Abstract
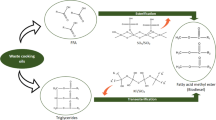
A heterogeneous catalyst, FeO x /SiO2, prepared by the pore-filling method, was found to be active in the transesterification of crude Jatropha oil with methanol. When the transesterification reaction was carried out with a reaction temperature of 220 °C, a catalyst amount of 15 wt%, a methanol/oil molar ratio of 218:1, and a reaction time of 3 h, the yield of fatty acid methyl esters (FAME) in the product exceeded 99.0 %, and met with EN standards for allowable contents of glycerine and mono-, di-, and tri-glycerides. The correlation between the FAME production activity and measured acidity of the FeO x /SiO2 catalysts showed that the transesterification reaction was promoted via the acidic function of these catalysts, which are less inhibited by coexisting free fatty acids in the feedstock triglycerides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The production of biodiesel, a renewable alternative fuel created from vegetable oils and animal fats through a chemical process, has increased dramatically in recent years. Biodiesel is a fuel composed of mono-alkyl esters of long-chain fatty acids derived from vegetable oils or animal fats by alcoholysis with low molecular weight alcohols. Homogeneous base catalysts, such as sodium hydroxide, potassium hydroxide or potassium methoxide, are usually used as catalysts for the alcoholysis reaction [1]. However, alkali catalysts are subject to a saponification reaction with the coexisting free fatty acids (FFA) contained in certain vegetable oils, such as high-temperature-pressed vegetable oils and partly oxidized vegetable oils. This makes it difficult to separate the produced glycerin from the biodiesel. Moreover, this process uses a large amount of water and produces a large amount of waste water. The use of environmentally friendly solid catalysts in the alcoholysis process offers a promising potential solution to these problems.
Heterogeneous catalysis has an important role to play in the commercialization of vegetable oil alcoholysis because of its advantages as a non-corrosive, environmentally friendly process that creates less waste water. Heterogeneous catalysts are much easier to separate from liquid products, and possess longer catalyst lifetimes [2]. Furthermore, a heterogeneous catalyst produces a higher purity byproduct glycerin stream, because washing or neutralizing is not required for the product stream. Recently, the French Institute of Petroleum (IFP) commercialized the first heterogeneously catalyzed biodiesel production process, known as the Esterfip-H™ process. This process operates in a continuous mode, with two successive fixed-bed reactors, and uses a spinel mixed oxide made of Zn and Al as a solid base catalyst [3]. A solid catalyst that does not leach any catalytic components has the advantages of no saponification, ease of separation, no water being required for fatty acid methyl ester (FAME) washing, etc. However, this catalyst still permits the use of feedstocks with low acid values, such as <0.25 % FFA, which is the level of refined vegetable oils.
In general, the cost of biodiesel is dominated by the cost of the feedstock. Therefore, it would be beneficial to use low-cost crude vegetable oils and waste triglycerides containing high FFA content in the production of FAME. For example, the FFA content is more than 15 wt% in brown greases [4, 5], 4–15 wt% in yellow greases [5], up to 15 wt% in crude Jatropha oil [6], 23 wt% in sludge palm oil [7], and 2 wt% in crude palm oil [8]. When using homogeneous base catalysts in the production of FAME from these feedstocks containing high FFA [1], an esterification reaction using acid catalysts will be necessary in advance of transesterification. Biodiesel production from Jatropha oil has been reviewed by Juan et al. [9]. Many solid acid catalysts have been investigated for use in the production of biodiesel from high acid value vegetable oils. Basically, acid catalysts are suitable for esterification but not good for transesterification. For example, Peng et al. [10] prepared SO4 2−/TiO2–SiO2 solid for the production of biodiesel from cottonseed oil with the addition of 10–80 wt% of oleic acid at 200 °C with 3 wt% catalyst concentration. A 90 % FAME yield was obtained in 90 min using cottonseed oil with 80 % oleic acid addition. However, a lower yield of 60 % was obtained under the same reaction conditions using cottonseed oil with 10 % oleic acid addition. The FAME yield thus increased with increasing oleic acid content. Therefore, a catalyst for the production of biodiesel from feedstocks containing a wide range of FFA is highly desirable. Other forms of biodiesel production using heterogeneous catalysts were reviewed by Semwal et al. [11].
