Abstract
The physicochemical properties of binary and ternary fat systems made of commercial samples of palm oil (PO) blended with anhydrous milk fat (AMF) and/or rapeseed oil (RO) were studied. Physical properties such as solid fat content by pulsed-Nuclear Magnetic Resonance (p-NMR), melting profile by differential scanning calorimetry (DSC), and polymorphism of the blends were investigated. Palm oil was then batch enzymatically interesterified for 27 h, using Lipozyme® TL IM as biocatalyst, and further blended with AMF and/or RO in the same way. The objective of the present work was to evaluate the effect of batch enzymatic interesterification (B-EIE) of palm oil on physical characteristics of the investigated fat blends. For that purpose, iso-solid diagrams have been constructed from p-NMR data. It was shown that B-EIE of palm oil modifies its melting behaviour, but also its polymorphic stability and miscibility with other fats. Under dynamic conditions, after B-EIE, the non-ideal behaviour (eutectic) detected at low temperatures in the ternary PO/AMF/RO system disappears in the corresponding EIE-PO/AMF/RO. After static crystallization followed by a tempering, the hardness of palm oil is increased after B-EIE, as well as the hardnesses of the blends containing this fat compared to the native one. Polymorphism stability of the binary and ternary fat systems is also modified after B-EIE compared to the corresponding native systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The texture of fat-containing products such as butter, shortening, margarine or creams and ice creams depends on factors like solid fat content (SFC), polymorphism and microstructure of the fats they contain. Palm oil, which is semisolid at 25–30 °C, is used worldwide as such or blended with other oils and fats to produce a wide variety of food products [1, 2]. However, the use of palm oil also presents some disadvantages, such as its tendency to slowly crystallize and form coarse crystals; post-hardening is also a drawback [3]. Interesterification has been used for many years to modify some physical properties of fats [4]. The use of this technique has significantly increased in the food industry over recent years. Interesterification is performed to exchange fatty acids between and within triacylglycerols (TAGs) [5]. These modifications result in changes in physicochemical properties of the fat, such as melting properties, SFC, oxidative stability, viscosity and functionality, compared to the original material [6].
Milk fat (MF) is usually viewed as a natural product that has excellent organoleptic properties in many food products [7]. Although butterfat has desirable properties such as butter flavor and palatability, it has also some disadvantages, such as poor spreadability at refrigerator temperature and cholesterol content. Dry fractionation of MF can produce new products having different physicochemical properties. Those products are suitable as food ingredients and can be used for a large range of applications [8]. Milk fat can also be blended with vegetable oils, for example, to produce a more spreadable product. Examples of such products are the butter-like spreads and butter-like shortenings sometimes called compound dairy blends. From an industrial point of view, the lower price of vegetable oils and fats compared to MF can reduce the costs relative to MF, while keeping the preferred taste of butter in the blends. A growing market for such products motivates the industry to develop new blends. According to Nor Hayati et al. [9], palm oil (PO) is a suitable vegetable oil that can be blended with milk fat. The use of PO or its fractions is increasing in the manufacture of ice cream, as they are cheaper than milk fat [2]. However, addition of vegetable oils to MF increases the complexity of the fat system and induces changes in microstructure, crystallization behaviour and polymorphism. Several butyric-vegetal mixed-fat blends have recently been characterized for their physical characteristics [10–12]. The effects of addition of rapeseed oil to MF were mainly ascribed to dilution (solvent) effects [10]. On the contrary, it was shown that the behaviour of compound blends made of MF or one of its industrial fractions and PO is clearly not ideal [11]; a eutectic effect was described.
Although lots of studies have been pursued on the effect of interesterification of different fat blends on their final properties, reports on the interesterification of palm oil itself are scarce [3, 13, 14].
