Abstract
A new, rapid, and direct method was developed for the determination of moisture content in biodiesel produced from various types of oils using Fourier transform infrared (FTIR) spectroscopy with an attenuated total reflectance (ATR) element. Samples of biodiesels used in this study were produced using sludge palm oil (SPO). The calibration set was prepared by spiking double-distilled water into dried biodiesel samples in ratios (w/w) between 0 and 10% moisture. Absorbance values from the wavelength regions 3,700–3,075 and 1,700–1,500 cm−1, and the partial least square (PLS) regression method were used to derive a FTIR spectroscopic calibration model for moisture content in biodiesel samples. The coefficient of determinations (R 2) for the models was computed by comparing the results obtained from FTIR spectroscopy against the values of the moisture concentrations (%) determined using the American Oil Chemists’ Society (AOCS) oven method Ca 2d-25. Same comparison was done using International Union of Pure and Applied Chemistry (IUPAC) distillation method 2.602. R 2 was 0.9793 and 0.9700 using AOCS and IUPAC methods, respectively. The standard error (SE) of calibration was 1.84. The calibration model was cross validated within the same set of samples, and the standard deviation (SD) of the difference for repeatability (SDDr) and accuracy (SDDa) of the FTIR method was determined. With its speed and ease of data manipulation, FTIR spectroscopy is a useful alternative method to other methods for rapid and routine determination of moisture content in biodiesel for quality control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although fats and oils and their products, such as biodiesel, are traded on a dry basis and packaging is generally made under optimum conditions, it is virtually impossible to keep liquid, semisolid or solid materials absolutely anhydrous despite their hydrophobic nature. The solubility of water in common fats and oils is varied, and is as high as 0.05–0.30% without physical evidence of its presence [1]. Biodiesel may contain small but problematic quantities of water. Although it is not miscible with water, it is, like ethanol, hygroscopic (absorbs water from atmospheric moisture) [1]. One of the reasons biodiesel can absorb water is the persistence of mono- and diacylglycerols left over from an incomplete reaction. These molecules can act as emulsifiers, allowing water to mix with the biodiesel [2]. In addition, there may be water that is residual from processing or resulting from storage tank condensation. Water contamination is also a potential problem when using certain chemical catalysts involved in the production process, substantially reducing the catalytic efficiency of base (high pH) catalysts such as potassium hydroxide. However, the supercritical methanol production methodology, whereby the transesterification process of oil feedstock and methanol is effectuated under high temperature and pressure, has been shown to be largely unaffected by the presence of water contamination during the production phase [2].
The presence of water in biodiesel is a problem because water can cause corrosion of fuel system components such as pumps, injectors and fuel line tubes [3]. Water can also negatively affect the heat of combustion, which leads to less power (increased fuel consumption) and may produce smoke. If water contamination in such amounts allows microbes to grow, especially upon storage, this may lead to affecting the engine components such as filters and pumps. At low temperatures <0 °C, water may freeze, affecting the quality of biodiesel negatively, just like gelling and nucleation [4, 5].
Previously, the amount of water contaminating biodiesel has been difficult to measure by taking samples. However, it is now possible to measure the water content using water-in-oil sensors. The moisture and volatile matter methods have been developed to provide accurate moisture assay in minimum test time. The American Oil Chemists’ Society (AOCS) official methods [6], such as hot-plate (Ca 2b-38), air oven (Ca 2c-25), vacuum oven (Ca 2d-25) and others involve the evaporation of water by heating with or without the use of a vacuum for the quickest drying without damaging solids by decomposition, charring or loss of weight as the result of pyrolysis of portions of the oil. Distillation method 2.602 of the IUPAC [7], official method Ca 2a-45 of the AOCS [2] and American Association of Cereal Chemists AACC method 44–51 [8] were used for determination of water in fats, oils and their products as entrainment methods. However, they are less accurate for less than 0.05% moisture. Most of the above-mentioned methods are rather time consuming and/or have been found to give varying results. In the modified hot-plate method of the AOCS Ca 2f-93 [9], the addition of acetone serves to avoid splattering of the fat or oil during evaporation of the moisture. The Karl-Fisher method [3] eliminates the need for heat, plus it is rapid and convenient when compared with older methods; however, it is environmentally hazardous because of using chemicals such as iodine, sulfur dioxide, pyridine, chloroform and anhydrous methanol. FTIR spectroscopy had been used to determine fat and/or moisture in high-fat products using 1-propanol as solvent [10].