In this work, we investigated iron oxide catalysts supported on silica for methanolysis of crude Jatropha oil containing 1.3 wt% of FFA. The effect of the iron content of FeO x /SiO2 with 0.07–7.0 wt% as Fe was studied, and the acidic properties of the FeO x /SiO2 catalysts were collated with their catalytic performances.
Materials and Methods
Materials
Ammonium iron(III) citrate (assay: as Fe 16.0–19.0 %), tricaprin (98 % purity), methanol (99.8 %) and n-heptane (99.0 %) were purchased from Wako Pure Chemical Industries, Ltd., Osaka; and Oleic acid (99.9 %) was purchased from Maruzen Chemical Company, Ltd., Tokyo. The SiO2 support material (CARiACT Q10, particle size 200–500 μm) was purchased from Fuji Silysia Chemical, Ltd., Aichi. The surface area and average pore diameter of the SiO2 were 300 m2/g and 18.6 nm, respectively. Degummed crude Jatropha oil (phosphorous content = 7 ppm) was obtained from the Thailand Institute of Scientific and Technological Research (TISTR), Bangkok. The acid value and water content of the oil were determined according to the JIS testing methods, i.e., JIS K2501 (http://www.webstore.jsa.or.jp/webstore/Com/FlowControl.jsp?lang=en&bunsyoId=JIS+K+2501%3A2003&dantaiCd=JIS&status=1&pageNo=0 ) and JIS K2275 (http://www.webstore.jsa.or.jp/webstore/Com/FlowControl.jsp?lang=en&bunsyoId=JIS+K+2275%3A1996&dantaiCd=JIS&status=1&pageNo=0), respectively. The properties of the oil are shown in Table 1. Most notably, the oil contains 1.3 wt% of FFA. The gas chromatograph (GC) standards for FAME containing methyl esters of oleic acids, triolein, diolein and monoolein, were purchased from Sigma-Aldrich Co., Ltd., Taufkirchen, Germany.
Catalyst Preparation
A series of FeO x /SiO2 catalysts with iron oxide loadings varying from 0.07 to 7.0 % as Fe were prepared by the pore-filling method, using an ammonium iron(III) citrate aqueous solution with the SiO2 support. Here, a prescribed amount of an ammonium iron(III) citrate was dissolved in water which amount was the same as the pore volume of SiO2 support, and then this aqueous solution was impregnated into the SiO2 support under vacuum. The samples were dried at 105 °C for 12 h and subsequently calcined at 500 °C for 3 h in N2 or O2. The catalysts were designated as FeX/SiO2–Y 2, where X and Y 2 are the weight percentage of iron and the atmosphere of calcination, respectively. The dispersion of the iron species (i.e., the number of surface Fe Lewis acid sites and metallic Fe sites per total amount of Fe) was measured by a pulse NO chemisorption method. The catalysts investigated in this study are summarized in Table 2.
Catalyst Characterization
The surface area and pore volumes were measured by N2 adsorption–desorption with a BELSORP 28SA (BEL Japan, Inc., Osaka). Powder samples were out-gassed in a He flow at 200 °C for 2 h before measurements were taken. The specific surface areas were calculated with the BET equation, and the mean pore size by the BJH method.
Powder X-ray diffraction (XRD) patterns were measured using an MXP-18 diffractometer (Bruker AXS) with Cu K radiation, operated at 40 kV and 150 mA. Raman spectra were obtained with a Raman spectrometer (LabRam Infinity, HORIBA, Ltd, Kyoto), with 632.81 nm excitation wavelength (laser power 30 mW) in the range 200–1,500 cm−1.
Iron dispersion over the catalysts was obtained with an NO chemisorption apparatus (Ohkura Riken Co., Ltd., Saitama). The catalysts were reduced at 573 K for 1 h in H2. Pulse NO chemisorption was performed using 10 % NO in He carrier gas.