Nor Hayati et al. [9] compared the melting characteristics and SFC of MF/PO stearin before and after enzymatic interesterification (EIE) by nuclear magnetic resonance (NMR) and differential scanning calorimetry (DSC). They found a better miscibility among the blended fats after EIE. Some authors recently studied the effect of chemical IE on blends of palm stearin/coconut oil/canola oil [15] or blends of palm stearin/palm kernel oil/soybean oil [16] to produce trans-free fats for possible use in margarines. To our knowledge, few studies concern blends made of an interesterified fat blended with another, especially palm oil.
Investigations reported herein are directed toward evaluation of the effect of incorporating batch enzymatically interesterified palm oil (using Lipozyme® TL IM as catalyst) into some binary and ternary fat blends. Phase behaviour of the binary and ternary blends was investigated to better understand the extent of compatibilities and incompatibilities between these fats and to highlight the effect of the EIE. As for fat crystal networks, hardness is an important macroscopic property that contributes to sensory perception of the final product; this property has also been measured. All the results were compared with those obtained for control blends containing native palm oil.
Materials and Methods
Materials
Whole standard anhydrous milk fat (AMF) was from industrial supply (S.A. Corman, Goé, Belgium). Palm oil (PO) was supplied by ADM International (Decatur, USA). Rapeseed oil (RO) was from Wilmar Edible Oils B.V. (Barendecht, The Netherlands).
Lipozyme® TL IM, a silica granulated Thermomyces lanuginosa lipase, was kindly supplied by Novozymes A/S (Bagsværd, Denmark).
Methods
Laboratory-Scale Batch Enzymatic Interesterification (B-EIE)
Enzymatic interesterification was conducted at the lab scale using a non-specific lipase as biocatalyst. Fresh Lipozyme® TL IM was first deaerated then dehydrated as recommended by Novozyme. Batch enzymatic interesterification was conducted during a period of 27 h, as follows: 5 kg of the feedstock was melted and transferred into the reactor. When the oil reached the temperature of 70 °C, the preconditioned enzyme was added into the system at a ratio of 4 % w/w to start the reaction. A stirrer set at 300 rpm ensured an homogenous distribution of the enzyme. IE product was filtered through a Büchner filter to remove the immobilized enzyme before fat analysis and further use. Two independent batches were produced.
TAG Determination by High Performance Liquid Chromatography (HPLC)
TAG composition of palm oil, before and after B-EIE, was analysed by reverse phase HPLC based on the official AOCS method Ce 5b-89 [17] including a differential refractometer as a detector. Minor practical adjustments were made in order to improve TAG separation in compliance with the said official method. All equipment—pump, column, autosampler and detector—was supplied by Waters (Zellik, Belgium) [18].
Sample Preparation
Various binary and ternary fat blends were prepared, crystallized and stored according to the method proposed by Danthine and Deroanne [19].
Physical Characterization
Solid Fat Content Determination
SFC was measured according to the standard serial non-tempered IUPAC 2.150 method [20] using a pulsed NMR Minispec-mq20 spectrometer (Bruker, Germany) and after crystallization followed by the tempering procedure. Automatic calibration was made daily using three standards (supplied by Bruker, Germany) containing 0.0, 31.3 and 74.8 %, respectively, of solids.
Melting Properties by Differential Scanning Calorimetry (DSC)
DSC analyses were carried out using a Q1000 DSC (TA Instruments, New Castle, USA) with a refrigerated cooling system (TA Instruments, New Castle, USA) and aluminium solid fat index (SFI) pans. Calibration was made with indium (m.p. 156.6° C) and n-dodecane (m.p. −9.56° C) standards. Nitrogen was used as a purge gas in order to prevent condensation in the cells. An empty aluminium SFI pan was used as a reference. Samples weighed between 2 and 4 mg. They were first heated to 80° C in the DSC pan to ensure complete melting, and held for 5 min at that temperature to erase thermal memory. Afterwards, they were quickly frozen at −60° C (cooling rate −25° C/min), and kept for 10 min at −60° C, in order to ensure complete solidification. Melting profiles were recorded from −60° to 70 °C at a heating rate of 5° C/min. All the DSC analyses were carried out in duplicate. The integration and peak temperature measurements were performed using the Universal Analysis Software version 4.2 (TA Instruments, New Castle, USA). The melting peaks were integrated with a linear baseline.