The objective of the present study is to use FTIR spectroscopy to develop a convenient, rapid method for the determination of the moisture content in biodiesel. The biodiesel samples used in this study were produced from sludge palm oil (SPO).
Materials and Methods
Crude biodiesel (CBD) samples were produced from sludge palm oil (SPO), which was provided by Sime Darby Sdn. Bhd. Malaysia. Samples were prepared in the laboratories of the Biotechnology Engineering Department (BTE) at the Faculty of Engineering, International Islamic University Malaysia (IIUM). Each CBD sample was dried in a vacuum oven for 2 ± 1 h. The drying was carried out at 75 °C with internal oven pressure of 13–15 kPa under nitrogen blanketing for 1 h. Heating was repeated several times, with addition of nitrogen gas on top of the oil prior to each heating to avoid oxidation, until constant weight after cooling to room temperature in desiccators containing activated silicates.
Twenty calibration samples in triplicate were derived by spiking double-distilled water in a certain ratio (w/w) covering the range of 0–10% moisture. Samples were neatly covered and agitated vigorously using Autovortex mixture SA1 (Stuart Scientific, UK) to ensure homogeneity of the samples.
Methods of Analysis
Distilled water was spiked in the purified and dried biodiesel to construct the calibration curve. Attenuated total reflectance (ATR) element (ZnSe) was used to obtain the spectra using the mid-IR region of 4,000–400 cm−1. The partial least square (PLS) regression method was used to derive a FTIR spectroscopic calibration model for moisture content in biodiesel samples.
AOCS Vacuum Oven Method
The calibration samples were analyzed in triplicate for determination of moisture content and volatile matter using the AOCS method Ca 2d-25 [6] in an Eyela Vacuum Drying Oven VOS 450SD (Rikakikai Co. Ltd., Tokyo, Japan).
IUPAC Distillation Method
The same samples were also analyzed in duplicate for moisture content using the IUPAC distillation method 2.602 (entrainment method) for determination of water [7].
Fourier Transform Infrared (FTIR) Spectroscopy Scanning
Each sample was shaken vigorously using an Autovortex Mixture SAI (Stuart Scientific, UK), and a few drops of the sample were placed on top of the ATR unit, which was fitted in the sample holder in the FTIR spectrophotometer. Scanning was carried out for each sample. ATR was rinsed at least three times with pure acetone then dried. Samples were scanned using an FTIR spectrophotometer (Perkin-Elmer Corp., Norwalk, CT) with a deuterated triglycine sulfate (DTGS) detector connected to a Perkin-Elmer model 100 professional computer. Infrared data system (IRDM) software was used during FTIR data collection. FTIR data were collected from 40 scans at a resolution of 2 cm−1 and strong apodization through the 4,000–600 cm−1 region. All spectra were referenced against the background air spectrum, and they were recorded as absorbance values at each data point.
The software program Spectrum version 3.02.01 Perkin-Elmer was used to construct a model of entire samples for calibration development. A partial least square (PLS) approach was developed for mathematical treatments of FTIR data [11].
The adequacy of FTIR calibration was assessed on the goodness of fit between distillation method data and FTIR prediction of moisture content values of the 20 samples used during the calibration process. The same comparison of fitness was done between the vacuum oven method data and FTIR prediction of moisture content values for the same samples. In both cases, the assessment of accuracy for the PLS equation was based on the standard error (SE) and the multiple correlation coefficient of determination (R 2) [12].
Validation
The “leave-one-out” cross-validation technique was used for the two test calibration models, and the accuracy of each equation was assessed according to the standard error of prediction (SEP) and coefficient of determination (R 2). The validation step was further investigated by using the mean difference (MD) and standard deviation of the difference (SDD) for repeatability and accuracy between the predicted FTIR data against moisture content values of the AOCS vacuum oven method and the IUPAC distillation method.
