fifth edition, Edited by Firestone, AOCS Press, Champaign Illinois, USA. Method for determination of saponification value (SV) Cd 3-25
Official Methods and Recommended Practices of the AOCS (2003) Fifth edition, Edited by Firestone, AOCS Press, Champaign Illinois, USA. Method for determination peroxide value (PV) Cd 8–53
Official Methods and Recommended Practices of the AOCS (2003) Fifth edition, Edited by Firestone, AOCS Press, Champaign Illinois, USA. Method for determination of iodine value Cd 1–25
Christie WW (1982) The preparation of derivatives of lipids. In: Lipid analysis, 2nd edn. Pergamon Press Ltd., Oxford, p 51
Prabhakara Rao PG, Jyothirmayi T, Karuna MSL, Prasad RBN (2010) Studies on lipid profiles and fatty acid composition of roe from rohu (Labeo rohita) and murrel (Channa striatus). J Oleo Sci 59:515–519
Goodrum JW (2002) Volatility and boiling points of biodiesel from vegetable oils and tallow. Biomass Bioenergy 22:205–211
Crabbe E, Nolasco-Hipolito CN, Kobayashi G, Sonomoto K, Ishizaki A (2001) Biodiesel production from crude palm oil and evaluation of butanol extraction and fuel properties. Process Biochem 37:65–71
Dai K, Hiroko I, Tadaatsu N (2002) Functions and potential applications of glycolipid bio-surfactants—from energy-saving materials to gene delivery carriers. J Biosci Bioeng 94:187–201
Kanu PJ (2011) Biochemical analysis of black and white sesame seeds from China. Am J Biochem Mol Biol 1:145–157
Narsing Rao G, Prabhakara Rao PG, Satyanarayana A (2012) Physico-chemical, amino acid and fatty acid composition of Sterculia urens L. seed. Food Hydrocolloid 28:320–324
Soh K, Choo YM, Cheng SF, Ma AN (2006) Recovery and conversion of palm olein-derived used frying oil to methyl esters for biodiesel. LOH J Oil Palm Res 18:247–252
Akbar E, Yaakob Z, Kamarudin SK, Ismail M, Salimon J (2009) Characteristics and composition of Jatropha curcas oil seed from Malaysia and its potential as biodiesel feedstock. Eur J Sci Res 29:396–403
Chang NW, Huang PC (1998) Effects of the ratio of polyunsaturated and monounsaturated fatty acid to saturated fatty acid on rat plasma and liver lipid concentrations. Lipids 33:481–487
Shaohua L, Guolong Y, Yuxiang M (2010) Chemical characteristics and fatty acid profile of foxtail millet bran oil. J Am Oil Chem Soc 87:63–67
Maria FG, Diana OL, Jose A, Juan CO, Nelson RG, Carlos AG (2009) Chemical quality evaluation of damaged jojoba seeds (Simmondsia chinensis). J Am Oil Chem Soc 86:65–70
Acknowledgments
The authors thank the Director, CSIR-CFTRI, Mysore and Director, CSIR-IICT, Hyderabad for their keen interest and supporting the work. We acknowledge the Ministry of Food Processing Industries, Govt. of India, New Delhi for financial assistance to develop tailor made annatto dye formulations for application in foods (Grant No. GAP-0363).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Prabhakara Rao, P., Narsing Rao, G., Jyothirmayi, T. et al. Characterisation of Seed Lipids from Bixa orellana and Trachyspermum copticum . J Am Oil Chem Soc 92, 1483–1490 (2015). https://doi.org/10.1007/s11746-015-2717-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-015-2717-1