Powder X-Ray Diffraction Analyses
The polymorphic forms of the blends were determined by powder X-ray diffraction using a D8 Advance diffractometer (Bruker, Germany) (λ Cu = 1.54178 Å, 40 kV, 30 mA) equipped with a Vantec (Bruker, Germany) detector, and a TTK450 low-temperature Chamber & TCU 110 Temperature Control Unit (Anton Paar, Graz, Austria) connected to a circulating water bath (Julabo, Germany). Diffraction patterns were recorded in the 2θ range 15°–27°. d-spacings were determined using the Bragg law. Analyses were performed isothermally at 15° C, after the tempering period. Each sample was run in triplicate.
Textural Measurements
Texture measurements of the products were carried out after the tempering at 15° C. Samples were analysed in a controlled temperature cabinet using a SMS TA.XT2i/5 texturometer with a cone probe (P/45C) (Stable Micro System, Surrey, UK). A constant speed penetration test was used, as previously described [19]. The probe penetrated the product (50 g) at a constant speed of 0.5 mm/sec to a distance of 5 mm.
At least five penetration tests were run on each sample; at least three samples of each blend were analysed. Coefficients of variation (C.V.) were all lower than 10 %.
Statistical
Values are expressed as the mean ± standard deviation (SD). Statistical significance of the difference between the groups was evaluated by one-way analysis of variance using Minitab 15 software (Minitab Inc., State College, Pennsylvania, USA).
Results and Discussion
Besides examining the physicochemical properties of the pure fats, their phase behaviour when blended in binary and ternary systems was investigated in order to determine their miscibility. Physical properties were investigated both under dynamic conditions and after a crystallization/tempering procedure.
Binary Blends
Dynamic Conditions
Blends Containing Rapeseed oil
The SFC melting profiles of the binary blends made of RO/PO and RO/EIE-PO are depicted in Fig. 1a, b. The behaviour of the binary PO/RO blend is of monotectic type. This type of behaviour is characteristic of eutectic systems, which shift to monotectic systems when the difference in the melting points of the two components is 20° C or above [21]. The melting points of the components involved in this blend are different by more than 20° C; RO is indeed completely liquid at 10° C. The solid fat content increase within the blends is totally due to the increase of PO content. The behaviour of the blend made of RO and EIE-PO is similar to the previous one (RO/PO): monotectic. After B-EIE, the SFC melting profile of PO (Curve 11, Fig. 1b) is shifted to higher values compared to the native oil (Curve 11, Fig. 1a); a broader melting profile is observed [3]. The higher amount of trisaturated TAG (SSS) after interesterification resulted in 10 % solids at 35° C (5 % for native PO), and 5 % of remaining solids at 40° C (0 % for native PO). SFC measured for the blends containing the EIE-fat was higher compared to the native corresponding ones. Moreover, blends containing more than 40 % of EIE-PO are not completely melted at 40° C, due to the higher SSS content after B-EIE (Table 1).