The acidity of the catalysts was measured by a calorimeter (CSA-450G, Tokyo Riko, Co., Ltd., Tokyo) at 50 °C, using ammonia as a probe molecule.
Reaction Procedure
Esterification and transesterification with the solid catalyst were carried out in a batch-type high-pressure reactor (Tama Seiki Kogyo Co., Ltd., Tokyo). The equipment consists of a high-pressure cylindrical chamber (inside diameter 1.8 cm; length 30 cm), an inserted glass tube (inside diameter 1.5 cm; length 26 cm), an electric furnace with four holes for the high-pressure cylindrical chamber, and a motor for shaking the furnace. For investigating the effect of FFA on transesterification and esterification of Jatropha oil, 10 wt% of oleic acid was blended into the original Jatropha oil. Initially, a solid catalyst, MeOH and the mixed oil were charged in a glass tube. The glass tube was then inserted into the cylindrical chamber, and this chamber was purged with N2 three times to remove the air, and then pressurized up to the N2 initial pressure of 1.0 MPa. The chamber was then continuously shaken at the appropriate temperature for 3 h. Under the highest reaction temperature condition of 220 °C, the maximum chamber pressure was 2.7 MPa (with a net methanol vapor pressure of 1.7 MPa) for the MeOH/oil molar ratio of 27. For the highest MeOH/oil ratio of 218, the respective amounts of oil and catalyst were adjusted to maintain a comparable vapor pressure of methanol; that is, the chamber pressure was 3.2 MPa (with a net methanol vapor pressure of 2.2 MPa). After the catalytic runs, all of the reaction products including the solid catalysts were recovered from the glass tube after washing the inside of glass tube with a minimum amount of MeOH, and filtered with a 1.0 μm filter. In earlier work we determined that the relative standard deviations (%), i.e., standard deviations divided by the mean values of those values, were within 5.3 %, so the subsequent results were determined by a single run.
Analytical Methods
The reaction solutions then obtained were further filtrated with a 0.2 μm filter, and then evaporated with a rotary evaporator to remove the methanol. The finally obtained BDF products were settled for 2 h to precipitate glycerin (G) at the bottom. The upper layer of the obtained solutions was removed for GC analysis. An Agilent 6890N GC (Agilent Technologies Inc., Santa Clara, California) system, equipped with an auto-injector, flame ionization detector (FID), and DB-5HT capillary column, was used to determine the components of G, mono-glycerides (MG), di-glycerides (DG), triglyceride (TG) and FFA, and calibrated using the standards of 1,2,4-butanetriol, 1,2,3-tricaproylglycerol and FAME of more than 99 % purity. The contents of G, MG, DG, TG, FAME, FFA and unknowns were directly measured by GC analysis, but the total sum was around 98.9–101.4. So, each measured data was corrected by converting the total sum % of measured data into 100 % in total sum as shown in Eq. 1:
Even under the highest temperature (220 °C) in our reaction experiments, GC data showed no olefin isomerization of the unsaturated esters; and no glycerin decomposition, due to its higher decomposition temperature of 290 °C. Under the reaction temperature of 220 °C, small amounts of MeOH would be decomposed [12] (probably <10 % if any), but most of the MeOH will be in a vapor phase during our reaction experiments.
Results and Discussion
Properties of Catalysts
The XRD and Raman spectra showed no peaks of iron oxide species, even for the catalysts with the highest Fe loadings (Fe7.1/SiO2–N2 and Fe7.1/SiO2–O2) (data are not shown here). This indicates that the iron species were well dispersed on the SiO2 support. One of the main reasons for this high dispersion of iron species could be the use of the iron precursor of ammonium iron(III) citrate, because several oxide peaks were observed after using iron(III) nitrate.