Results and Discussion
Moisture Content Measured by AOCS Vacuum Oven and IUPAC Distillation Methods
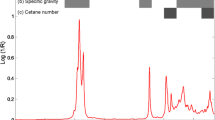
The range of triplicate moisture content of the CBD data set was from 0 to 10% for calibration and cross validation. The means and standard deviation (SD) for the moisture content values of calibration and cross-validation data set are 4.885, 0.860 and 4.740, 0.570 respectively, for the AOCS method and 4.739, 0.850 and 4.632, 0.450 respectively, for the IUPAC method, as shown in Table 1. Figure 1 shows the spectrum of pure dried biodiesel produced from SPO that showed peaks at 2,920, 2,852, 1,743, 1,464, 1,377, 1,159, 1,117 and 722 cm−1 for functional groups C–H, C=O, C=C, C–O and C–C, respectively, which was similar to other vegetable oils [10–12].
Absorption Bands of Water
Hydrogen (H)-bonded stretching vibration of the O–H group has highly characteristic absorption in the frequency region of 3,150–3,500 cm−1, as reported by van de Voort et al. [13]. The absorption at the same above region in the spectrum of KBr was assigned to water [14]. Che Man and Mirghani [11] were able to establish a correlation between moisture content in crude palm oil and FTIR spectra at the 3,074–3,700 cm−1 region. Wadumesthrige et al. [15] showed an IR absorption peak at 3,450 cm−1, attributed to the O–H stretching of carboxylic acids in oxidized biodiesel.
Water has HO–H vibration at the frequency of 1,650 cm−1 in fatty foods, as reported by van de Voort et al. [10] and by Safar et al. [16]. Moisture content as water % in anhydrous methanol (MeOH) had been measured by using the water absorption at 1,650 cm−1 in their FTIR spectra [17]. The spectra of non-dried KBr pellets showed some risk of interference from –OH bending modes of water contaminating the KBr at the 1,650–1,670 cm−1 region [18]. The spectra of water have been shown by many researchers ([13, 19, 20]. The mean spectrum of CBD spiked by water is shown in the correlation spectrum (Fig. 2), which showed the –OH stretching absorption of H-bonding hydroxyl groups at the 3,075–3700 cm−1 region, however, not for the varying amount of the –OH-containing compounds, such as mono- and diacylglycerols, free fatty acids and hydroperoxides as in oxidized oil samples, if any [21–24]. The 1,500–1,700 cm−1 region in the correlation spectrum (Fig. 2) showed a correlation band for water, but this was less intense than the 3,075–3,700 cm−1 region; however, both regions were selected for this study. A weak band at 1,640–1,615 cm−1 for H–OH bending vibration was determined by Nakanishi and Solomon [25].
Figure 2 shows the mathematical correlation between spectral changes with the changes in the moisture content as described by Fuller [26]. The two regions at 3,075–3,700 and 1,500–1,700 cm−1 proved that there is a good correlation between absorbance and moisture content obtained from either of the two classical methods, AOCS or IUPAC. A mathematical correlation between spectral (absorption) changes with changes in moisture content values (0–10%) is shown in Fig. 3. A variance spectrum obtained from the calibration set is shown in Fig. 4. It was determined by calculating the average absorbance at each wave number position over the entire calibration data set and then calculating the square root of variance about the mean of the entire data set generating the variance spectrum.
FTIR calibration models were obtained using these wavelength regions and PLS regression for the prediction of moisture content for the CBD samples. Using the calibration data set, the SEC for the model was 0.87; the multiple correlation coefficient of the determination (R 2) was 0.9793. A good linear regression with the AOCS vacuum oven method-determined moisture content values against the FTIR spectroscopic predicted values was obtained (Y = 1.0404x), and gave an intercept and slope that were not significantly different (P > 0.05) from 0.0 and 1.0, respectively (Fig. 5).
Another calibration model was obtained using the IUPAC method and the FTIR spectroscopic predicted values using the same wavelength regions of 3,075–3,700 and 1,500–1,700 cm−1, and the PLS regression to predict moisture content for the same CBD set of samples. The SEC for this model was 0.95 and R 2 was 0.97. A linear regression of moisture content values against FTIR predicted values was obtained (Y = 0.9788x + 0.1), which also gave an intercept and slope that were not significantly different (P > 0.05) from 0.0 and 1.0, respectively (Fig. 6).
The covariance or coefficient of variation (CV) is used to describe the accuracy of the FTIR calibration, which is used to express the average of the product of deviation for each data point in the data set pair. Clark et al. [27] reported that a CV less than 20% is acceptable for most analytical purposes. The CV of both equations (Figs. 5, 6) in this study to predict moisture content in CBD was about 9–11%.