a,b: SFC melting profiles by p-NMR of the binary blends made of a) RO/PO (curve 1: 100 %PO, curve 2: 90 %PO, curve 3: 80 %PO, curve 4: 70 %PO, curve 5: 60 %PO, curve 6: 50 %PO, curve 7: 40 %PO, curve 8: 30 %PO, curve 9: 20 %PO, curve 10: 10 %PO, curve 11: 100 %RO); and b) RO/EIE-PO (curve 1: 100 %B-EIE-PO, curve 2: 90 %PO, curve 3: 80 %PO, curve 4: 70 %PO, curve 5: 60 %PO, curve 6: 50 %PO, curve 7: 40 %PO, curve 8: 30 %PO, curve 9: 20 %PO, curve 10: 10 %PO, curve 11: 100 %RO)
The DSC melting profiles of the binary blends made of RO/PO and RO/EIE-PO are depicted in Fig. 2a and b. The DSC melting curve of pure rapeseed oil (Curve 7, Fig. 2a) is made of a main very low melting peak (VLMP), in accordance with literature data [22]. The addition of palm oil to RO leads to the appearance of a higher melting peak. The temperature of this peak gradually increases with the amount of palm oil present in the blend (see arrows, Fig. 2a). Besides this higher melting peak, the VLMP tends to be split into a low melting (LM) and a medium melting (MM) region upon addition of palm oil, the MM then being incorporated in the LM region present in the DSC melting curve of pure palm oil (shoulders on the right side). As a result of interesterification, the DSC melting profile of EIE-palm oil (Curve 1, Fig. 2b) is significantly modified compared to the one of the corresponding native oil (Curve 1, Fig. 2a). Three distinct melting areas (LM-MM-HM) (Curve 1, Fig. 2a) were observed, while only two were present in the DSC melting profile of the native PO (Curve 1, Fig. 2a): a low melting (LM) between −30° and 14 °C followed by a higher temperature broad melting area (HM) between 14 and 42 °C, in agreement with previous results [18]. The modification of the melting behaviour is due to the random redistribution of the fatty acids on the glycerol during interesterification (Table 1). A significant increase of the trisaturated TAG (SSS) content is observed upon interesterification from 7.8 to 12.9 %; the triUnsaturated TAG (UUU) content is also highly increased in the interesterified products: from 7.6 to 13.1 %. Both monoUnsaturated (SUS) and diUnsaturated (SUU) TAG contents are decreased accordingly (from 46.0 to 40.4 % and from 38.6 to 33.6 %, respectively). As a result of these modifications in the TAGs composition, the LM area observed in the native oil is now split in a low melting (LM) (−30 to 5 °C) and a medium melting area (MM) (5° to 15 °C). The HMP present in the DSC melting profile of the native oil remains, although it is now shifted to higher temperatures (15–45 °C instead of 14–42 °C), in agreement with recent literature data [3, 13]. Besides the shift to higher temperatures, the intensity of the HM region is also increased. The relative partial melting enthalpy of the HMP ((ΔH HMP/ΔH Total)*100) is increased from ~40 to ~70 % after B-EIE. These temperature/intensity increases and enthalpy modifications in the HM region are obviously related to the higher SSS content in the interesterified product (Table 1) [13].
a, b, c, d: DSC melting profiles of a) RO/PO blend (from bottom to top: curve 1: 100 %PO, curve 2: 80 %PO, curve 3: 70 %PO, curve 4: 50 %PO, curve 5: 70 %PO, curve 6: 20 %PO, curve 7: 100 %RO) b) RO/EIE-PO blend (from bottom to top: curve 1: 100 % EIE-PO, curve 2: 70 %EIE-PO, curve 3: 50 %EIE-PO, curve 4: 30 %EIE-PO, curve 5: 20 %EIE-PO, curve 6: 100 %RO) c) AMF/PO blend (from bottom to top 100 %PO, 90 %PO, 80 %PO, 70 %PO, 60 %PO, 50 %PO, 40 %PO, 30 %PO, 20 %PO, 10 %PO, 100 %AMF) d) AMF/EIE-PO blend (from bottom to top 100 %EIE-PO, 90 %EIE-PO, 80 %EIE-PO, 70 %EIE-PO, 60 %EIE-PO, 50 %EIE-PO, 40 %EIE-PO, 30 %EIE-PO, 20 %EIE-PO, 10 %EIEPO, 100 %AMF)
Regarding the binary blend made of RO and B-EIE-PO, it also presents a higher melting peak due to the presence of the interesterified fat (see arrows, Fig. 2b). The intensity of this peak is higher compared to the native system, with corresponding peak temperatures also being slightly higher. The shape of the low/medium melting region is also modified in the system containing the B-EIE-PO, compared to the RO/PO system.
