Abstract
Chemical interesterification of different lipid materials has considerable potential for the production of a wide variety of special fats with improved functional and nutritional properties. The present study aimed to evaluate the chemical interesterification of blends of high-oleic sunflower oil (HOSO) and fully hydrogenated palm oil (FHPO) in the ratios (% w/w) of 80:20, 70:30, 60:40 and 50:50. The blends were characterized in triacylglycerol composition, melting point, solid fat content and crystallization behavior, and some applications in food products were suggested. The interesterification altered the solid fat content, melting point and crystallization isotherm of the samples, after the levels of trisaturated triacylglycerols decreased and disaturated–monounsaturated and monosaturated–diunsaturated triacylglycerol contents increased, due to the randomization of fatty acids. The modification in the triacylglycerol composition promoted greater miscibility between the HOSO and FHPO fractions, creating new application possibilities for the food industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The objective of interesterification as a technological process is to modify the plasticity of oil and fat mixtures by changing their triacylglycerol composition to improve crystallization and melting characteristics of the blends for industrial applications. The triacylglycerol composition is modified by rearranging the fatty acids among the glycerol molecules, and therefore, not altering the initial fatty acid composition. In the chemical interesterification reaction, the fatty acids exchange on the triacylglycerol molecules occurs in a random way, that is, the equilibrium of the fatty acids possible arrangements in the glycerol molecule is reached at the end of the process [1, 2]. Thus, chemical interesterification is an important technological process for the production of zero-trans fats for different industrial purposes. The interesterified fats can be used in various applications by replacing the partial hydrogenated fats; consequently, interesterification can successfully substitute the partial hydrogenation process [3–5].
Fully hydrogenated palm oil presents high palmitic acid content, which has a natural tendency to crystallize in the β′ form, thus providing an aerating capability [6] and also favorable melting and crystallization characteristics to some fat products [7]. Apart from the fact that interesterification is an alternative for developing fats with zero trans isomers, the use of high-oleic sunflower oil could reduce the risk of coronary heart disease due to the reduced susceptibility of low-density lipoprotein (LDL) to oxidation [8].
Studies carried out by Ahmadi and Marangoni [9] showed that the chemical interesterification of blends of high-oleic sunflower oil and fully hydrogenated rapeseed and soybean oils could be used as an alternative to partial hydrogenation to produce plastic fats suitable for commercial applications in cookies. Dian et al. [10] demonstrated that chemical interesterification significantly modified the triacylglycerol composition, and consequently the solid fat content of the palm stearin/sunflower oil blends, contributing to the production of interesterified blends with characteristics suitable for margarines and fat spreads applications. According to Lida et al. [11], the chemical interesterification of palm oil, sunflower oil and/or palm kernel olein blends could provide an alternative for the design of new fats with specific solid profiles.
The objective of the present study was to evaluate the chemical interesterification of binary blends of high-oleic sunflower oil and fully hydrogenated palm oil, at different ratios, in order to produce fats with plastic characteristics desirable for different food applications. Triacylglycerol composition, solid fat content and crystallization behavior of the chemically interesterified blends were analyzed and the results were compared with those of the non-interesterified blends.
Experimental Procedures
Materials
Raw Materials
Refined high-oleic sunflower oil (HOSO) was provided by Pepsico (SP, BRA), and fully hydrogenated palm oil (FHPO) was provided by A. Azevedo Indústria e Comércio de Óleos Ltda (SP, BRA). Sodium methoxide powder (99 %, provided by Sigma-Aldrich––St. Louis, MO, USA) was the catalyst for the chemical interesterification reaction. The blends were prepared in the following ratios: high-oleic sunflower oil and fully hydrogenated palm oil at 80:20, 70:30, 60:40 and 50:50 (% w/w).
Methods
Chemical Interesterification
On a laboratory scale, 100 g of each blend was added 0.4 % (w/w) of sodium methoxide, and stirred at 500 rpm for 20 min at 100 °C in a closed vessel, according to the optimized methods performed by Grimaldi et al. [12]. After the reaction time, distilled water and 5 % citric acid solution were added to stop the reaction. The interesterified samples were washed with distilled water (80 °C) to remove impurities, and then dried under a vacuum at 110 °C for 30 min. The samples were subsequently clarified (80 °C for 20 min) by using 0.5 % Tonsil Optimum 380 FF (Química Sumex, Mexico City, Mexico) bleaching earth.