The “leave-one-out” cross-validation technique was approved to provide an estimate of the overall accuracy of predictions. The intercept and slope of the linear regression line are shown in Fig. 7. Plotting with the classical IUPAC distillation method determination versus the FTIR predicted moisture content value for the cross validation (Y = 0.9721x) was not significantly different (P > 0.05) from 0.0 and 1.0, respectively, with an SEP of 0.974 and R 2 of 0.9592. The mean difference MDa and standard deviation of difference SDDa were 0.0574 and 0.0183, respectively (Table 2).
When the “leave-one-out” cross-validation set was predicted with the calibration model of the AOCS vacuum oven method, the SEP was 1.823, R 2 was 0.9586, MDa was 0.0214, and SDDa was 0.0529. The intercept and slope for the linear regression line (Y = 1.2037x − 0.1532) were not significantly different (P > 0.05) from 0.0–1.0, respectively.
Table 2 compares the obtained data in terms of MD, SDD for accuracy (a) and repeatability (r) between duplicates for each of the two classical methods and FTIR-predicted results. The two classical methods and FTIR have comparable MDr and SDDr. The result in terms of MDa and SDDa indicated that the moisture content of CBD could be measured with good accuracy by FTIR spectroscopy. Comparison among the three methods used in this study is exemplified in Table 3, which shows the rapidity and sensitivity, small amount of sample, and that no chemicals were used in the FTIR method compared to the classical ones.
From this study, we hypothesize that the 3,075–3,700 and 1,500–1,700 cm−1 regions can be used to develop a calibration model to predict the moisture content in CBD samples. It could be concluded that FTIR spectroscopy equipped with an attenuated total reflectance (ATR) element could be used to develop equations for the determination of the moisture content in crude biodiesel (CBD). Absorbance values from the 3,075–3,700 and 1,500–1,700 cm−1 wavelength regions were found to be the best regions for the determination of moisture content in biodiesel samples. The coefficient of determinations (R 2) was 0.9793 and 0.97 for biodiesels produced from sludge palm oil (SPO) using the AOCS and IUPC methods, respectively. The calibration models were cross validated within the same set of samples, and the standard deviation (SD) of the difference for repeatability (SDDr) and accuracy (SDDa) of the FTIR method were found to be very low, indicating that FTIR spectroscopy is a useful alternative method to wet chemical methods for simple, rapid, accurate and cost-effective determination of the moisture content in biodiesel produced from sludge palm oil (SPO).
References
Sonntag NOV (1982) Analytical methods, in Bailey’s industrial oil and fat products, vol. II, 4th edn edn. Wiley, New York, pp 484–487
“Biodiesel Flower Power: Facts * Arguments * Tips” (PDF). At: http://www.biodiesel.org/resources/reportsdatabase/reports/gen/20040101_gen-331. pdf. by: Union zur Förderung von Oel- und Proteinpflanzen e. V. Reinhardtstraße 18 10117 Berlin www.ufop.de
Jones D (1996) Principles and prevention of corrosion, 2nd edn. Prentice Hall, Upper Saddle River
Mendez-Villuendas E, Bowles R (2007) Surface nucleation in the freezing of gold nanoparticles. Phys Rev Lett 98(18):185503
Young RG, Lovell PA (1991) Introduction to Polymers, 2nd edn. CRC Press, NY
American Oil Chemists’ Society (1989) AOCS vacuum oven method (Ca 2d-25) In: D. Firestone Official and tentative methods of the American Oil Chemists’ Society, American Oil Chemists’ Society; Champaign IL
International Union for Pure and Applied Chemistry (IUPAC) (1979) Standard Methods for Analysis of Oils, Fats and Derivatives, 6th edn. In: C. Paquot, International Union for Pure and Applied Chemistry (IUPAC), Commission on Oils, Fats and Derivatives, Pergamon Press, Methods (2.601 & 2.602), pp. 152–156
American Association of Cereal Chemist’s, (1995) Approved Methods of the AACC, 9th edn. the Association: St. Paul, MN., Method 44-15A