Blends Containing AMF
The DSC melting profiles of the AMF/PO and AMF/EIE-PO blends are shown in Fig. 2c and d, respectively. The typical DSC melting curve of AMF contains three main endothermic regions (LM-MM-HM) [23]. In the AMF-PO blend, the temperature of the high melting peak (HM) is gradually changing with the amount of palm oil (from 34° C for pure AMF (curve 1) to 38° C for pure PO (curve 11), see arrow, Fig. 2c). For intermediate compositions, a medium melting peak (MM) is clearly observed (see Fig. 2c). This medium melting peak is more intense and thinner in the blends made of 70 % AMF–30 %PO (Curve 8) and 60 % AMF–40 %PO (Curve 5). The shape of the melting curve is changing within the low melting (LM) region upon PO addition. This low melting peak is more pronounced for blends containing more than 60 % of PO. After B-EIE (Fig. 2d), the evolution of the high melting peak is also gradual from 0 to 100 % EIE-PO. However, the temperature increase is more pronounced compared to the previous binary blend: from 34 °C for pure AMF (curve 1) to 42 °C for pure EIE-PO (curve 11) (see arrow Fig. 2d).
Due to the TAG composition modifications resulting from B-EIE, contrarily to the former blend made of AMF and native PO, the evolution of the LMP is more gradual from 0 to 100 % EIE-PO. The peak temperature varies from 5 °C for pure AMF (curve 1) to −3 °C for pure EIE-PO (curve 11) (see arrow Fig. 2d). In view of those results, it is obvious that the behaviour of the binary blend involving AMF is modified after B-EIE of PO. The interesterification of palm oil has changed its miscibility with AMF.
Regarding the SFC melting curves, at 10 °C the SFC of AMF and PO are similar. However, at that temperature, all the binary combinations contain a lesser amount of solid fat compared to both pure components (data not shown). At higher temperatures, this is not the case anymore: all the profiles are located between those of the pure fats [11, 24]. SFC of a fat blend can sometimes be lower than that of both pure fats due to interactions between the two fats [21]. To highlight this phenomenon and better compare both systems (AMF/PO and AMF/EIE-PO systems), their miscibility was investigated by means of iso-solid diagrams (temperature/composition). Figure 3a and b show the iso-solid diagrams constructed for AMF blended with PO and EIE-PO, respectively; the iso-solid lines presented correspond to 30 and 40 % of SFC. The results showed clear evidence of a eutectic interaction that occurred in the binary system made of AMF and PO. Indeed, depletion in the iso-solid diagram has been observed for compositions close to 70 % PO, especially at low temperatures. The eutectic effect is due to TAG complexity of those native fats, leading to immiscibility [11, 21]. However, after B-EIE of PO, the behaviour of the binary blend is modified: the strong depletion observed in the 40 % SFC iso-solid line has disappeared, due to rearrangements of FA after PO enzymatic IE (Fig. 3b). The new TAG composition obtained after B-EIE has modified the compatibility of the two fats. For example, after B-EIE, the amount of POP is significantly decreased (Table 1). The ratio between symmetric/asymmetric TAG is also changed (data not shown), and those TAG changes modify the compatibility of the oil when mixed with AMF. At higher temperatures, the iso-solid diagrams revealed continuous solid solutions for both systems. The iso-solid lines were approximately linear. Those results are similar to those of Nor Hayati et al. (2000), who found a better miscibility among the blended fats after interesterification of palm oil stearin and AMF by a sn-1,3-specific lipase [9]. B-EIE of PO modifies its melting behaviour and also its miscibility with other fats, depending on the type of oil. Under dynamic conditions, combined with rapeseed oil, the behaviour of the blend is not modified after B-EIE of PO; the values are simply higher. On the contrary, with AMF, besides an increase in the values, the type of interaction is modified.