Analyses
The peroxide value (PV) and free fatty acid (FFA) content were determined by AOCS methods Cd 8b-90 and Ca 5a-40, respectively [13], and the results were expressed as the means of three determinations.
Fatty acid composition was determined by AOCS method Ce 1-62 [13]. The methyl esters were prepared according to Hartman and Lago [14]. The analyses were performed by capillary gas chromatography (Agilent 6850 series GC system, Santa Clara, CA, USA) with capillary column DB-23 (50 % cyanopropyl-methyl polysiloxane, 60 m long, 0.25 mm internal diameter, 0.25 μm film). Conditions of analysis: oven temperature set at 110 °C for 5 min, then programmed to increase to 215 °C at a rate of 5 °C/min, and 215 °C for 24 min; detector temperature: 280 °C; injector temperature: 250 °C; carrier gas: helium; split injection ratio of 1:50; injection volume: 1.0 μL. The qualitative composition was determined by comparison of retention times with those of fatty acid standards. Samples were analyzed in duplicate and the values reported as the means of two injections.
The iodine value (IV) was calculated from the fatty acid composition according to AOCS method Cd 1c-85 [13].
Triacylglycerol composition was determined in duplicate according to AOCS method Ce 5-86 [13] by capillary gas chromatography (Agilent 6850 series GC system, Santa Clara, CA, USA) with a capillary column DB-17HT (Agilent catalog no. 122-1811, 50 %-methyl-phenyl polysiloxane, 15 m long, 0.25 mm internal diameter and 0.15 μm film). Conditions of analysis: split injection ratio of 1:100; column temperature: set at 250 °C and programmed to increase to 350 °C at a rate of 5 °C/min; carrier gas: helium at a flow rate of 1.0 mL/min; injector temperature: 360 °C; detector temperature: 375 °C; injection volume: 1.0 μL; sample concentration: 10 mg/mL in tetrahydrofuran. The identification of the triacylglycerol groups was performed by comparison of retention times [15].
Solid fat content (SFC) was determined using a nuclear magnetic resonance spectrometer Bruker pc 120 Minispec (Silberstreifen, Rheinstetten, Germany), according to AOCS direct method Cd 16b-93 [13]. The samples were first melted at 70 °C for 10 min and then cooled at 0 °C for 90 min, in a high precision TCON 2000 dry bath (Duratech, Carmel, IN, USA). After this initial preparation, the samples were held at each measuring temperature (10, 20, 25, 30, 35, 40, 45, 50, 55 and 60 °C) for 30 min before SFC measurements. Each result was expressed as the mean of two determinations. The isosolid diagrams were constructed with data provided by SFC analyses [16, 17].
The melting point (MP) was obtained from the SFC curves measured by NMR as the temperature corresponding to 4 % SFC [18, 19], calculated by polynomial equations using an equation software.
Free fatty acids and partial triacylglycerols (diacylglycerols and monoacylglycerols) were removed from the system according to the methodology described by Farmani et al. [20].
Determination of lipid classes (triacylglycerols, diacylglycerols, monoacylglycerols and free fatty acids) was performed by a liquid chromatography Perkin Elmer type 250 instrument (Waltham, MA, USA) equipped with a Sicon Analytic refractive index detector (Hitachi High Technologies America, Schaumburg, IL, USA), and two columns (500 Å, 300 × 7.8 mm and 100 Å, 300 × 7.8 mm) (Jordi Gel DVB, Apple Valley, MN, USA). Conditions of analysis: samples diluted at a ratio of 1:100 (v/v) in tetrahydrofuran; mobile phase: tetrahydrofuran at a flow rate of 1.0 mL/min; injection volume: 20.0 μL. The analyses were performed in duplicate and the values reported as means.
Isothermal crystallization was determined by the increase in SFC as a function of crystallization time, monitored by nuclear magnetic resonance spectrophotometry (NMR) (Bruker pc120 Minispec, Silberstreifen, Rheinstetten, Germany) with the reading compartment stabilized at 25 °C. Prior to data acquisition, samples were melted (100 °C for 15 min) and kept at 70 °C for 1 h in a high precision dry bath (TCON 2000, Duratech, Carmel, IN, USA) to eliminate all previous crystallization memory [21]. Each result was expressed as the mean of two determinations.
Results and Discussion
Quality Characteristics of the Raw Materials
The quality characteristics of the raw materials complied with the Brazilian legislation [22], which established the limits of 0.3 % and 10 mequiv O2/kg of FFA and PV for refined fats and oils, respectively. HOSO and FHPO presented FFA values of 0.03 and 0.15 %, respectively, while HOSO showed a PV of 3.0 mequiv O2/kg.