Hartman L, Jablonka FH (1992) A modified moisture and volatile matter determination in fats and oils. J Am Oil Chem Soc 69:1276
van de Voort FR, Sedman J, Ismail AA (1993) A rapid FTIR quality-control method for determining fat and moisture in high-fat products. Food Chem 48:213–221
Che Man YB, Mirghani MES (2000) Rapid method for determining moisture content in crude palm oil by Fourier transform infrared spectroscopy. J Am Oil Chem Soc 77:631–637
Dubois J, van de Voort FR, Sedman J, Ismail AA, Ramaswamy HR (1996) Quantitative Fourier transform infrared analysis for anisidine value and aldehydes in thermally stressed oils. J Am Oil Chem Soc 73:787–794
van de Voort FR, Ismail AA, Sedman J, Dubois J, Necodemo T (1994) The determination of the peroxide value by Fourier transform infrared FTIR spectroscopy. J Am Oil Chem Soc 71:921–926
Cross AD, Jones RA (1969) An introduction to practical infrared spectroscopy, 3rd edn. Butterworths, London, p 83
Wadumesthrige K, Smith JC, Wilson JR, Salley SO, Simon Ng KY (2008) Investigation of the parameters affecting the cetane number of biodiesel. J Am Oil Chem Soc 85:1073–1081
Safar M, Bertrand D, Robert P, Devaux MF, Genot C (1994) Characterization of edible oils, butters and margerines by Fourier transform infrared spectroscopy with attinuated total reflectance. J Am Oil Chem Soc 71:371–377
Ismail AA, van de Voort FR, Emo G, Sedman J (1993) Rapid quantitative determination of free fatty acids in fats and oils by Fourier transform infrared spectroscopy. J Am Oil Chem Soc 70:335–341
Painter P, Starsinic M, Coleman M (1985) Determination of functional groups in coal by Fourier transform interferometry. In: Ferraro JR, Basile LJ (eds) Fourier transform infrared spectroscopy applications to chemical systems, vol. 4. Academic Press, London, pp 189–200
Bell RJ (1971) Introduction to Fourier transform spectroscopy. Academic Press, London, pp 189–191
Luinge HJ, Hop E, Lutz ETG, van Hemert JA, de Jong EAM (1993) Determination of Fat Protein and Lactose Content of Milk Using FTIR Spectroscopy. Anal. Chem. Acta 284, 419, (In: McDonald RE, and Mossoba MM (eds) New Techniques and Applications in Lipid Analysis, (1997) AOCS Press, Champaign, Illinois, p. 14)
Aryee ANA, van de Voort FR, Simpson BK (2009) FTIR determination of free fatty acids in fish oils intended for biodiesel production. Process Biochem 44:401–405
Setiowaty G, Che Man YB, Selamat J, Moh F (2000) Quantitative determination of peroxide value in thermally oxidized palm olein by Fourier transform infrared spectroscopy. Phytochem Anal 11:1–5
Mirghani MES, Che Man YB, Jinap S, Baharin BS, Bakar J (2002) Rapid method for determining malondialdehyde as secondary oxidation product in palm olein system by Fourier transform infrared spectroscopy. Phytochem Anal 13:195–201
Mirghani MES, Kabashi NA, Qudsieh IY, Elfaki FA (2008) A new method for the determination of toxic dye using FTIR spectroscopy. IIUM Engineering J 9:17–38
Nakanishi K, Solomon PH (1977) Infrared absorption spectroscopy, 2nd edn. Holden-Day, Inc., San Francisco, p 6
Fuller MP, Ritter GL, Draper CS (1988) Partial least–square quantitative analysis of infrared spectroscopic data. Part I: algorithm implementation. Appl Spectrosc 42:217–227
Clark DH, Mayland HF, Lamb RC (1987) Mineral analysis of forages with near infrared reflectance (NIR) spectroscopy. J Agron 97:485–490
Acknowledgments
The authors thank the International Islamic University Malaysia (IIUM) for providing the funding for this study. The authors are grateful to the Department of Biotechnology Engineering, Faculty of Engineering, for allowing the use laboratory facilities such as the FTIR spectrometer and other instruments.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Mirghani, M.E.S., Kabbashi, N.A., Alam, M.Z. et al. Rapid Method for the Determination of Moisture Content in Biodiesel Using FTIR Spectroscopy. J Am Oil Chem Soc 88, 1897–1904 (2011). https://doi.org/10.1007/s11746-011-1866-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-011-1866-0