Tempered Conditions
Investigations towards hardness, SFC and polymorphism were conducted after static crystallization and further storage at 15° C.
Polymorphism of the Pure Fats
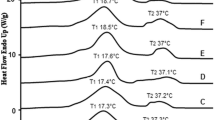
Results obtained for pure AMF, PO and EIE-PO are presented in Fig. 4. RO is not represented, as it is completely liquid under the same conditions.
After static crystallization and further storage for 48 h at 15 °C, both pure AMF and PO were crystallized in a β′-form. The polymorphic form of AMF (Fig. 4, curve 1) is β′2, and that of PO is β′1-form (Fig. 4, curve 2), in accordance with literature data [11, 25]. After interesterification, fatty acid redistributions create new intersolubility and this modifies polymorphism within the TAGs. The polymorphic stability of EIE-PO is modified compared to the native oil: besides the β′1-form, some β crystals are present after storage at 15 °C (Fig. 4, curve 3). This is due to the increase of SSS content upon interesterification (Table 1).
The SFC increase linked to the higher SSS content and the polymorphic behaviour modification can explain the differences observed in hardness between native and EIE-PO measured after the same crystallization and tempering procedures.
Textural Data
Blends Containing Rapeseed Oil
The hardness of the blends containing RO as a function of the amount of PO or B-EIE-PO is presented in Fig. 5a. It was not possible to measure the texture of the blends containing less than 40 % of hard fat (PO or BEIE-PO), due to the high amount of liquid oil in those blends. It is clear that the hardness of the blends containing the interesterified fat is significantly higher compared to the reference, whatever the hard fat concentration.
Blends Containing AMF
The hardness of the blends containing AMF as a function of the amount of PO or B-EIE-PO is presented in Fig. 5b. It is obvious that, as it was for blends containing RO, the hardness of the blends containing the interesterified fat is significantly higher compared to the reference, whatever the hard fat concentration. The hardness of all the blends, except two: 80 % B-EIE-PO/20 % AMF and 20 % B-EIE-PO/80 % AMF, is significantly different from that of pure AMF. Moreover, discontinuities are clearly observed within the textural data for both types of blends (see straight lines).
The SFC was measured after the same crystallization and tempering procedures for all the binary blends. Results are presented in Fig. 6. Two local minima/maximum appear in the SFC line for the AMF/PO blend, meaning again that the behaviour of this blend is not ideal. On the contrary, the evolution of the SFC line for the AMF/EIE-PO blend, under the same conditions, is more gradual, indicating again that the miscibility is better.
In order to understand the textural data, the polymorphism of both binary blends has been investigated under the same conditions; X-ray diffraction (XRD) patterns are presented in Fig. 5b, together with textural data. A β′-form is present in both blends (AMF/PO and AMF/EIE-PO) for all the compositions. However, the type of β′ sub-form observed gradually changes from β′2 for pure milk fat to β′1 for pure palm fat (mix of β′1 and β for pure B-EIE-PO). Besides a β′-form, a β-form (characteristic peak of the β-form at 4.59 Å) tends to clearly appear in some intermediate compositions. Discontinuities observed within the textural data are linked to polymorphic behaviour changes. Indeed, discontinuities correspond to β′2/β′1 transitions and β appearance/disappearance (see straight lines in Fig. 5b).
Moreover, as just stated, after B-EIE, the polymorphic behaviour of palm oil is modified compared to the native oil: a blend of β′1-form and β-form is observed after the same tempering. However, in the binary AMF/EIE-PO blend, the relative amount of β-form detected in the intermediate compositions is much lower compared to the corresponding initial PO/AMF blend (see Fig. 7). This is due to changes in the intersolubility behaviour within the new TAGs after EIE.
As observed under dynamic conditions, combined with rapeseed oil, the behaviour of the blend after static crystallization is not modified after B-EIE of PO; the values are simply higher. On the contrary, with AMF, besides an increase in the values, the type of interaction is modified; the miscibility of the AMF/EIE-PO system is increased due to B-EIE of PO.