Fatty Acid Composition
Table 1 shows the fatty acid compositions and iodine values of the raw materials and blends. According to O’Brien [23], sunflower oil and HOSO have 14–39.4 % and 75–85 % of oleic acid content, respectively. In this study, HOSO had a high oleic acid content (76.1 %), as expected, followed by linoleic acid (14.1 %). The FHPO was mainly comprised of stearic (55.5 %) and palmitic (42.4 %) acids.
The percentages of unsaturated fatty acids in the HOSO and FHPO were 91.4 and 0.1 %, respectively, and ranged from 45.9 to 73.2 % in the blends. Neither the raw materials nor the blends contained trans fatty acids, considered nutritionally undesirable fatty acids [19].
Triacylglycerol Composition
The triacylglycerol composition is an important parameter for evaluating interesterified products, since it can be an important tool for characterizing the physical changes and crystalline form of the developed product [12]. Table 2 shows the main triacylglycerols of the raw materials and blends before and after interesterification. HOSO contained 14 main triacylglycerol species, while FHPO contained about 6 species. OOO was the predominant TAG species in HOSO (61.4 %), whereas the predominant species in FHPO were PStSt (41.7 %), PPSt (39.3 %) and StStSt (11.4 %).
The study of the blends before and after interesterification suggested that the PPSt content was reduced during the reaction, whereas the StOO content increased from 2.6 to 15.1 %. Species with 56 or more carbon number was extinct upon interesterification. After randomization, the distribution of fatty acids on the TAG molecules changed and new species were formed including POP, PPLi, PStO, StStO and StOLi. The triacylglycerol composition indicated that the randomization process occurred in a consistent way, which is highly important since it allows industry to modify these fats and target their applications [23].
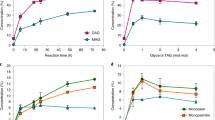
Figure 1 shows the trisaturated (SSS), disaturated–monounsaturated (SUS), monosaturated–diunsaturated (SUU), and triunsaturated (UUU) triacylglycerol contents before and after the interesterification process. As expected, HOSO presented 99.4 % of unsaturated triacylglycerols (SUU and UUU), whereas FHPO contained 99.5 % trisaturated triacylglycerols. Among the blends, Fig. 1 clearly exhibits an accentuated decrease in the content of SSS triacylglycerols for the interesterified samples. The UUU triacylglycerol content was also reduced whereas the SUS and SUU contents significantly increased. A similar effect on the triacylglycerol composition was found by Silva et al. [24], in which study the chemical interesterification of olive oil and palm stearin blends were evaluated.
After interesterification, the blends showed more balanced compositions especially the 60:40 and 50:50 (HOSO: FHPO) blends, in which SUS species (30.8 and 36.1 %, respectively) were not present before interesterification. In general, interesterification resulted in the formation and increased contents of intermediate melting point triacylglycerols, as also shown by Ribeiro et al. [17], who investigated the chemical interesterification of soybean oil and fully hydrogenated soybean oil.
Melting Point (MP)
According to Karabulut et al. [18], the MP of fats and oils change with the length of the fatty acid chain, degree of unsaturation, trans fatty acid content and position of the fatty acids in the glycerol molecule. Table 3 shows the MP of the binary blends before and after interesterification.
The interesterified blends showed a wider range of MP (21.9–46.1 °C) if compared to the non-interesterified blends (48.5–54.4 °C). The chemical interesterification reduced the MP of the HOSO:FHPO blends due to a decrease in high MP triacylglycerols (SSS), specially PPSt and PStSt. Similar changes in the MP of binary and ternary blends due to the fatty acids randomization have also been reported in the literature [17, 18, 20]. Although the MP is not considered a determinant factor in the development of fats, it is also associated with functional applications of a specific fat.