The NO chemisorption data are shown in Table 2. NO adsorbed on the coordinatively unsaturated Fe2+ and Fe3+ cationic sites (Lewis acidic sites), as well as on the metallic Fe sites, appeared on the iron oxide species, and negligible adsorption occurred over the SiO2 surface. Therefore, the NO uptake based on the total amount of Fe loading offers a good indication of iron species dispersion. The dispersion of the FeX/SiO2–N2 catalysts was highly dependent on the amount of Fe loading, but consistently exceeded 29 % with the exception of the Fe0.07/SiO2–N2 catalyst. The lower dispersion at lower Fe loadings suggests that some strong interaction between highly dispersed iron species and the SiO2 support resulted in stabilization of the iron species and a decrease in the number of coordinatively unsaturated Fe cation sites. On the other hand, a higher dispersion of more than 40 % was obtained after calcination of the FeX/SiO2 catalysts under an O2 atmosphere.
Effect of Fe Loading on Catalytic Activity
Figure 1 shows the effect of the amount of iron loading in the FeX/SiO2–Y 2 catalysts on catalytic activity. The iron content (X) varied within the range of 0.07–7.1 wt% in the FeX/SiO2–N2 samples, and 2.1–7.1 wt% in the FeX/SiO2–O2 samples. The initial SiO2 was also tested and represented an Fe loading of 0 wt%. The tests were conducted under reaction conditions of 200 °C, 3 h, and 15 wt% of catalyst. In the case of the ironless sample, SiO2, the transesterification procedure generated a 45.0 % yield of FAME. In the case of the FeX/SiO2–N2 catalysts, small amounts of Fe loading, up to 0.35 wt%, dramatically increased the FAME yield (65.9 and 75.5 % FAME yield for Fe loading of 0.07 and 0.35 wt%, respectively). Up to an Fe loading of 2.1 wt%, the transesterification reaction continually increased, but began to equilibrate above 2.1 wt% (FAME yield of 89.1 and 91.7 % for Fe loading of 2.1 and 7.1 wt%, respectively). As shown in Fig. 1, the catalyst calcination atmosphere also affected the FAME yield, with calcination under a N2 atmosphere producing 10 % higher FAME yields than calcination under O2. Accordingly, for the FeX/SiO2 catalysts, the optimal iron loading is more than 4 wt%, preferably 7 wt%, and calcination under a N2 atmosphere is preferable.
This suggests that low valent Fe sites will be more effective in the transesterification reaction than highly oxidized Fe3+ sites. After the pre-reduction of Fe7.1/SiO2–O2 catalysts under the H2 flow, FAME yield increased from 81.0 (no H2 reduction) to 87.5 % (reduction temperature of 300 °C); and decreased to 78.0 % with the H2 reduction temperature of 500 °C. These volcanic relationships also suggest that the most active Fe species will be of the low valent sort.
As shown in Table 2 above, dispersion of the iron oxide species was 34 and 29 % for Fe4.2/SiO2–N2 and Fe7.1/SiO2–N2, respectively; compared to 45 and 40 % for Fe4.2/SiO2–O2 and Fe7.1/SiO2–O2, respectively. Taking these dispersion values into account, the coordinatively unsaturated Fe sites appearing after calcination under a N2 atmosphere were superior in FAME production activity, despite the lesser number of coordinatively unsaturated Fe sites.
Effect of Catalyst Content on Catalytic Performance
The influence of the amount of Fe4.2/SiO2–N2 catalyst on the FAME yield was investigated at a 27:1 molar ratio of methanol to oil, at 200 °C for 3 h. The experiments were carried out with various amounts of catalyst (1–35 wt% in relation to the amount of oil) and without catalyst (0 wt% in relation to the amount of oil). The results are shown in Fig. 2.
The transesterification reaction producing FAME was highly dependent upon the amount of catalyst applied. With no catalyst, the transesterification procedure produced almost a zero percent yield of FAME; but the mere presence of the catalyst dramatically increased the reaction rate. As shown in Fig. 2, when the amount of catalyst was increased from 1 to 25 wt%, the corresponding yield increased gradually from 61.9 % to a maximum value of 94.3 %. The FAME yield slightly decreased at 35 wt% of catalyst, but this may be due to a mixing problem involving the reactants and the solid catalyst in the reactor. Accordingly, in this reaction, the optimal amount of catalyst is considered to be from 15 to 25 wt%.