Ternary Blends
Dynamic Conditions
Under dynamic conditions, comparison between the ternary iso-solid diagrams (exemplified here at 10 °C) shows that SFC of the blends containing EIE-PO (Fig. 8b) was higher compared to blends containing native PO (Fig. 8a). After B-EIE, SFC measured at 10 °C did not show any eutectic interactions in the ternary system, as illustrated in Fig. 8b. This again demonstrates a better miscibility among the fats involved in this new ternary system. The former eutectic interaction observed in the ternary PO/AMF/RO (Fig. 8a) blend disappeared. This is due to variations in the TAG composition after B-EIE of PO, leading to new intersolubility, as described for the binary blends containing AMF.
Tempered Conditions
Investigations towards polymorphism were conducted after static crystallization and further storage at 15 °C. All the measurements were carried out at 15 °C.
The polymorphic behaviours of ternary systems involving RO, AMF and PO or its B-EIE form, have also been compared in a same way. The ternary diagrams obtained are depicted in Fig. 9a and b.
The polymorphic stability of the ternary blend is also modified after B-EIE, due to fatty acids redistribution that creates new intersolubility behaviour within the TAG and modifies polymorphism.
Conclusions
Enzymatic interesterification modifies the TAG composition of fats, leading to new physicochemical properties of the final products. In this study, it was shown that batch enzymatic interesterification of palm oil modifies its melting behaviour, but also its polymorphic stability and miscibility with other fats, especially with anhydrous milk fat, which is one of the most complex fats. Indeed, after static crystallization and storage at 15 °C, the β-form is detected in EIE-PO, but not in the native PO. However, when blended with AMF, the β-form is mainly detected in PO-containing blends (not B-EIE-PO). This unexpected β-form is due to a compound formation between some AMF and PO TAGs. After B-EIE, fatty acid reorganizations within the TAG decrease this tendency.
Abbreviations
- AMF:
-
Anhydrous milk fat
- B-EIE:
-
Batch Enzymatic interesterification
- DSC:
-
Differential scanning calorimetry
- EIE-PO :
-
Enzymatically interesterified palm oil
- HMP:
-
High melting peak
- HMA:
-
High melting area
- LMP:
-
Low melting peak
- LMA:
-
Low melting area
- MF:
-
Milk fat
- MMP:
-
Medium melting peak
- MMA:
-
Medium melting area
- PO:
-
Palm oil
- RO:
-
Rapeseed oil
- SFC:
-
Solid fat content
- SFI:
-
Solid fat index
- TAG:
-
Triacylglycerol
References
Khatoon S., Khan M.I., Jeyarani T. (2012) Enzymatic interesterification of palm and coconut stearin blends. International J Food Sci Tech 1-7
Wan Rosnani AI, Nor Aini I (2007) Physico-chemical characteristics of palm-based oil blends for Ice cream. Palm Oil Dev 46:8–14
De Clercq N, Danthine S, Nguyen M, Gibon V, Dewettinck K (2012) Enzymatic interesterification of palm oil and fractions: monitoring the degree of interesterification using different methods. J Am Oil Chem Soc 89:219–229
Marangoni A, Rousseau D (1995) Engineering triacylglycerols: the role of interesterification. Trends in Food Sci Technol 6:329–335
Xu X, Guo Z, Zhang H, Vikbjerg A, Damstrup M (2006) Chemical and enzymatic interesterification of lipids for use in food. In: Gunstone FD (ed) Modifying lipids for use in food. Woodhead, Cambridge, pp 234–272
Lee Y, Tang T, Lai O (2012) Health benefits, enzymatic production, and application of medium- and long-chain triacylglycerol (MLCT) in food industries: a review. J Food Sci 77:137–144