Solid Fat Content (SFC)
Figure 2 shows the SFC of the binary blends before and after chemical interesterification. The SFC of the non-interesterified mixtures was directly related to the concentrations of FHPO and HOSO. Interesterified blends showed reduced SFC at all measured temperatures, indicating that the blends became more fluid. At 35 °C, the non-interesterified blends had SFC varying from 15.0 to 45.5 %, and after randomization the SFC values considerably decreased (0.4–19 %). These SFC reductions are important to the sensory properties of fat products at the human body temperature due to the possibility of higher SFC (>4 %) cause waxy sensation in the mouth. These results are linked to the decrease of trisaturated (SSS) triacylglycerols and simultaneous increase of disaturated–monounsaturated (SUS) and monosaturated–diunsaturated (SUU) triacylglycerols, represented mainly by the species POP/PStO/StStO and POO/StOO, respectively, as shown in Table 2 and Fig. 1. The interesterified blends of the presented study exhibited a wide plasticity range. According to Ribeiro et al. [19], the increase in the SUS and SUU contents caused by interesterification may improve technological functionality and sensory characteristics, contributing for the use of interesterified fats in the food industry.
Isosolid Curve Diagrams
The SFC can also be used as a tool for verifying the compatibility of fats in different binary systems as continuous solid solutions, eutectic systems and monotectic systems. The degree of compatibility among different fats is generally associated with the preferred polymorphic form of each triacylglycerol on the fat system [25]. Figure 3 shows the isosolid curve diagrams plotted from the SFC data of the blends, before and after interesterification. The isosolid phase behavior was evaluated from 12 to 22 % of SFC (before interesterification) and from 2 to 12 % of SFC (after interesterification) as a function of temperature and the addition of fully hydrogenated palm oil to high-oleic sunflower oil. The lack of depression of isosolid lines probably inferred that the HOSO:FHPO blends did not exhibit eutectic behavior. Figure 3a suggests monotectic interactions that were attenuated (Fig. 3b) because of the random rearrangement of the fatty acids on the glycerol molecule. According to Timms [25], the monotectic systems occur when high MP triacylglycerols are dissolved in liquid triacylglycerols, presenting differences in MP above 20 °C between both components, as also verified in the current work for FHPO and HOSO. The monotectic system was also found in studies of binary blends carried out by Braipson-Danthine and Deroanne [16] and Ribeiro et al. [17].
The linear behavior observed after interesterification (Fig. 3b) shows that the process reduced incompatibility, probably due to the formation of a more stable and common polymorphism crystal to the mixture, thus the interesterified blends may provide a greater range of industrial applications in comparison to the non-interesterified blends [26].
Diacylglycerol Effects on the Crystallization Properties of Interesterified Blends
Randomization of the fatty acids on the glycerol molecule during the chemical interesterification results in a considerable formation of diacylglycerols, which have a fundamental role in the quality of various products, since they can affect crystallization kinetics. In some cases, the presence of these minor lipids can favor crystallization and, in others, an inhibition effect is observed [27]. For instance, the addition of 30 and 50 % of palm-based diacylglycerol in palm oil increased significantly the melting point and crystallization onset temperature, however an addition of 2 or 5 % of diacylglycerols did not alter these parameters, according to Saberi et al. [28]. Smith et al. [29] reviewed several publications about the effects of diacylglycerol on the fat crystallization. It was reported that diacylglycerols as other minor components as free fatty acids and monoacylglycerols can vary considerably among fats and even among different batches of the same material. Diacylglycerol presence in different lipid matrix can either induce or inhibit the crystal formation depending on the fat system and also on the diacylglycerol type and quantity in the sample [29]. Therefore, diacylglycerol effects in each lipid system should be considered as an important factor for evaluation.
Table 4 shows the FFA and partial acylglycerol contents of the HOSO:FHPO blends after interesterification. Considering the raw materials used for the preparation of the blends before interesterification, high oleic sunflower oil did not contain considerably monoacylglycerol/diacylglycerol whilst fully hydrogenated palm oil presented ~5 % of diacylglycerols and 95 % of triacylglycerols in its composition.
The 60:40 HOSO:FHPO interesterified blend, which had the highest diacylglycerol content (9.6 %), was submitted to purification to remove the FFA and partial acylglycerols. Removal of diacylglycerols was performed with the addition of an equal volume of 96 % ethanol (at approximately 50 °C) to the melted sample and by posterior separation of the ethanol phase. This procedure was repeated five times and traces of ethanol were removed by evaporation with a pure nitrogen stream [20]. After purification, the blend presented 93.5, 5.8 and 0.7 % of triacylglycerol, diacylglycerol and monoacylglycerol contents, respectively, achieving an approximately 60 % reduction in the diacylglycerol level for the 60:40 HOSO:FHPO interesterified blend.