Effect of the MeOH/oil Ratio on Catalytic Performance
The influence of the methanol/oil molar ratio on the FAME yield was tested with 15 wt% of the Fe7.1/SiO2–N2 catalyst for 3 h, at 200 and 220 °C. The results obtained are illustrated in Fig. 3.
Clearly, the conversion rate increased with increasing methanol. When the molar ratio was 54:1 (tested at 200 °C), the FAME yield was 96.9 %, which exceeded the EN standard of more than 96.5 %. However, the reaction solution contained 2.35 wt% of MG, which did not meet the EN standard of <0.8 wt%. As the 200 °C curve in Fig. 3 demonstrates, when the methanol/oil molar ratio was increased from 109 to 218, the corresponding FAME yields were 98.1 and 97.7 %. However, the respective reaction solutions were composed of 1.28 and 1.34 wt% of MG, which also did not meet the EN standard. The 220 °C curve in Fig. 3 shows that the FAME yield at each methanol/oil molar ratio was greater than the corresponding yield at 200 °C. Table 3 shows the glyceride content in the reaction solutions at 220 °C, and the corresponding EN standard values. When the methanol/oil molar ratio was 109, the MG content was 0.97 wt%, which still did not meet the EN standard. However, when the methanol/oil molar ratio was 218, the content of MG, DG, TG, G, and total glycerin finally met the EN standard. The excess methanol present in the reaction can be recovered by simple distillation, but this would result in an increase in the cost of the biodiesel production process.
The transesterification reaction stoichiometrically requires 3 moles of methanol for each mole of TG. However, in practice the methanol loading needs to be high enough to shift the equilibrium favorably to the products. In general, acid-catalyzed transesterification needs high molar ratios of methanol to oil (30–150:1), due to its relatively slow reaction rate [13] in comparison to that involving alkaline catalysts. According to our results, a methanol/oil molar ratio of 218 was required to shift the equilibrium in order to meet the MG, DG and TG content values of the EN standard; and this ratio of 218:1 was fixed for the following tests.
Without question, future work in this area should include an attempt to reduce the MeOH/oil ratio in iron-based catalysts.
Effect of Reaction Temperature on Catalytic Performance
In order to study the influence of the reaction temperature on the FAME yield, experiments were conducted using 15 wt% of the Fe7.1/SiO2–N2 catalyst, with a methanol/oil molar ratio of 218, for 3 h, at various temperatures ranging from 190 to 220 °C. As can be seen in Fig. 4, the FAME yield was 91.0 % at 190 °C, and increased with the reaction temperature, reaching 99 % at 210 °C. The glyceride content in the reaction solution also met EN standards. This indicates that higher temperatures can increase the reaction rate. However, reaction temperatures above 210 °C did not produce a significant increase in the FAME yield, and this means that the transesterification reaction had reached equilibrium conditions with respect to the glyceride/MeOH relation.
Reusability of Catalysts
Reusability is a highly important characteristic of a given catalyst because it reduces the cost of the overall production process. In order to assess its reusability, the same Fe7.1/SiO2–N2 catalyst was used in four 3-h tests. The tests were carried out under the following reaction conditions: methanol to oil molar ratio of 218:1, 15 wt% of catalyst, at 220 °C. The used catalyst was separated from the reaction mixture by decantation, washed with acetone, and heated at 105 °C overnight, before it was reused in a second reaction cycle. FAME yields were maintained above 99 % in all the reactions. The important chemical content values for meeting the EN standard were those of the glycerides analyzed in the experiments concerning the methanol/oil molar ratio (Table 3). The glyceride content results for the product FAME, over the four runs, are shown in Fig. 5. TG could not be detected by GC in any of the reactions. The MG and G levels remained constant through all the reactions. However, the DG level slightly increased as the catalyst was reused over three cycles and became equilibrated. In any case, all the glyceride levels remained under the EN standard through all the reactions. The experimental results indicate that Fe7/SiO2–N2 is a stable and durable catalyst.