Hartel R (1996) Applications of milk-fat fractions in confectionery products. J Am Oil Chem Soc 73:945–953
De BK, Hakimji M, Patel A, Sharma D, Desai H, Kumar T (2007) Plastic fats and margarines through fractionation, blending and interesterification of milk fat. Eur J Lipid Sci Techol 109:32–37
Nor Hayati I, Aminah A, Mamot S, Nor Aini I, Noor Lida HM, Sabariah S (2000) Melting characteristic and solid fat content of milk fat and palm stearin blends before and after enzymatic interesterification. J Food Lipids 7:175–193
Kaufmann N, Andersen U, Wiking L (2012) The effect of cooling rate and rapeseed oil addition on the melting behavior, texture and microstructure of anhydrous milk fat. Int Dairy J 25:73–79
Danthine S (2012) Physicochemical and structural properties of compound dairy fat blends. Food Res Int 48:187–195
Kaufmann N, Kirkensgaards J, Andersen U, Wiking L (2013) Shear and rapeseed oil addition affect the crystal polymorphic behavior of milk fat. J Am Oil Chem Soc 90:871–880
Danthine S., De Clercq N., Dewettinck K., Gibon V. Monitoring batch lipase catalyzed interesterification of palm oil and fractions by differential scanning calorimetry. J Thermal Analysis Calorimetry (doi 10.1007/s10973-014-3645-2)
De BK, Patel JD (2010) Modification of palm oil by chemical and enzyme catalyzed interesterification. J Oleo Sci 59(6):293–298
Soares FASDM, da Silva RC, Hazzan M, Capacla IR, Viccola ER, Maruyama JM, Gioielli LA (2012) Chemical interesterification of blends of palm stearin, coconut oil, and canola oil: physicochemical properties. J Agric Food Chem 60:1461–1469
Siti HMF, Norizzah AR, Zaliha O (2013) Effect of interesterification on the physicochemical, microstructural and thermal properties of palm stearin, palm kernel oil and soybean oil blends. Food Chem 137:8–17
AOCS (1997) Official Methods and Recommended Practices. In: Firestone D (ed) 5th Edn. AOCS, Champaign
Braipson-Danthine S, Gibon V (2007) Comparative analysis of triacylglycerol composition, melting properties and polymorphic behavior of palm oil and fractions. Eur J Lipid Sci Technol 109:359–372
Braipson-Danthine S, Deroanne C (2006) Determination of solid fat content (SFC) of binary fat blends and use of these data to predict SFC of selected ternary fat blends containing low-erucic rapeseed oil. J Am Oil Chem Soc 83:571–581
IUPAC (1987) Standard Methods for the Analysis of Oils, Fats and Derivatives, 7th edn. Blackwell Scientific Publications, London
Timms RE (1984) Phase behaviour of fats and their mixtures. Prog Lipid Res 23:1–38
Danthine S, Deroanne C (2003) Physical and textural characteristics of hydrogenated low-erucic acid rapeseed oil and low-erucic acid rapeseed oil blends. J Am Oil Chem Soc 80(11):1069–1075
tenGrotenhuis E, van Aken G, van Malssen K, Schenk H (1999) J Am Oil Chem Soc 76:1031–1039
Abd El-Aziz M, Mahran GA, Asker AA, Sayed AF, El-Hadad SS (2013) Blending of butter oil with refined palm oil: impact on physicochemical properties and oxidative stability. Int J Dairy Sci 8(2):36–47
Noor Lida HMD, Md Ali AR (1998) Physico-chemical characteristics of palm-based oil blends for the production of reduced fat spreads. J Am Oil Chem Soc 75(11):1625–1631
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Danthine, S., Lefébure, E., Trinh, H.N. et al. Effect of Palm Oil Enzymatic Interesterification on Physicochemical and Structural Properties of Mixed Fat Blends. J Am Oil Chem Soc 91, 1477–1487 (2014). https://doi.org/10.1007/s11746-014-2494-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-014-2494-2