For evaluating the diacylglycerol effects on the crystallization properties of the interesterified blends, the isothermal crystallization behavior of the 60:40 blend were determined before and after the purification procedure (Fig. 4). No differences were found in the crystallization behavior of the 60:40 interesterified blends before and after removal of diacylglycerols, probably because the diacylglycerols formed during the interesterification did not significantly interfere with the crystal formation kinetics at 25 °C in this sample. Due to this result, the FFA and partial acylglycerol removal was considered unnecessary for the interesterified blends developed in the present study and, therefore, was not performed for the other samples.
Isothermal Crystallization
Figure 5 shows the isothermal crystallization curves at 25 °C for the different fractions of high-oleic sunflower oil and fully hydrogenated palm oil, before and after chemical interesterification. Table 5 shows the values for the induction period (τSFC) and the maximum solid fat content (SFCmax) for all blends.
The values for SFCmax and τSFC for the non-interesterified blends varied from 19 to 48 % and from 4 to 7 min, respectively, whereas the interesterified blends showed SFCmax values between 6 and 28 % and τSFC values between 6 and 13 min. The crystallization kinetics of the blends showed an increase in the induction period for crystal formation and a decrease in the SFCmax of the interesterified HOSO:FHPO blends compared to the initial mixtures.
Considering the exposed physico-chemical properties of the fat systems obtained from chemical interesterification of HOSO and FHPO, some possible food applications can be suggested for these specific interesterified blends. The interesterified 80:20 HOSO: FHPO blend presented SFC curve similar to the range showed to use in soft tub margarines formulation [30], while the interesterified 70:30 and 50:50 HOSO: FHPO blends exhibited SFC profiles suitable for use in margarines [24, 31] and puff pastry margarines [31], respectively. The interesterified 60:40 HOSO:FHPO blend showed a SFC profile that suggests potential application in the formulation of biscuit fillings [32].
Conclusions
Chemical interesterification modified the solid fat contents, melting points and isothermal crystallization behaviors of the binary HOSO:FHPO blends in all proportions studied, due to the reduction in SSS and increase in SUS and SUU triacylglycerol contents. Changes in the triacylglycerol composition improved miscibility between high-oleic sunflower oil and fully hydrogenated palm oil. The interesterified 80:20; 70:30; 60:40; and 50:50 HOSO:FHPO blends are attractive as potential applications for soft tub margarines, margarines, biscuit fillings and puff pastry margarines, respectively, with the additional advantage of the high-oleic sunflower oil health benefits. Further studies related to a direct application of the developed fats among the suggested uses should be considered for confirming an appropriate behavior of these blends in each formulation.
References
Sreenivasan B (1978) Interesterification of fats. J Am Oil Chem Soc 55:796–805
Konishi H, Neff WE, Mounts TL (1993) Chemical interesterification with regioselectivity for edible oils. J Am Oil Chem Soc 70:411–415
Wainwright B (2000) Specialty fats and oils. In: O’Brien RD, Farr WE, Wan PJ (eds) Introduction to fats and oils technology. AOCS Press, Champaign, pp 505–511
Dijkstra AJ (2004) Edible oil processing, quo vadis. Eur J Lipid Sci Tech 106:77–78
Scrimgeour CM, Harwood JL (2007) Fatty acid and lipid structure. In: Gunstone FD, Harwood JL, Dijkstra AJ (eds) The lipid handbook. Taylor & Francis Group, Boca Raton, pp 1–36
Nor Aini I, Miskandar MS (2007) Utilization of palm oil and palm products in shortenings and margarines. Eur J Lipid Sci Tech 109:422–432
Mayamol PN, Balachandran C, Samuel T, Sundaresan A, Arumughan C (2009) Zero trans shortening using rice bran oil, palm oil and palm stearin through interesterification at pilot scale. Int J Food Sci Tech 44:18–28
Ashton EL, Best JD, Ball MJ (2001) Effects of monosaturated enriched sunflower oil on the CHD risk factors including LDL size and copper-induced LDL oxidation. J Am Coll Nutr 20:320–326
Ahmadi L, Marangoni AG (2009) Functionality and physical properties of interesterified high oleic shortening structured with stearic acid. Food Chem 117:668–673