Effect of Co-existing FFA on Catalytic Performance
In order to confirm whether the Fe7.1/SiO2–N2 catalyst is active even in the presence of FFA, 10 % oleic acid was added to the Jatropha oil as a feedstock. Here, the reaction conditions were as follows: 15 wt% of catalyst to feed oil; a methanol/oil molar ratio of 27:1; and a reaction temperature of 200 °C. A lower methanol/oil ratio was employed, in comparison with the ratio of 218:1 above, in order to distinguish the effect of the FFA. The resulting FAME yield was 94.1 wt% (MG = 3.78 wt%, DG = 0.88 wt%, TG = 0.59 wt%, FFA = 0.33 wt% and unknowns = 0.32 wt%) with 11.3 wt% of FFA, compared to 91.7 wt% (MG = 4.89 wt%, DG = 1.56 wt%, TG = 1.32 wt%, FFA = 0.21 wt% and unknowns = 0.34 wt%) for the original Jatropha oil with 1.3 wt% of FFA. These results show that FeX/SiO2–N2 catalysts are acid tolerant, and that their acidic function appears to promote the transesterification reaction.
Effect of Acidity on Catalytic Performance
Before discussing the effect of the acidity of the iron catalysts, let us consider the effects of catalyst acidity on the transesterification reaction by using the reference acidic supports of SiO2–Al2O3 (Al2O3 content of 28 wt% and surface area of 369 m2/g) and Al2O3 (surface area of 204 m2/g). Classifying acidity measured by using NH3 adsorption calorimetry [14] into three kinds for convenience—that is, strong (with a heat of adsorption over 110 kJ/mol), medium (heat of adsorption between 110 and 90 kJ/mol), and weak (heat of adsorption between 90 and 70 kJ/mol) [15]—we may summarize their acidic properties as follows: the amount of strong acidity was 0.17, 0.17 and 0 mmol/g-cat for SiO2–Al2O3, Al2O3 and SiO2, respectively; the amount of strong and medium acidity was 0.38, 0.25 and 0 mmol/g-cat for SiO2–Al2O3, Al2O3 and SiO2, respectively; the amount of strong, medium and weak acidity was 0.90, 0.37 and 0.05 mmol/g-cat, respectively. After the preliminary transesterification experiments using rapeseed oil as a feedstock, FAME yields were 18.9, 13.1 and 50.4 % for SiO2–Al2O3, Al2O3 and SiO2, respectively. SiO2 support gave the highest FAME yields, despite the almost negligible acidity. This suggests that the silanol bond over the SiO2 dissociates into –SiO− and contributes to methoxide anion formation, followed by transesterification. On the other hand, SiO2–Al2O3, whose main acidity is of the Brønsted type, showed higher FAME yields than those of Al2O3, whose main acidity is of the Lewis type (along with lower amounts of Brønsted acidity) [16]. This suggests that Brønsted sites will contribute to the formation of protonated triglycerides followed by the nucleophilic attack by MeOH.
The acidity of the iron catalysts supported on SiO2 is shown in Fig. 6, but the acidic properties of SiO2 were the same as those of Fe0.07/SiO2–N2. When the iron loading was increased from 0.07 to 2.1 wt%, the acidity dramatically increased, as shown in Fig. 6. At iron loading values above 2.1 wt%, the acidity increased gradually, but was increasingly equilibrated. Comparing Fe4.2/SiO2–N2 and Fe4.2/SiO2–O2, the catalyst calcined in N2 was more acidic than that calcined in O2. These results show very similar tendencies to the results in Fig. 2; that is, a significant increase in FAME yield up to 2.1 wt% of Fe loading, and a slight increase in FAME yield over 2.1 wt% of Fe loading. The acidic properties of Fe7.1/SiO2–N2 were similar to those of the Al2O3 support, but they differed in respect of the redox properties of the metal cation in the coordinatively unsaturated sites (Lewis acidic sites)—that is, in the redox properties of the Fen+ cation compared with Al3+ cation [17]. This point will be discussed below.
Figure 7 shows the relationship between the FAME yield and catalyst acidity. A strong correlation was obtained between the FAME yield and both the strong and combined strong-plus-medium acidity levels, but it is not clear which acidic sites would be most effective in promoting transesterification. However, the acidic function of the FeX/SiO2–Y 2 catalysts was confirmed to promote the FAME formation reaction.