Dian NLHM, Sundram K, Idris NA (2007) Effect of chemical interesterification on triacylglycerol and solid fat contents of palm stearin, sunflower oil and palm kernel olein blends. Eur J Lipid Sci Tech 109:147–156
Lida HMDN, Sundram K, Siew WL, Aminah A, Mamot S (2002) TAG composition and solid fat content of palm oil, sunflower oil, and palm kernel olein blends before and after chemical interesterification. J Am Oil Chem Soc 79:1137–1144
Grimaldi R, Gonçalves LAG, Ando MY (2005) Optimization of chemical interesterification of palm oil––(in Portuguese). Quim Nova 28:633–636
AOCS (2009) Official methods and recommended practices of the American Oil Chemists’ Society. AOCS, Champaign
Hartman L, Lago RC (1973) Rapid preparation of fatty acid methyl esters from lipids. Lab Pract 22:475–476
Antoniosi Filho NR, Mendes OL, Lanças FM (1995) Computer prediction of triacylglycerol composition of vegetable oils by HRGC. Chromatographia 40:557–562
Braipson-Danthine S, Deroanne C (2006) Determination of solid fat content (SFC) of binary fat blends and use of these data to predict SFC of selected ternary fat blends containing low-erucic rapeseed oil. J Am Oil Chem Soc 83:571–581
Ribeiro APB, Grimaldi R, Gioielli LA, Gonçalves LAG (2009) Zero trans fats from soybean oil and fully hydrogenated soybean oil: physico-chemical properties and food applications. Food Res Int 42:401–410
Karabulut I, Turan S, Ergin G (2004) Effects of chemical interesterification on solid fat content and slip melting point of fat/oil blends. Eur Food Res Tech 218:224–229
Ribeiro APB, Masuchi MH, Grimaldi R, Gonçalves LAG (2009) Chemical interesterification of soybean oil and fully hydrogenated soybean oil: influence of the reaction time––(in Portuguese). Quim Nova 32:939–945
Farmani J, Hamedi M, Safari M, Madadlou A (2007) Trans-free Iranian vanaspati through enzymatic and chemical transesterification of triple blends of fully hydrogenated soybean, rapeseed and sunflower oils. Food Chem 102:827–833
Campos R (2005) Experimental methodology. In: Marangoni AG (ed) Fat crystal networks. Marcel Dekker, New York, pp 267–349
Brazil (2005) National Health Surveillance Agency (ANVISA––Agência Nacional de Vigilância Sanitária) Ministério da Saúde. Resolução RDC n° 270, de 22 de Setembro de 2005. Regulamento Técnico para Fixação de Identidade e Qualidade de Óleos e Gorduras Vegetais. Diário Oficial da União, Brasília
O’Brien RD (2004) Fats and oils––formulating and processing for applications, 2nd edn. CRC Press, New York
Silva RC, Soares DF, Lourenço MB, Soares FASM, Silva KG, Gonçalves MIA, Gioielli LA (2010) Structured lipids obtained by chemical interesterification of olive oil and palm stearin. Food Sci Tech 43:752–758
Timms RE (1984) Phase behaviour of fats and their mixtures. Prog Lipid Res 23:1–38
Rousseau D, Marangoni AG (2002) The effects of interesterification on the physical properties of fats. In: Marangoni AG, Narine SS (eds) Physical properties of lipids. CRC Press, Boca Raton, pp 479–565
Metin S, Hartel RW (2005) Crystallization of fats and oils. In: Shahidi F (ed) Bailey’s industrial oil and Fat products. Wiley Interscience, New York, pp 45–76
Saberi AH, Lai OM, Toro-Vázquez JF (2011) Crystallization kinetics of palm oil in blends with palm-based diacylglycerol. Food Res Int 44:425–435
Smith KW, Bhaggan K, Talbot G, van Malssen KF (2011) Crystallization of fats: influence of minor components and additives. J Am Oil Chem Soc 88:1085–1101
Petrauskaite V, De Greyt W, Kellens M, Huyghebaert A (1998) Physical and chemical properties of trans-free fats produced by chemical interesterification of vegetable oil blends. J Am Oil Chem Soc 75:489–493
Dijkstra AJ (2007) Modification processes and food uses. In: Gunstone FD, Harwood JL, Dijkstra AJ (eds) The lipid handbook. CRC Press, Boca Raton, pp 263–353
Ghotra BS, Dyal SD, Narine SS (2002) Lipid shortenings: a review. Food Res Int 35:1015–1104
Acknowledgments
The authors thank the Brazilian research funding agencies FAPESP, CNPq and CAPES for the financial support.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Masuchi, M.H., Gandra, K.M., Marangoni, A.L. et al. Fats from Chemically Interesterified High-Oleic Sunflower Oil and Fully Hydrogenated Palm Oil. J Am Oil Chem Soc 91, 859–866 (2014). https://doi.org/10.1007/s11746-014-2420-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-014-2420-7