Effect of the amounts of strong acidity or the total amounts of strong and medium acidity on FAME yields of the transesterification reaction with FeX/SiO2–Y 2 catalyst (X = 0.07–7.1, Y = N2: X = 4.2, Y = O2). Reaction conditions: methanol/oil molar ratio 27:1, catalyst/oil mass ratio 15 %, reaction time 3 h, reaction temperature 200 °C
Connell and Dumesic measured the IR absorption frequencies of the band for pyridine adsorbed on 0.5 wt% Fe/SiO2 treated in H2 at 220 °C [18]. They concluded that the acidity decreases in the following manner: coordinatively unsaturated Fe3+ sites > coordinatively unsaturated Fe2+ sites ≫ SiO2.
Therefore, in the case of our FeX/SiO2 catalysts, both of the coordinatively unsaturated Fe cation sites will co-exist for the Lewis acidic sites. The lower acidity of the Fe4.2/SiO2–O2 catalyst in comparison with Fe4.2/SiO2–N2 would be due to the formation of less acidic discrete Fe2O3 phases over the SiO2 in the case of the former catalyst, and to the formation of more reduced Fe oxide species and stronger interaction between the Fe oxide species and the SiO2, affecting Fe–O coordination, in the case of the latter catalyst.
As noted above, the properties of the coordinatively unsaturated Fe sites are discussed from the viewpoint of the acidic function of the catalysts; but these sites will also contribute to the MeOH activation, forming a methoxide anion through the coordination of methanol oxygen with the Fen+ cation. In addition, it is presumed that some redox properties of the Fen+ sites will promote the methanol adsorption, and the subsequent activation of MeOH.
The transesterification process involves three steps: first, a nucleophilic attack on a carboxylic carbon of the triglyceride (TG) by a methoxide anion, followed by a rupture of the ester bond between the fatty acid monoester and the di-glyceride (DG); second, a similar reaction, but with the formation of a fatty acid monoester and mono-glyceride (MG) from the DG; third, a similar reaction, but with the formation of a fatty acid monoester and glyceride from the MG. In this transesterification reaction, alkaline catalysts are superior in promoting methoxide anion formation from the methanol. In contrast, acidic catalysts are less active in methoxide anion formation, but could activate the carbonyl bonds via H+ addition (Brønsted acidic sites) or via coordination of the carbonyl oxygen with the coordinatively unsaturated Fe cation sites (Lewis acidic sites), and thereby promote transesterification. In addition, the coordinatively unsaturated Fe sites will participate in methoxide anion formation, and promote the subsequent transesterification reaction. Therefore, as shown in Figs. 6 and 7, an increase in the number of Lewis acidic sites would result in the promotion of FAME formation via transesterification, in addition to the transesterification performance of SiO2 itself. However, it is not clear whether strong acidic sites (heat of adsorption of NH3 > 110 kJ/mol) or medium strength acidic sites (90 kJ/mol < heat of adsorption of NH3 < 110 kJ/mol) are more effective in promoting the transesterification reaction. Stronger Lewis acidic sites would appear to be preferable owing both to their activation of the carboxyl carbon via coordination of the carbonyl oxygen with the coordinatively unsaturated Fe cation sites, and to their contribution to the MeOH activation.
Conclusions
Transesterification of crude Jatropha oil with methanol was performed using iron oxide catalysts supported on silica as heterogeneous catalysts. Under the reaction conditions of a reaction temperature of 220 °C, a catalyst amount of 15 wt% to Jatropha oil, a methanol/oil molar ratio of 218:1, and a reaction time of 3 h, a Fe2O3 (Fe = 7.1 wt%)/SiO2 catalyst, calcined under a N2 flow, produced a high yield of FAME in the product (>97.0 %), and met the EN standards for allowable contents of glycerine and mono-, di-, and tri-glycerides. Moreover, the advantages offered by this catalyst were not inhibited by the presence of coexisting FFA, and the catalyst could be used for four cycles without loss of activity or leaching. The correlation between the FAME production activity and measured acidity of the FeO x /SiO2 catalysts showed that the transesterification reaction was promoted via the catalysts’ acidic function, which would be apparent over the coordinatively unsaturated Fe oxide species dispersed over the SiO2.
References
Ma F, Hanna MA (1999) Biodiesel production: a review. Bioresour Technol 70:1–15
Gryglewicz S (1999) Rapeseed oil methyl esters preparation using heterogeneous catalysts. Bioresour Technol 70:249–253
Bournay L, Casanave D, Delfort B, Hillion G, Chodorge JA (2005) New heterogeneous process for biodiesel production: a way to improve the quality and the value of the crude glycerin produced by biodiesel plants. Catal Today 106:190–192
Kim M, DiMaggio C, Yan S, Wang H, Salley SO, Simon Ng KY (2011) Performance of heterogeneous ZrO2 supported metaloxide catalysts for brown grease esterification and sulfur removal. Bioresour Technol 102:2380–2386
Ngo HL, Zafiropoulos NA, Foglia TF, Samulski ET, Lin W (2008) Efficient two-step synthesis of biodiesel from greases. Energy Fuels 22:626–634
Berchmans HJ, Hirata S (2008) Biodiesel production from crude Jatropha curcas L. seed oil with a high content of free fatty acids. Bioresour Technol 99:1716–1721
Hayyan A, Alam MZ, Mirghani MES, Kabbashi NA, Hakimi NINM, Siran YM, Tahiruddin S (2011) Reduction of high content of free fatty acid in sludge palm oil via acid catalyst for biodiesel production. Fuel Process Technol 92:920–924
Jitputti J, Kitiyanan B, Rangsunvigit P, Bunyakiat K, Attanatho L, Jenvanitpanjakul P (2006) Transesterification of crude palm kernel oil and crude coconut oil by different solid catalysts. Chem Eng J 116:61–66
Juan JC, Kartika DA, Wub TY, Hin TYY (2011) Biodiesel production from jatropha oil by catalytic and non-catalytic approaches: an overview. Bioresour Technol 102:452–460
Peng BX, Shu Q, Wang JF, Wang GR, Wang DZ, Han MH (2008) Biodiesel production from waste oil feedstocks by solid acid catalysis. Process Saf Environ Protect 86:441–447
Semwal S, Arora AK, Badoni RP, Tuli DK (2011) Biodiesel production using heterogeneous catalysts. Bioresour Technol 102:2151–2161
Brown JC, Gulari E (2004) Hydrogen production from methanol decomposition over Pt/Al2O3 and ceria promoted Pt/Al2O3 catalysts. Catal Commun 5:431–436
Lotero E, Liu YJ, Lopez DE, Suwannakarn K, Bruce DA, Goodwin JG Jr (2005) Synthesis of biodiesel via acid catalysis. Ind Eng Chem Res 44:5353–5363
Restola G, Auroux A (2010) Surface acid–base characterization of containing group IIIA catalysts by using adsorption microcalorimetry. In: Occeli ML (ed) Advances in fluid catalytic cracking. CRC Press, Boca Raton, pp 199–256
Mochizuki T, Toba M, Morita Y, Yoshimura Y (2008) Effect of Yb loading on aromatic hydrogenation activity of Pd-Pt/USY zeolite catalysts. J Jpn Pet Inst 51:58–64
Tanabe K (1970) Solid acids and bases. Kodansha/Academic Press, Tokyo/New York
(2004) Thermodynamic Database MALT for Windows, MALT Group, Kagaku Gijutsu-Sha, Tokyo
Connell G, Dumesic JA (1986) Acidic properties of binary oxide catalysts. J Catal 101:103–113
Acknowledgments
This work was supported by the Japan Science and Technology Agency, and the Science and Technology Research Partnership for Sustainable Development.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Suzuta, T., Toba, M., Abe, Y. et al. Iron Oxide Catalysts Supported on Porous Silica for the Production of Biodiesel from Crude Jatropha Oil. J Am Oil Chem Soc 89, 1981–1989 (2012). https://doi.org/10.1007/s11746-012-2101-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-012-2101-3